Effects of Sphaeropsis Blight on Rhizosphere Soil Bacterial Community Structure and Soil Physicochemical Properties of Pinus sylvestris var. mongolica in Zhanggutai, China
Abstract
:1. Introduction
2. Materials and Methods
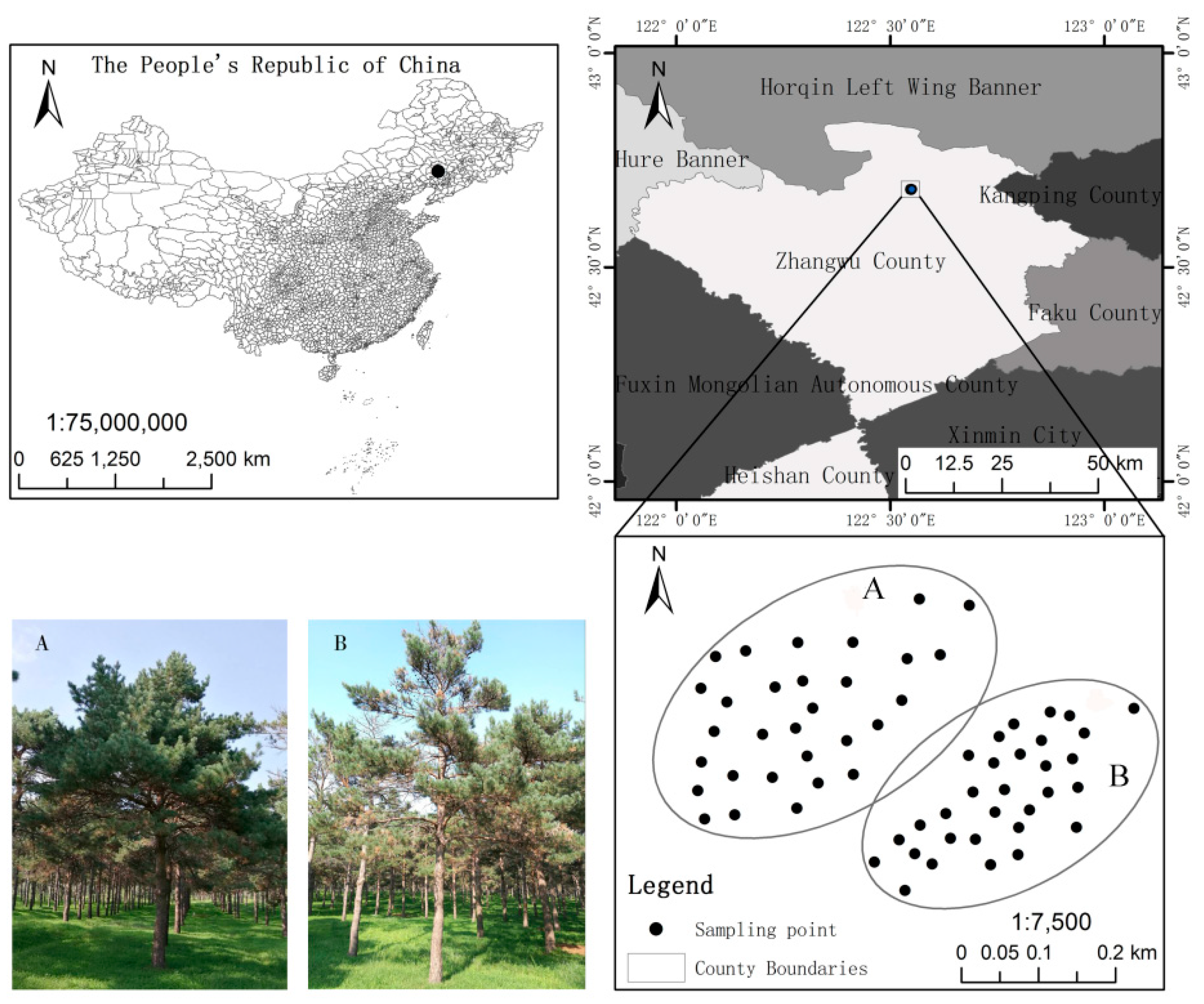
2.1. Site Description
2.2. Soil Sampling
2.3. Analysis of Physicochemical Properties of Soil
2.4. DNA Extraction
2.5. PCR Amplification and Illumina MiSeq Sequencing
2.6. Bioinformatic Analysis
2.7. Data Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Bacterial Community Diversity
3.3. Soil Bacterial Community Structure
3.4. Relationship between Soil Bacterial Community Diversity and Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gans, J. Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Vance, G.; Pendall, E.; Stahl, P. Timber harvesting alters soil carbon mineralization and microbial community structure in coniferous forests. Soil Boil. Biochem. 2008, 40, 1901–1907. [Google Scholar] [CrossRef]
- Madsen, E.L. Microorganisms and their roles in fundamental biogeochemical cycles. Curr. Opin. Biotechnol. 2011, 22, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Nacke, H.; Thürmer, A.; Wollherr, A.; Will, C.; HoDac, L.; Herold, N.; Schöning, I.; Schrumpf, M.; Daniel, R. Pyrosequencing-Based Assessment of Bacterial Community Structure Along Different Management Types in German Forest and Grassland Soils. PLoS ONE 2011, 6, 17000. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.O.; Frohne, P.S.; Kennedy, A.C. Dynamics of a Soil Microbial Community under Spring Wheat. Soil Sci. Soc. Am. J. 2002, 66, 826. [Google Scholar] [CrossRef]
- Doran, J.W.; Sarrantonio, M.; Liebig, M.A. Soil Health and Sustainability. Adv. Agron. 1996, 56, 1–54. [Google Scholar]
- Ben, J.J.L.; Chin-A-Woeng, T.F.C.; Bloemberg, G.V. Microbe–plant interactions: Principles and mechanisms. Antonie Van Leeuwenhoek 2002, 81, 373–383. [Google Scholar]
- HU, J.L. Scientific connotation and ecological service function of soil microbial diversity. Acta Pedol. Sin. 2008, 5, 892–900. [Google Scholar]
- Pace, N.R. A Molecular View of Microbial Diversity and the Biosphere. Science 1997, 276, 734–740. [Google Scholar] [CrossRef]
- Torsvik, V.; Goksøyr, J.; Daae, F.L. High diversity in DNA of soil bacteria. Appl. Environ. Microbiol. 1990, 56, 782–787. [Google Scholar] [Green Version]
- Agnelli, A.; Ascher, J.; Corti, G.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G.; Ascher-Jenull, J. Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Boil. Biochem. 2004, 36, 859–868. [Google Scholar] [CrossRef]
- Hackl, E.; Zechmeister-Boltenstern, S.; Bodrossy, L.; Sessitsch, A. Comparison of Diversities and Compositions of Bacterial Populations Inhabiting Natural Forest Soils. Appl. Environ. Microbiol. 2004, 70, 5057–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackl, E.; Pfeffer, M.; Donat, C.; Bachmann, G.; Zechmeisterboltenstern, S. Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Boil. Biochem. 2005, 37, 661–671. [Google Scholar] [CrossRef]
- Li, D.F.; Yang, R.F. Next-generation sequencing technologies and the application in microbiology—A review. Acta Microbiol. Sin. 2011, 51, 445–457. [Google Scholar]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Seo, T.S.; Bai, X.; Kim, D.H.; Meng, Q.; Shi, S.; Ruparel, H.; Li, Z.; Turro, N.J.; Ju, J. Four-color DNA sequencing by synthesis on a chip using photocleavable fluorescent nucleotides. Proc. Natl. Acad. Sci. USA 2005, 102, 5926–5931. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.X.; Meng, P. Countermeasures for the decline and sustainable development of artificial pure forest land of pinus camphora in sandy land of Liaoning province. Prot. For. Sci. Technol. 2005, 3, 86–87. [Google Scholar]
- Chen, F.S.; Zeng, D.H.; Fan, Z.P.; Chen, G.S.; Yu, Z.Y.; Zhao, Q. Available nitrogen in forest soil of Pinus sylvestris var. mongolica plantations in Zhanggutai sandy lands. J. Beijing For. Univ. 2005, 27, 6–11. [Google Scholar]
- Zeng, D.H.; You, W.Z.; Fan, Z.P.; Liu, M.G. Analysis of natural regeneration barriers of Pinus sylvestris var. mongolica plantation on sandy land. J. Appl. Ecol. 2002, 13, 257–261. [Google Scholar]
- Chang, Z.L.; Wu, X.G.; Liu, G.R.; Li, S.H.; Wang, E.L. The causes and countermeasures of fattening and dying of pinus camphora in Liaoning province. J. Liaoning For. Sci. Technol. 2003, 5, 31–34. [Google Scholar]
- Wu, X.G.; Liu, G.R.; Liu, G.R.; Li, S.H.; Tan, X.W. The causes and countermeasures of fattening and dying of pinus camphora in Liaoning province. J. Inn. Mong. For. Sci. Technol. 2003, 3, 16–20. [Google Scholar]
- Song, X.D. Preliminary discussion on the causes and prevention of the death of pinus camphora. Liaoning For. Sci. Technol. 1994, 3, 70–71. [Google Scholar]
- Jiao, S.R. Report on the causes of the early decline of Pinus slyvestris var. mongolica shelterbelt and its preventative and control measures in Zhanggutai of Liaoning province. Sci. Silvae Sin. 2001, 37, 131–138. [Google Scholar]
- Jiao, S.R. Introduction & a fforestation of Pinus sylvestris var. mongolica in Zhanggutai area in Liaoning province. Prot. For. Sci. Technol. 2009, 6, 10–14. [Google Scholar]
- Zhang, Y.X. Relationship between growth of Pinus sylvestris var. mongolica and weather soil in Zhanggutai area. J. Liaoning Agric. Coll. 2008, 10, 2. [Google Scholar]
- Jiao, S.R. Studie s on the hydrologic dynamics in the pine plantations in Zhanggutai, Liaoning Province. Acta Phytoecol. Geobot. Sin. 1987, 10, 296–307. [Google Scholar]
- Zeng, D.H.; Hu, Y.L.; Chang, S.X.; Fan, Z.P. Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil 2009, 317, 121–133. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, Y.P.; Liu, S.L. Research on Rhizosphere Soil Properties of Main Tree Species of Sand-fixation Forest in Zhanggutai. J. Desert Res. 2004, 24, 74–78. [Google Scholar]
- Zhang, X.L.; Yang, S.J.; Zhang, B.X.; Bai, X.F. Research on rhizosphere soil properties of Pinus sylvestris var. mongolica with different infection grades. For. Res. Chin. Acad. For. 2006, 19, 88–92. [Google Scholar]
- Zhou, F.Y. The research of effect of different age of stand and density of sand Pinus svlvestris var mongolica artificial forest of Liaoning Zhanggutai on height growth. J. Jilin For. Sci. Technol. 2017, 46, 4–8. [Google Scholar]
- Wei, X.T.; Lei, Z.Y.; Han, H. Soil moistures characteristics of different—Aged Pinus sylvestris var. Mongolica artificial forests in Zhanggutai sandy area. J. Arid Land Resour. Environ. 2016, 30, 115–121. [Google Scholar]
- Gu, Y. The Facts that Led the Degradation of Artificial San-Fixation Pinus Svlvestris var. Mongolica and Its Prevention Measure; Liaoning Project Technology University: Liaoning, China, 2009. [Google Scholar]
- Collignon, C.; Uroz, S.; Turpault, M.P.; Frey-Klett, P. Seasons differently impact the structure of mineral weathering bacterial communities in beech and spruce stands. Soil Boil. Biochem. 2011, 43, 2012–2022. [Google Scholar] [CrossRef]
- Shanmugam, V.; Verma, R.; Rajkumar, S.; Naruka, D.S. Bacterial diversity and soil enzyme activity in diseased and disease free apple rhizosphere soils. Ann. Microbiol. 2011, 61, 765–772. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.; Tisserand, E.; Cébron, A.; Turpault, M.P.; Buée, M.; De Boer, W.; Leveau, J.H.J.; Frey-Klett, P. Specific impacts of beech and Norway spruce on the structure and diversity of the rhizosphere and soil microbial communities. Sci. Rep. 2016, 6, 27756. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Plant Analysis, 3rd ed.; Agricultural Press of China: Beijing, China, 2000; pp. 25–109. [Google Scholar]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637–638, 1400–1412. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Boil. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Peng, W.; Bo, C.; Hua, Z. High throughput sequencing analysis of bacterial communities in soils of a typical Poyang Lake wetland. Acta Ecol. Sin. 2017, 37, 1650–1658. [Google Scholar] [Green Version]
- Fu, Y.J.; Zhang, J.L.; Hou, X.Q. Comparative analysis of fungi diversity in rizospheric and non-rhizospheric soil from cypripedium macranthum estimated via high-throughput sequencing. Acta Agric. Boreali Occident. Sin. 2019, 28, 1–7. [Google Scholar]
- Yang, L.B.; Sui, X.; Zhu, D.G.; Cui, F.X.; Li, B.J.; Song, R.Q.; Ni, H.W. Study on fungal communities characteristics of different Larix gmelini forest typesin cold temperate zone. J. Cent. South Univ. For. Technol. 2017, 37, 76–84. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Timmy, S.; Yarza, P.; Peplies, P.; Glo¨ckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F.; Nilsson, H. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.T.; Yang, L.B.; Xu, N.; Chai, C.R.; Wang, J.F.; Fu, X.L. Effect of simulation nitrogen depositions on bacterial diversity of Deyeuxia angustifoliain wetland of Sanjiang Plain. Pratacultural Sci. 2016, 33, 589–598. [Google Scholar]
- Yang, L.B.; Sui, X.; Zhu, D.G.; Cui, F.X.; Li, J.B.; Song, R.Q.; Ni, H.W. Study on soil microbial biomass and community composition of Larix gmelinii in cold temperate zone. J. Cent. South Univ. For. Technol. 2018, 38, 67–75. [Google Scholar]
- Ji, P.; Rhoads, W.J.; Edwards, M.A.; Pruden, A. Impact of water heater temperature setting and water use frequency on the building plumbing microbiome. ISME J. 2017, 11, 1318–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Sun, X.X.; Zhang, X.C.; Zhang, S.; Lu, J.; Xia, Y.M. The interactions between gut microbiota and entomopathogenic fungi: A potential approach for biological control of Blattella germanica (L.). Pest Manag. Sci. 2018, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, B.; Tan, J.; Zhu, L.; Lou, D.; Cen, X. Comparative analysis of the gut microbiota of black bears in china using high-throughput sequencing. Mol. Genet. Genom. 2017, 292, 407–414. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, J.H.; Friedman, C.R.; Wang, H.F. Variation of Soil Bacterial Communities in a Chronosequence of Rubber Tree (Hevea brasiliensis) Plantations. Front. Plant Sci. 2017, 8, 849. [Google Scholar] [CrossRef]
- Schimel, D.; Melillo, J.M.; Tian, H.Q.; McGuire, A.D.; Kicklighter, D.; Rosenbloom, N. Contribution of Increasing CO2 and Climate to Carbon Storage by Ecosystems in the United States. Science 2000, 287, 2004–2006. [Google Scholar] [CrossRef]
- Preston-Mafham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles–a critique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar]
- I Burd, G.; Dixon, D.G.; Glick, B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, J.; Shim, H.; Bai, Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environ. Int. 2007, 33, 406–413. [Google Scholar] [CrossRef]
- Cleyet-Marcel, J.C.; Larcher, M.; Bertrand, H. Plant growth enhancement by rhizobacteria. In Nitrogen Assimilation by Plants: Physiological, Biochemicval and Molecular Aspects; Morot Gaudry, J.F., Ed.; Science Publishers: Plymouth, UK, 2001; pp. 185–197. [Google Scholar]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida Indoleacetic Acid in Development of the Host Plant Root System. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Dey, R.; Pal, K.K.; Bhatt, D.M.; Chauhan, S.M. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Pasternak, J.J. Plant growth promoting bacteria. In Molecular Biotechnology Principles and Applications of Recombinant DNA, 3rd ed.; Glick, B.R., Pasternak, J.J., Eds.; ASM Press: Washington, DC, USA, 2003; pp. 436–454. [Google Scholar]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Boil. 2001, 28, 897. [Google Scholar] [CrossRef]
- Liu, C.L.; Zuo, W.Y.; Zhao, Z.Y.; Qiu, L.H. Bacterial diversity of different successional stage forest soils in Dinghushan. Acta Microbiol. Sin. 2012, 52, 1489–1496. [Google Scholar]
- Lin, Y.T.; Hu, H.W.; Whitman, W.B.; David, C.C.; Chu, C.Y. Comparison of Soil Bacterial Communities in a Natural Hardwood Forest and Coniferous Plantations in Perhumid Subtropical Low Mountains. Bot. Stud. 2014, 55, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Wang, L.; Yang, J.; Ma, X.S.; Wang, W.B.; Dong, Y.F. Understory vegetation patterns and soil characteristics of a Pinus thunbergii plantation in mountainous land of Shandong Province. J. Zhejiang A&F Univ. 2018, 35, 209–218. [Google Scholar]
- Song, X.D.; Zhang, R.S.; Wang, E.L.; Xu, G.J.; Liu, M. Ecological and economical benefits of agroforestry system in Pinus sylvestris var. mongolica plantation in sandy land. J. Liaoning For. Sci. Technol. 2014, 2, 1–4. [Google Scholar]
- Song, X.D.; Xu, G.J.; Chen, J.Y.; Wu, X.G.; Liu, J.R.; Sun, H.J. Silvicultural measures for controlling sphaeropsis shoot blight of Mongol Scotch pine in sandy area. For. Pest Dis. 2008, 3, 30–33. [Google Scholar]
- Jang, P.; Ye, J.R. Isolation and Screen of Antagonistic Bacteria Against Sphaeropsis and Bioassay on Seedling of Pine. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2007, 31, 144–146. [Google Scholar]
- Huang, J.F. Effect of Biocontrol to Shoot Blight Disease by Burkholderia Cenocepacia Strain NSM-05; Nanjing Forestry University: Nanjing, China, 2017. [Google Scholar]
- Tang, X. Screening of Antagonistic Bacteria against Sphaeropsis Sapines and Mechanism of Antagomism; Nanjing Forestry University: Nanjing, China, 2017. [Google Scholar]
- Dinesh, K. Maheshwari. Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–448. [Google Scholar]
- Poole, E.J.; Bending, G.D.; Whipps, J.M.; Read, J.D. Bacteria associated with Pinus sylvestris–Lactarius rufus ectomycorrhizas and their effects on mycorrhiza formation in vitro. New Phytol. 2001, 151, 743–751. [Google Scholar] [CrossRef]
- 8Wu, X.Q.; Huo, L.L.; Sheng, J.M.; Ren, J.H.; Zheng, L.; Chen, D.; Ye, J.R. Effects of ectomycorrhizal fungus Boletus edulis and mycorrhiza helper Bacillus cereuson the growth and nutrient uptake by Pinus thunbergii. Biol. Fertil. Soils 2012, 48, 385–391. [Google Scholar]
- Barabote, R.D.; Xie, G.; Leu, D.H.; Normand, P.; Necsulea, A.; Daubin, V.; Me’digue, C.; Adney, W.S. Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus 11B provides insights into its ecophysiological and evolutionary adaptations. Genome Res. 2009, 19, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, A.; Grohmann, K.; Himmel, M.; Leighton, L.; Updegraff, D.M. Isolation and Characterization of Acidothermus cellulolyticus gen. nov., sp. nov., a New Genus of Thermophilic, Acidophilic, Cellulolytic Bacteria. Int. J. Syst. Bacteriol. 1986, 36, 435–443. [Google Scholar] [CrossRef]
- Adney, W.S. Thermal tolerant exoglucanase from Acidothermus cellulolyticus. U.S. Patent 7,393,673, 1 July 2008. [Google Scholar]
- Tucker, M.P.; Grohmann, K.; Himmel, M.E.; Mohagheghi, A. Thermostable purified endoglucanases from the rmophilic bacterium acidothermus cellulolyticus. U.S. Patent 5,110,735, 5 May 1992. [Google Scholar]
- Karpunina, L.V.; Soboleva, E.F. Effect of the Rhizobium leguminosarum252 Agglutinins on the Activity of Certain Enzymes in Plant Cells. Microbiology 2001, 70, 295–298. [Google Scholar] [CrossRef]
- Verma, J.P.; Yadav, J.; Tiwari, K.N. Application of, Rhizobium, sp. BHURC01 and Plant Growth Promoting Rhizobactria on Nodulation, Plant Biomass and Yields of Chickpea (Cicer arietinum L.). Int. J. Agric. Res. 2010, 5, 148–156. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, X.; Smith, D.L.; Bai, Y.; Zhou, X.; Smith, D.L. Crop Ecology, Management & Quality Enhanced Soybean Plant Growth Resulting from Coinoculation of Bacillus Strains with Bradyrhizobium japonicum. Crop Sci. 2003, 43, 1774–1781. [Google Scholar]
- Biekejian, B.; Deng, Y.X.; Yin, D.C.; Song, R.Q. Screening of Ectomycorrhizal Fungi for Promoting the Growth of Seedlings of Pinus sylvestris var. mongolica. For. Sci. Technol. 2017, 42, 31–33. [Google Scholar]
- Deng, X.; Song, X.S.; Yin, D.C.; Song, R.Q. Effect of inoculating Phialocephala fortinii D575 and Suillus luteus N94 on the growth of Pinus sylvestris var. mongolica and its resistant to damping–off. Chin. For. Pests 2017, 36, 21–25. [Google Scholar]
- Song, R.Q.; Deng, X.; Song, X.S. Progress of Researches on Interaction Mechanism between Ectomycorrhizal Fungi and Mycorrhizal Helper Bacteria. J. Jilin Agric. Univ. 2016, 38, 379–384. [Google Scholar]
- Yin, D.; Song, R.; Qi, J.; Deng, X. Ectomycorrhizal fungus enhances drought tolerance of Pinus sylvestris var. mongolica seedlings and improves soil condition. J. For. Res. 2018, 29, 1775–1788. [Google Scholar] [CrossRef]
- Yin, D.C.; Deng, X.; Song, R.Q. Synergistic effects between Suilllus luteus and Trichoderma virens on growth of Korean spruce seedlings and drought resistance of Scotch pine seedlings. J. For. Res. 2016, 27, 193–201. [Google Scholar] [CrossRef]
- Song, R.Q.; Yin, D.C.; Deng, X. Physiological responses of Pinus sylvestris var. mongolica seedlings to the interaction between Suillus luteus and Trichoderma virens. New Biotechnol. 2014, 69, 334–342. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhuo, Y.B.; Yin, Y.; Wei, Y.W.; Qin, S.J.; Zhu, W.X. Soil bacterial community structure characteristics in coniferous forests of Montane Regions of eastern Liaoning Province, China. Acta Ecol. Sin. 2019, 39, 997–1008. [Google Scholar]
- Zhang, Y.G.; Cong, J.; Lu, H.; Li, G.L.; Qu, Y.Y.; Su, X.J.; Zhou, J.Z.; Li, D.Q. Community structure and elevational diversity patterns of soil Acidobacteria. J. Environ. Sci. 2014, 26, 1717–1724. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Zhou, L.; Fu, S. Soil microbial characteristics and the influencing factors in subtropical forests. Acta Ecol. Sin. 2016, 36, 8–15. [Google Scholar] [CrossRef]
- Wu, Y.T.; Gutknecht, J.; Nadrowski, K.; Geißler, C.; Kühn, P.; Scholten, T.; Both, S. Relationships Between Soil Microorganisms, Plant Communities, and Soil Characteristics in Chinese Subtropical Forests. Ecosystems 2012, 15, 624–636. [Google Scholar] [CrossRef]
- Yang, X.Q.; Han, Y.Z. Spatial variations of soil organic carbon and nitrogen of forestland in Guandi mountain. For. Res. Beijing 2011, 24, 223–229. [Google Scholar]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. Crit. Rev. Plant Sci. 2010, 19, 1–30. [Google Scholar] [CrossRef]
- Shoebitz, M.; Ribaudo, C.M.; Pardo, M.A.; Cantore, M.L.; Ciampi, L.; Curá, J.A. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Boil. Biochem. 2009, 41, 1768–1774. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Hafeez, F.Y.; Malik, K.A. Manual on Biofertilizer Technology; NIBGE: Punjab, Pakistan, 2000; pp. 35–37. [Google Scholar]
- Wang, S.; Zhang, L.P.; Zhang, Y.; Hao, F.F.; Xiao, J.X.; Hu, D.N. Screening, identification and phosphate solubilizing capability of phosphate solubilizing bacteria in rhizosphere of Camellia oleifera Abel at red soil region. For. Res. 2015, 3, 17. [Google Scholar]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Rock phosphate-solubilizing Actinomycetes: Screening for plant growth-promoting activities. World J. Microbiol. Biotechnol. 2008, 24, 2565–2575. [Google Scholar] [CrossRef]
- Hamdali, H.; Hafidi, M.; Virolle, M.J.; Ouhdouch, Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl. Soil Ecol. 2008, 40, 510–517. [Google Scholar] [CrossRef]
- Probanza, A.; García, J.L.; Palomino, M.R.; Ramos, B.; Gutierrez-Mañero, F.J. Pinus pinea L. seedling growth and bacterial rhizosphere structure after inoculation with PGPR Bacillus (B. licheniformis CECT 5106 and B. pumilus CECT 5105). Appl. Soil Ecol. 2002, 20, 75–84. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Plant growth-promoting bacteria: A potential tool for arid mangrove reforestation. Trees 2002, 16, 159–166. [Google Scholar] [CrossRef]
- Rojas, A.; Holguin, G.; Glick, B.R.; Bashan, Y. Synergism between Phyllobacterium sp. (N2-fixer) and Bacillus licheniformis (P-solubilizer), both from a semiarid mangrove rhizosphere. FEMS Microbiol. Ecol. 2001, 35, 181–187. [Google Scholar] [CrossRef]





| Sample | pH | MC % | OM g/kg | TN g/kg | NH4+ mg/kg | NO3− mg/kg | AP mg/kg | TP g/kg | AK mg/kg | TK g/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| CK-5 | 5.16 ± 0.18D | 6.16 ± 0.06C | 21.30 ± 0.45A | 0.66 ± 0.01A | 8.87 ± 0.02A | 5.05 ± 0.01A | 177.79 ± 0.39A | 0.34 ± 0.00B | 101.27 ± 2.67A | 3.31 ± 0.00A |
| CK-7 | 5.39 ± 0.15B | 10.53 ± 0.20A | 16.74 ± 0.34C | 0.48 ± 0.02B | 6.77 ± 0.10B | 3.39 ± 0.04B | 97.37 ± 0.78C | 0.36 ± 0.00A | 84.70 ± 1.20B | 2.90 ± 0.00B |
| CK-9 | 5.49 ± 0.03C | 8.33 ± 0.03B | 19.55 ± 0.57B | 0.25 ± 0.00D | 5.98 ± 0.05D | 2.34 ± 0.01D | 60.46 ± 0.39E | 0.28 ± 0.00E | 75.69 ± 0.85C | 2.32 ± 0.00D |
| SS-5 | 5.52 ± 0.06C | 4.19 ± 0.14D | 15.34 ± 0.00D | 0.38 ± 0.01C | 6.24 ± 0.03C | 3.32 ± 0.07B | 114.08 ± 0.39B | 0.33 ± 0.00C | 81.62 ± 0.11B | 2.72 ± 0.02C |
| SS-7 | 5.50 ± 0.12C | 8.02 ± 0.05B | 13.91 ± 0.00E | 0.24 ± 0.00D | 5.21 ± 0.02E | 2.56 ± 0.03C | 70.95 ± 0.39D | 0.32 ± 0.00D | 70.97 ± 0.28D | 2.65 ± 0.05C |
| SS-9 | 5.63 ± 0.15A | 6.20 ±0.06 C | 15.27 ± 0.23D | 0.19 ± 0.00E | 4.12 ± 0.01F | 1.66 ± 0.06E | 53.86 ± 0.67F | 0.27 ± 0.00F | 62.78 ± 0.12E | 2.13 ± 0.02E |
| Sample | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|
| CK-5 | 6.44 ± 0.03C | 0.006 ± 0.00A | 3202.52 ± 50.50B | 3203.13 ± 38.47B | 0.99 ± 0.00A |
| CK-7 | 6.56 ± 0.06B | 0.004 ± 0.00A | 3302.69 ± 59.63AB | 3336.45 ± 64.93AB | 0.98 ± 0.00A |
| CK-9 | 6.78 ± 0.02A | 0.003 ± 0.00A | 3387.62 ± 54.59A | 3388.09 ± 79.06A | 0.98 ± 0.00A |
| SS-5 | 6.63 ± 0.03B | 0.004 ± 0.00A | 3250.22 ± 50.61AB | 3287.08 ± 39.94AB | 0.99 ± 0.00A |
| SS-7 | 6.55 ± 0.01B | 0.004 ± 0.00A | 3281.72 ± 16.85AB | 3286.57 ± 26.90AB | 0.99 ± 0.00A |
| SS-9 | 6.74 ± 0.00A | 0.003 ± 0.00A | 3310.71 ± 16.13AB | 3285.12 ± 14.68AB | 0.98 ± 0.00A |
| Sample | Proteobacteria | Actinobacteria | Acidobacteria | Chloroflexi | Firmicutes | Verrucomicrobia |
| CK-5 | 26.41 ± 1.78A | 22.60 ± 1.69AB | 15.87 ± 1.75A | 6.23 ± 0.62A | 12.20 ± 0.62B | 4.96 ± 1.09A |
| CK-7 | 32..48 ± 4.11A | 20.49 ± 1.93B | 17.36 ± 2.95A | 7.37 ± 1.44A | 3.51 ± 0.33A | 3.57 ± 1.66A |
| CK-9 | 28.72 ± 0.65A | 24.06 ± 0.30AB | 17.38 ± 0.82A | 6.30 ± 0.05A | 4.53 ± 0.25A | 4.40 ± 0.28A |
| SS-5 | 28.51 ± 1.58A | 25.59 ± 0.99A | 15.18 ± 1.18A | 7.18 ± 0.53A | 6.14 ± 0.85A | 5.00 ± 0.91A |
| SS-7 | 29.80 ± 1.87A | 22.13 ± 0.80AB | 18.53 ± 2.42A | 6.68 ± 0.14A | 5.01 ± 0.46A | 4.33 ± 1.05A |
| SS-9 | 25.45 ± 1.21A | 20.51 ± 0.26B | 21.13 ± 1.45A | 7.19 ± 0.62A | 4.38 ± 1.59A | 6.55 ± 0.96A |
| Sample | Gemmatimonadetes | Nitrospirae | Saccharibacteria | Bacteroidetes | Planctomycetes | other |
| CK-5 | 2.65 ± 0.24B | 1.57 ± 0.07B | 1.68 ± 0.13C | 1.35 ± 0.17AB | 1.97 ± 0.44A | 2.51 ± 0.23BC |
| CK-7 | 4.01 ± 0.46A | 2.84 ± 0.47A | 3.86 ± 0.28A | 1.44 ± 0.25AB | 0.84 ± 0.25B | 2.22 ± 0.22C |
| CK-9 | 3.84 ± 0.20A | 2.90 ± 0.21A | 2.38 ± 0.11C | 1.80 ± 0.09A | 0.86 ± 0.03B | 2.83 ± 0.13B |
| SS-5 | 3.14 ± 0.29AB | 2.90 ± 0.15A | 1.13 ± 0.12D | 1.21 ± 0.10B | 1.49 ± 0.27AB | 2.52 ± 0.07BC |
| SS-7 | 3.80 ± 0.38A | 2.97 ± 0.26A | 2.15 ± 0.09BC | 1.25 ± 0.04B | 0.81 ± 0.09B | 2.53 ± 0.17BC |
| SS-9 | 3.26 ± 0.07AB | 3.40 ± 0.24A | 2.11 ± 0.08BC | 1.48 ± 0.07AB | 1.17 ± 0.13B | 3.37 ± 0.04A |
| Diversity Index | pH | MC | OM | TN | NH4+ | NO3− | AP | TP | AK | TK |
|---|---|---|---|---|---|---|---|---|---|---|
| Shannon | 0.784 | −0.028 | −0.198 | −0.801 | −0.717 | −0.837 * | −0.803 | −0.832 * | −0.741 | −0.930 ** |
| Simpson | −0.907 * | −0.183 | 0.500 | 0.901 * | 0.872 * | 0.950 ** | 0.953 ** | 0.688 | 0.886 * | 0.944 ** |
| Ace | 0.576 | 0.490 | −0.084 | −0.699 | −0.571 | −0.754 | −0.833 * | −0.654 | −0.625 | −0.767 |
| Chao | 0.507 | 0.539 | −0.128 | −0.554 | −0.434 | −0.609 | −0.721 | −0.364 | −0.471 | −0.593 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halifu, S.; Deng, X.; Song, X.; An, Y.; Song, R. Effects of Sphaeropsis Blight on Rhizosphere Soil Bacterial Community Structure and Soil Physicochemical Properties of Pinus sylvestris var. mongolica in Zhanggutai, China. Forests 2019, 10, 954. https://doi.org/10.3390/f10110954
Halifu S, Deng X, Song X, An Y, Song R. Effects of Sphaeropsis Blight on Rhizosphere Soil Bacterial Community Structure and Soil Physicochemical Properties of Pinus sylvestris var. mongolica in Zhanggutai, China. Forests. 2019; 10(11):954. https://doi.org/10.3390/f10110954
Chicago/Turabian StyleHalifu, Saiyaremu, Xun Deng, Xiaoshuang Song, Yuning An, and Ruiqing Song. 2019. "Effects of Sphaeropsis Blight on Rhizosphere Soil Bacterial Community Structure and Soil Physicochemical Properties of Pinus sylvestris var. mongolica in Zhanggutai, China" Forests 10, no. 11: 954. https://doi.org/10.3390/f10110954




