Detection and Microscopy of Alnus glutinosa Pollen Fluorescence Peculiarities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pollen Samples
2.2. Pollen Exposures to O3
2.3. Pollen Fluorescence Data Collection
2.3.1. Fluorescence by Microscope
2.3.2. Fluorescence by Automatic Pollen Recognition Device
2.4. Data Analysis
2.4.1. Image Analysis
2.4.2. Principal Component Analysis
3. Results
3.1. Recognition of Ozone Effect on Pollen under Fluorescence Microscope
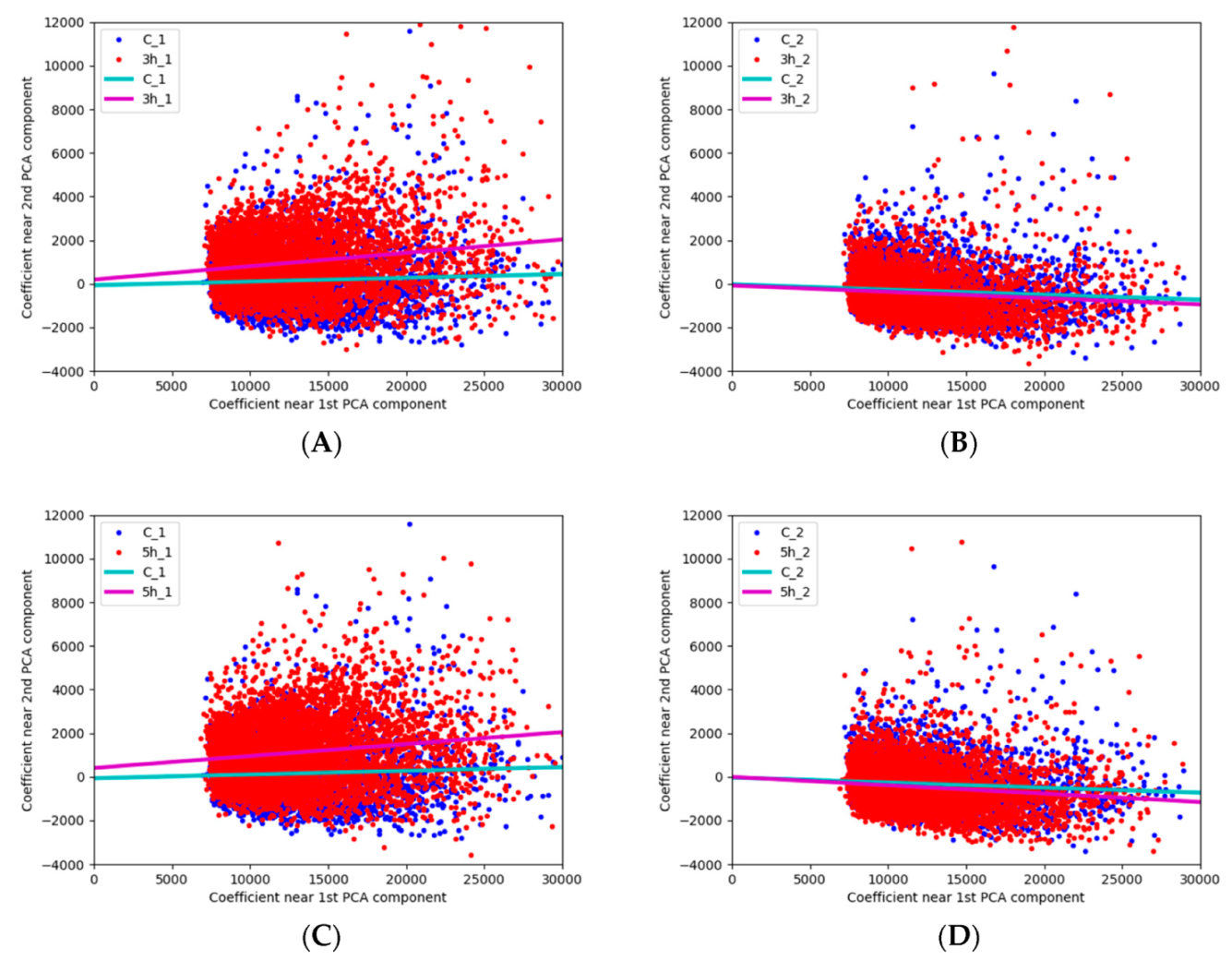
3.2. Possibilities of the Automatic Particle Detector in Assessing Ozone Effect on Pollen
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boucher, A.; Hidalgo, P.J.; Thonnat, M.; Belmonte, J.; Galan, C.; Bonton, P.; Tomczak, R. Development of a semi-automatic system for pollen recognition. Aerobiologia 2002, 18, 195–201. [Google Scholar] [CrossRef]
- Mitsumoto, K.; Yabusaki, K.; Kobayashi, K.; Aoyagi, H. Development of a novel real-time pollen-sorting counter using species-specific pollen autofluorescence. Aerobiologia 2010, 26, 99–111. [Google Scholar] [CrossRef]
- Kiselev, D.; Bonacina, L.; Wolf, J.P. Individual bioaerosol particle discrimination by multi-photon excited fluorescence. Opt. Express 2011, 19, 24516–24521. [Google Scholar] [CrossRef] [PubMed]
- Asbeck, F. Fluoreszierender Blütenstaub. Naturwissenschaften 1955, 42, 632. [Google Scholar] [CrossRef]
- Van Gijzel, P. Autofluorescence of fossil pollen and spores with special reference to age determination and coalification. Leidse Geol. Meded. 1967, 40, 261–317. [Google Scholar]
- Sivaguru, M.; Urban, M.A.; Fried, G.; Wesseln, C.J.; Mander, L.; Punyasena, S.W. Comparative performance of airyscan and structured illumination superresolution microscopy in the study of the surface texture and 3D shape of pollen. Microsc. Res. Tech. 2018, 81, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Schultz, E.; Burkhardt, H. Automated pollen recognition using 3D volume images from fluorescence microscopy. Aerobiologia 2002, 18, 107–115. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Iacopino, D.; Healy, D.A.; O’Sullivan, D.; Sodeau, J.R. The intrinsic fluorescence spectra of selected pollen and fungal spores. Atmos. Environ. X 2011, 45, 6451–6458. [Google Scholar] [CrossRef]
- Stanley, W.R.; Kaye, P.H.; Foot, V.E.; Barrington, S.J.; Gallagher, M.; Gabey, A. Continuous bioaerosol monitoring in a tropical environment using a UV fluorescence particle spectrometer. Atmos. Sci. Lett. 2011, 12, 195–199. [Google Scholar] [CrossRef]
- Crouzy, B.; Stella, M.; Konzelmann, T.; Calpini, B.; Clot, B. All-Optical automatic pollen identification: Towards an operational system. Atmos. Environ. 2016, 140, 202–212. [Google Scholar] [CrossRef]
- Šaulienė, I.; Šukienė, L.; Daunys, G.; Valiulis, G.; Vaitkevičius, L.; Matavulj, P.; Brdar, S.; Panic, M.; Sikoparija, B.; Clot, B.; et al. Automatic pollen recognition with the Rapid-E particle counter: The first-level procedure, experience and next steps. Atmos. Meas. Tech. 2019, 2, 1–33. [Google Scholar] [CrossRef]
- Buters, J.T.M.; Antunes, C.; Galveias, A.; Bergmann, K.C.; Thibaudon, M.; Galán, C.; Schmidt-Weber, C.; Oteros, J. Pollen and spore monitoring in the world. Clin. Transl. Allergy 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R.; EAS QC Working Group. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar] [CrossRef]
- Gottardini, E.; Rossi, S.; Cristofolini, F.; Benedetti, L. Use of Fourier transform infrared (FT-IR) spectroscopy as a tool for pollen identification. Aerobiologia 2007, 23, 211–219. [Google Scholar] [CrossRef]
- Mitsumoto, K.; Yabusaki, K.; Aoyagi, H. Classification of pollen species using autofluorescence image analysis. J. Biosci. Bioeng. 2009, 107, 90–94. [Google Scholar] [CrossRef]
- Oteros, J.; Pusch, G.; Weichenmeier, I.; Heimann, U.; Möller, R.; Röseler, S.; Traidl-Hoffmann, C.; Schmidt-Weber, C.; Buters, J.T. Automatic and online pollen monitoring. Int. Arch. Allergy Immunol. 2015, 167, 158–166. [Google Scholar] [CrossRef]
- Sofiev, M. On possibilities of assimilation of near-real-time pollen data by atmospheric composition models. Aerobiologia 2019, 35, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Kasprzyk, I. Comparative study of seasonal and intradiurnal variation of airborne herbaceous pollen in urban and rural areas. Aerobiologia 2006, 22, 185–195. [Google Scholar] [CrossRef]
- Tormo Molina, R.; Silva Palacios, I.; Muñoz RodrÍguez, A.F.; Tavira Muñoz, J.; Moreno Corchero, A. Environmental factors affecting airborne pollen concentration in anemophilous species of Plantago. Ann. Bot. 2001, 87, 1–8. [Google Scholar] [CrossRef]
- García-Mozo, H.; Oteros, J.A.; Galán, C. Impact of land cover changes and climate on the main airborne pollen types in Southern Spain. Sci. Total Environ. 2016, 548, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lebourgeois, F.; Delpierre, N.; Dufrêne, E.; Cecchini, S.; Macé, S.; Croisé, L.; Nicolas, M. Assessing the roles of temperature, carbon inputs and airborne pollen as drivers of fructification in European temperate deciduous forests. Eur. J. For. Res. 2018, 137, 349–365. [Google Scholar] [CrossRef] [Green Version]
- Sofiev, M.; Siljamo, P.; Ranta, H.; Rantio-Lehtimäki, A. Towards numerical forecasting of long-range air transport of birch pollen: Theoretical considerations and a feasibility study. Int. J. Biometeorol. 2006, 50, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Skjøth, C.A.; Sommer, J.; Stach, A.; Smith, M.; Brandt, J. The long-range transport of birch (Betula) pollen from Poland and Germany causes significant pre-season concentrations in Denmark. Clin. Exp. Allergy 2007, 37, 1204–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siljamo, P.; Sofiev, M.; Ranta, H. An approach to simulation of long-range atmospheric transport of natural allergens: An example of birch pollen. In Air Pollution Modeling and Its Application XVII; Springer: Boston, MA, USA, 2007; pp. 331–339. [Google Scholar] [CrossRef]
- Smith, M.; Skjøth, C.A.; Myszkowska, D.; Uruska, A.; Puc, M.; Stach, A.; Balwierz, Z.; Chlopek, K.; Piotrowska, K.; Kasprzyk, I.; et al. Long-Range transport of Ambrosia pollen to Poland. Agric. For. Meteorol. 2008, 148, 1402–1411. [Google Scholar] [CrossRef]
- Veriankaitė, L.; Siljamo, P.; Sofiev, M.; Šaulienė, I.; Kukkonen, J. Modelling analysis of source regions of long-range transported birch pollen that influences allergenic seasons in Lithuania. Aerobiologia 2010, 26, 47–62. [Google Scholar] [CrossRef]
- De Weger, L.A.; Pashley, C.H.; Šikoparija, B.; Skjøth, C.A.; Kasprzyk, I.; Grewling, Ł.; Thibaudon, M.; Magyar, D.; Smith, M. The long distance transport of airborne Ambrosia pollen to the UK and the Netherlands from Central and south Europe. Int. J. Biometeorol. 2016, 60, 1829–1839. [Google Scholar] [CrossRef] [Green Version]
- Sofiev, M. On impact of transport conditions on variability of the seasonal pollen index. Aerobiologia 2017, 33, 167–179. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Melnikova, E.V. Pollen chemosensitivity to ozone and peroxides. Russ. J. Plant Physiol. 2001, 48, 74–83. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Yashin, V.A.; Kuchin, A.V. Fluorescent analysis for bioindication of ozone on unicellular models. J. Fluoresc. 2015, 25, 595–601. [Google Scholar] [CrossRef]
- Albertine, J.M.; Manning, W.J.; DaCosta, M.; Stinson, K.A.; Muilenberg, M.L.; Rogers, C.A. Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PLoS ONE 2014, 9, e111712. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.; Jochner, S.; Gilles, S.; McIntyre, M.; Buters, J.T.; Schmidt-Weber, C.; Behrendt, H.; Ring, J.; Menzel, A.; Traidl-Hoffmann, C. High environmental ozone levels lead to enhanced allergenicity of birch pollen. PLoS ONE 2013, 8, e80147. [Google Scholar] [CrossRef] [PubMed]
- Kanter, U.; Heller, W.; Durner, J.; Winkler, J.B.; Engel, M.; Behrendt, H.; Pfeifer, M.; Mayer, K. Molecular and immunological characterization of ragweed (Ambrosia artemisiifolia L.) pollen after exposure of the plants to elevated ozone over a whole growing season. PLoS ONE 2013, 8, e61518. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Ernst, D. Effects of NO2 and ozone on pollen allergenicity. Front. Plant Sci. 2016, 7, 91. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Paoletti, E.; Lazzeri, P.; Pepponi, G. Pollen viability for air pollution bio-monitoring. J. Atmos. Chem. 2004, 49, 149–159. [Google Scholar] [CrossRef]
- Pasqualini, S.; Tedeschini, E.; Frenguelli, G.; Wopfner, N.; Ferreira, F.; D’Amato, G.; Ederli, L. Ozone affects pollen viability and NAD(P)H oxidase release from Ambrosia artemisiifolia pollen. Environ. Pollut. 2011, 159, 2823–2830. [Google Scholar] [CrossRef]
- Zhu, C.; Farah, J.; Choël, M.; Gosselin, S.; Baroudi, M.; Petitprez, D.; Visez, N. Uptake of ozone and modification of lipids in Betula Pendula pollen. Environ. Pollut. 2018, 242, 880–886. [Google Scholar] [CrossRef]
- Ministry of Environment. Lithuania’s Forests. Available online: http://www.amvmt.lt/index.php/nacionaline-misku-inventorizacija2/leidiniai/lietuvos-miskai (accessed on 6 September 2019).
- Šaulienė, I.; Šukienė, L.; Kainov, D.; Greičiuvienė, J. The impact of pollen load on quality of life: A questionnaire-based study in Lithuania. Aerobiologia 2016, 32, 157–170. [Google Scholar] [CrossRef]
- Mäkinen, Y.; Rantio-Lehtimaki, A. Diurnal variation of airborne fungal spores in Turku, Finland, in 1974. Rep. Aerobiol. Lab. Univ. Turku 1979, 1, 1–27. [Google Scholar]
- Jochner-Oette, S.; Simmons, M.; Jetschni, J.; Menzel, A. Impacts of land clearance by fire on spatial variation of mountain cedar pollen concentrations in Texas. Landsc. Urban Plan. 2017, 162, 178–186. [Google Scholar] [CrossRef]
- Sulmont, G. (Ed.) The Pollen Content of the Air: Identification Key; Studios Bouquet: Saint-Étienne, France, 2011. [Google Scholar]





| Years | Average Monthly Air Temperature (°C) | Total Precipitation Per Month (mm) | ||||
|---|---|---|---|---|---|---|
| January | February | March | January | February | March | |
| 2018 | −1.6 | −6.6 | −2.1 | 51.1 | 15.8 | 12.8 |
| 2019 | −4.0 | 1.3 | 3.1 | 54.0 | 38.4 | 31.8 |
| Name of the Group of Experimental Variant | Abbreviation | Exposure to Ozone | Storage | Duration of Storage Until the Start of the Experiment, Months | |
|---|---|---|---|---|---|
| Cumulative Concentration, ppm | Exposure Time, h | ||||
| Control | CS_1 | 0 | 0 | In the refrigerator | 5 |
| C_1 | 0 | 0 | Indoors | 1 | |
| C_2 | 5 | ||||
| 3-h exposure | 3 h_1 | 20.5 | 3 | Indoors | 1 |
| 3 h_2 | 5 | ||||
| 5-h exposure | 5 h_1 | 24.1 | 5 | Indoors | 1 |
| 5 h_2 | 5 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šaulienė, I.; Šukienė, L.; Daunys, G.; Valiulis, G.; Lankauskas, A.; Kokina, I.; Gerbreders, V.; Gavarāne, I. Detection and Microscopy of Alnus glutinosa Pollen Fluorescence Peculiarities. Forests 2019, 10, 959. https://doi.org/10.3390/f10110959
Šaulienė I, Šukienė L, Daunys G, Valiulis G, Lankauskas A, Kokina I, Gerbreders V, Gavarāne I. Detection and Microscopy of Alnus glutinosa Pollen Fluorescence Peculiarities. Forests. 2019; 10(11):959. https://doi.org/10.3390/f10110959
Chicago/Turabian StyleŠaulienė, Ingrida, Laura Šukienė, Gintautas Daunys, Gediminas Valiulis, Alfredas Lankauskas, Inese Kokina, Vjačeslavs Gerbreders, and Inese Gavarāne. 2019. "Detection and Microscopy of Alnus glutinosa Pollen Fluorescence Peculiarities" Forests 10, no. 11: 959. https://doi.org/10.3390/f10110959
APA StyleŠaulienė, I., Šukienė, L., Daunys, G., Valiulis, G., Lankauskas, A., Kokina, I., Gerbreders, V., & Gavarāne, I. (2019). Detection and Microscopy of Alnus glutinosa Pollen Fluorescence Peculiarities. Forests, 10(11), 959. https://doi.org/10.3390/f10110959






