Dynamics and Vertical Distribution of Roots in European Beech Forests and Douglas Fir Plantations in Bulgaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Plots Description
2.2. Soil Sampling and Analysis
2.3. Root Sampling and Analysis
2.4. Estimation of Annual Fine Root Production and Turnover Rates
2.5. Statistical Analyses
3. Results
3.1. Root Stocks and Root Size Classes Allocation
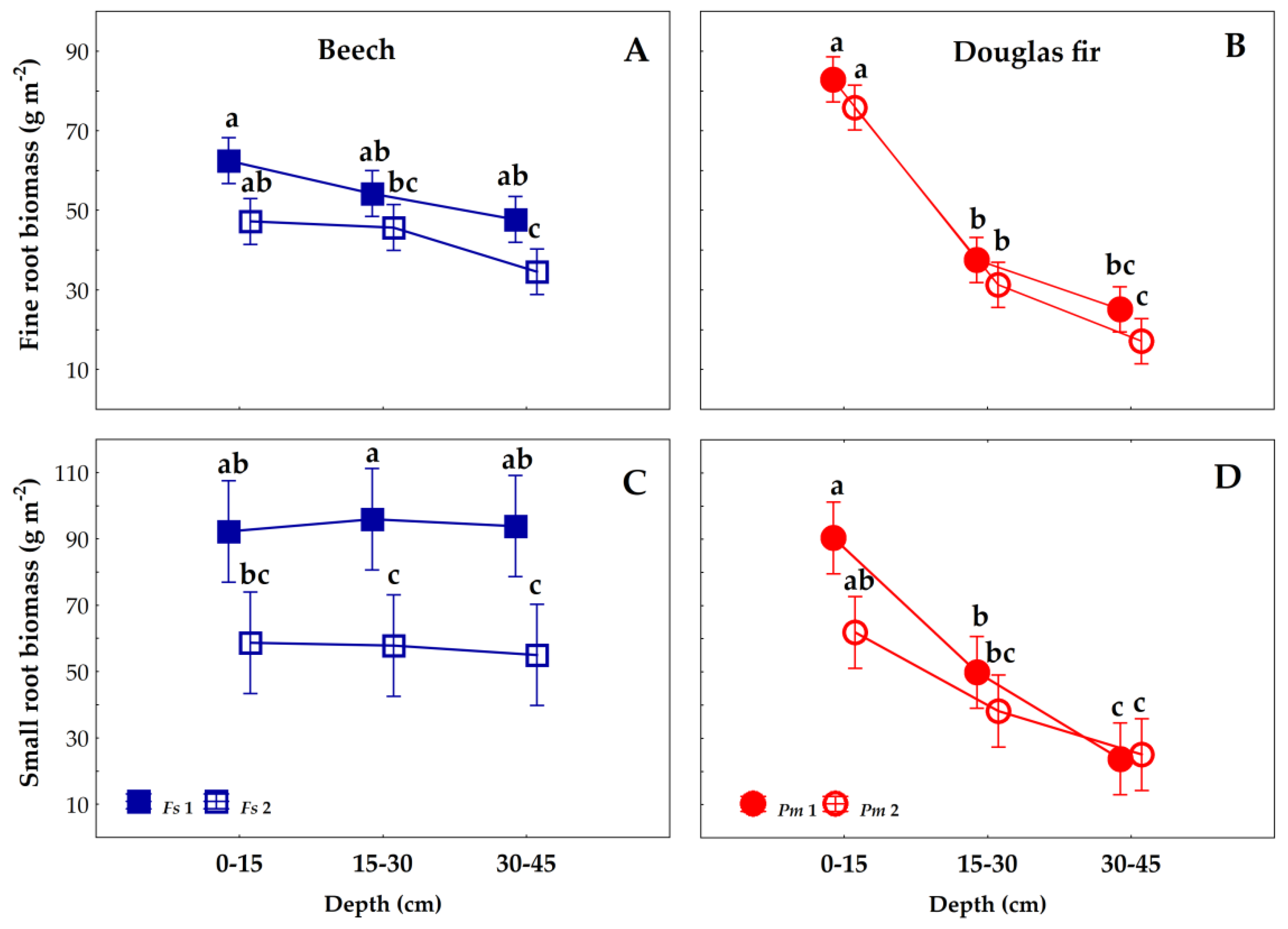
3.2. Fine and Small Roots Biomass/Necromass Vertical Distribution
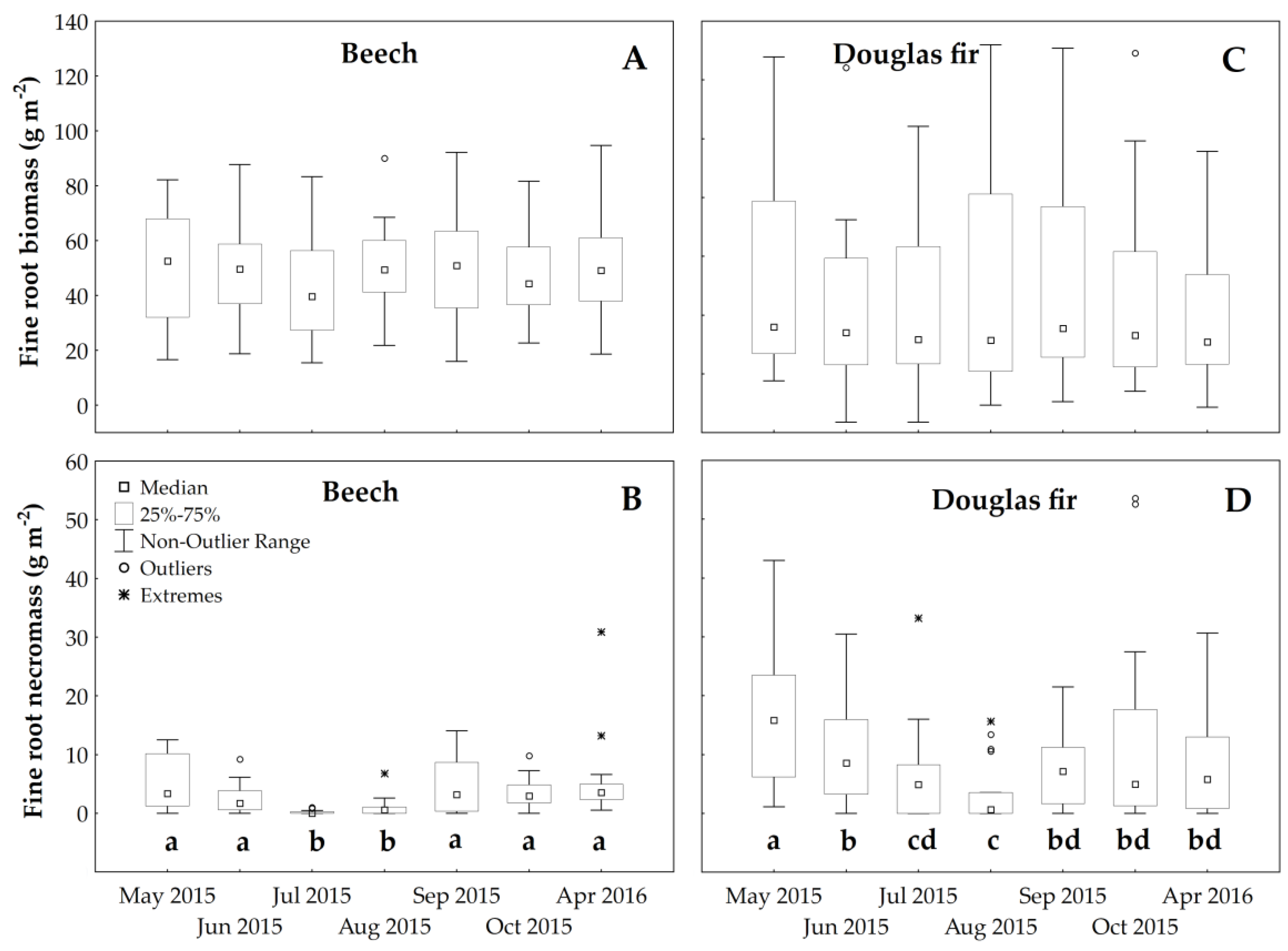
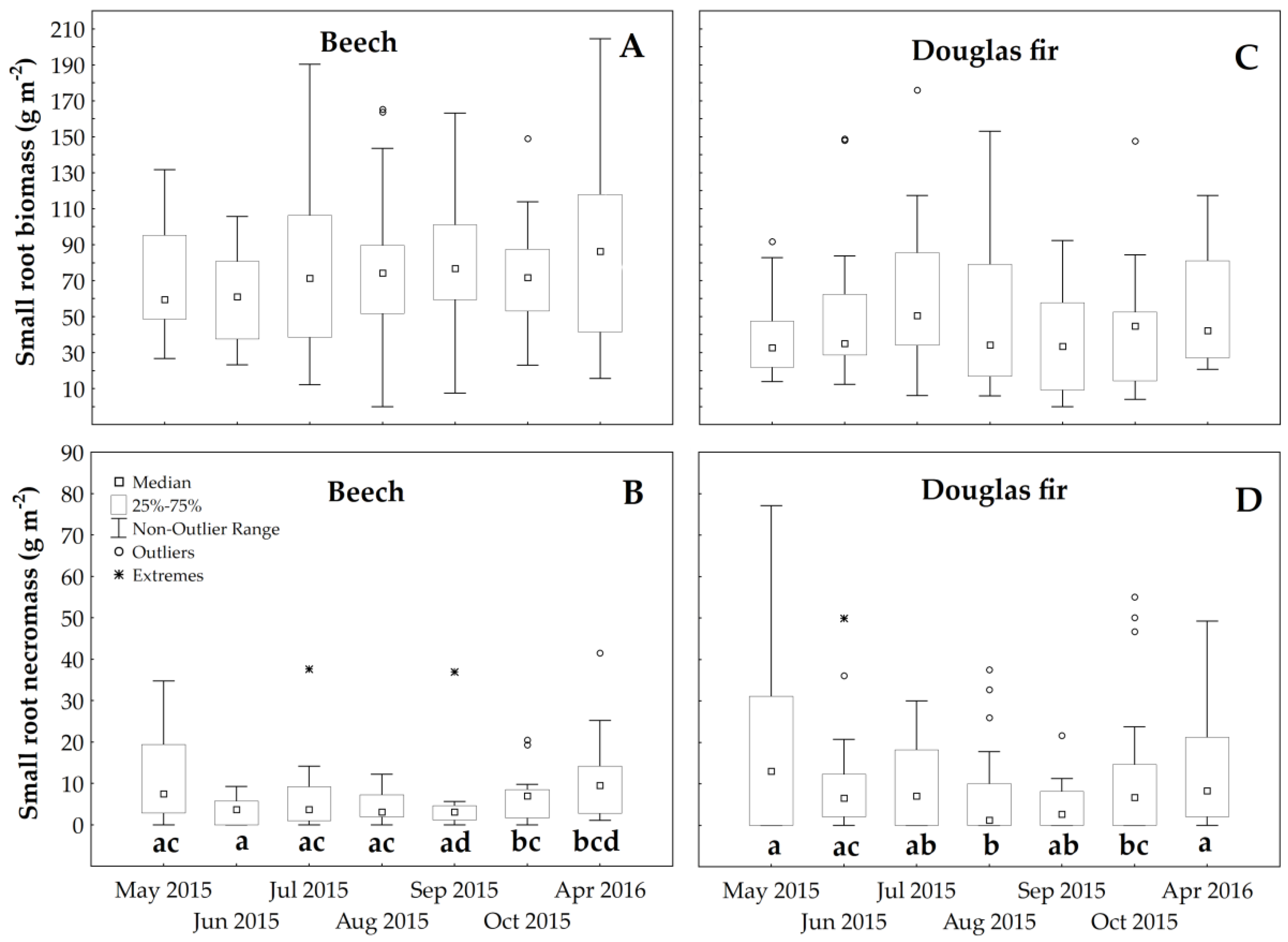
3.3. Root Temporal Dynamics
3.4. Root Annual Production and Turnover Rates
4. Discussion
4.1. Standing Root Stocks
4.2. Root traits Vertical Distribution
4.3. Root Dynamics
4.4. Root Annual Production and Turnover Rates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Coutts, M.P. Root architecture and tree stability. In Tree Root Systems and Their Mycorrhizas; Springer: Dordrecht, The Netherlands, 1983; pp. 171–188. [Google Scholar]
- Erktan, A.; McCormack, M.L.; Roumet, C. Frontiers in root ecology: Recent advances and future challenges. Plant Soil 2018, 424, 1–9. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A.; Hendrix, P.F. Fundamentals of Soil Ecology, 2nd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2004; ISBN 9780121797263. [Google Scholar]
- Vogt, K.A.; Vogt, D.J.; Bloomfield, J. Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. In Root Demographics and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems; Springer: Dordrecht, The Netherlands, 1998; pp. 687–720. [Google Scholar]
- Lauenroth, W.K.; Gill, R. Turnover of Root Systems. In Root Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 61–89. [Google Scholar]
- Majdi, H.; Pregitzer, K.; Morén, A.S.; Nylund, J.E.; Ågren, G.I. Measuring fine root turnover in forest ecosystems. Plant Soil 2005, 276, 1–8. [Google Scholar] [CrossRef]
- Lukac, M. Fine Root Turnover. In Measuring Roots; Mancuso, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 363–373. [Google Scholar]
- Addo-Danso, S.D.; Prescott, C.E.; Smith, A.R. Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. For. Ecol. Manag. 2016, 359, 332–351. [Google Scholar] [CrossRef]
- Persson, H. Fine-root production, mortality and decomposition in forest ecosystems. Vegetatio 1980, 41, 101–109. [Google Scholar] [CrossRef]
- Persson, H.A. The distribution and productivity of fine roots in boreal forests. Plant Soil 1983, 71, 87–101. [Google Scholar] [CrossRef]
- Makkonen, K.; Helmisaari, H.S. Seasonal and yearly variations of fine-root biomass and necromass in a Scots pine (Pinus sylvestris L.) stand. For. Ecol. Manag. 1998, 102, 283–290. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Makkonen, K.; Kellomäki, S.; Valtonen, E.; Mälkönen, E. Below- and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. For. Ecol. Manag. 2002, 165, 317–326. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Pajuste, K. Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: Comparison of soil core and ingrowth core methods. For. Ecol. Manag. 2005, 212, 264–277. [Google Scholar] [CrossRef]
- Hendricks, J.; Hendrick, R.; Wilson, C.; Mitchell, R.; Pecot, S.; Guo, D. Assessing the patterns and controls of fine root dynamics: An empirical test and methodological review. J. Ecol. 2006, 94, 40–57. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Bergeron, Y.; Paré, D. Differences in fine root productivity between mixed- and single-species stands. Funct. Ecol. 2011, 25, 238–246. [Google Scholar] [CrossRef]
- Danjon, F.; Stokes, A.; Bakker, M.R. Root Systems of Woody Plants. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 29-1–29-26. [Google Scholar]
- Bakker, M.R.; Turpault, M.P.; Huet, S.; Nys, C. Root distribution of Fagus sylvatica in a chronosequence in western France. J. For. Res. 2008, 13, 176–184. [Google Scholar] [CrossRef]
- Böhm, W. Excavation Methods. In Methods of Studying Root Systems; Springer: Berlin/Heidelberg, Germany, 1979; pp. 5–19. [Google Scholar]
- Jackson, D.S.; Jackson, D.S.; Chittenden, J. Estimation of dry matter in Pinus radiata root systems. 1. Individual trees. N. Z. J. For. Sci. 1981, 11, 164–182. [Google Scholar]
- Persson, H.; Ahlström, K.; Clemensson-Lindell, A. Nitrogen addition and removal at Gårdsjön—Effects on fine-root growth and fine-root chemistry. For. Ecol. Manag. 1998, 101, 199–205. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Derome, J.; Nojd, P.; Kukkola, M. Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol. 2007, 27, 1493–1504. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Li, F.; Jin, G. Fine root biomass, production and turnover rates in plantations versus natural forests: Effects of stand characteristics and soil properties. Plant Soil 2019, 436, 463–474. [Google Scholar] [CrossRef]
- Pregitzer, K.S. Fine roots of trees—A new perspective. New Phytol. 2002, 154, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voß, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching out in roots: Uncovering form, function, and regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef] [Green Version]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 2014, 95, 2224–2235. [Google Scholar] [CrossRef]
- Radville, L.; McCormack, M.L.; Post, E.; Eissenstat, D.M. Root phenology in a changing climate. J. Exp. Bot. 2016, 67, 3617–3628. [Google Scholar] [CrossRef] [Green Version]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef] [Green Version]
- Iversen, C.M.; McCormack, M.L.; Powell, A.S.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; et al. A global Fine-Root Ecology Database to address below-ground challenges in plant ecology. New Phytol. 2017, 215, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhart, T.; Walcher, S.; Hasenauer, H.; van Loo, M. Genetic diversity and adaptive traits of European versus American Douglas-fir seedlings. Eur. J. For. Res. 2017, 136, 811–825. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Hermann, R.K.; Lavender, D.P. Douglas-fir planted forests. New For. 1999, 17, 53–70. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Bauhus, J. Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Chang. Biol. 2017, 23, 5108–5119. [Google Scholar] [CrossRef]
- Eckhart, T.; Pötzelsberger, E.; Koeck, R.; Thom, D.; Lair, G.J.; van Loo, M.; Hasenauer, H. Forest stand productivity derived from site conditions: An assessment of old Douglas-fir stands (Pseudotsuga menziesii (Mirb.) Franco var. menziesii) in Central Europe. Ann. For. Sci. 2019, 76, 19. [Google Scholar] [CrossRef] [Green Version]
- Forest Europe. State of Europe’s Forests 2015 Report. Available online: https://www.foresteurope.org/docs/fullsoef2015.pdf (accessed on 8 December 2019).
- Executive Forest Agency, Ministry of Agriculture and Foods. Distribution of the forested area by tree species and age in 2015. Report. 2016. (in Bulgarian). Available online: http://www.iag.bg/data/docs/otchet2.pdf (accessed on 8 December 2019).
- Popov, E.; Hinkov, G.; Velichkov, I.; Stefanova, P. Productivity of Douglas fir plantations in Bulgaria and the necessity for its utilization. For. Sci. 2018, 54, 3–23. [Google Scholar]
- Denk, T.; Grimm, G.; Stögerer, K.; Langer, M.; Hemleben, V. The evolutionary history of Fagus in western Eurasia: Evidence from genes, morphology and the fossil record. Plant Syst. Evol. 2002, 232, 213–236. [Google Scholar] [CrossRef]
- Packham, J.R.; Thomas, P.A.; Atkinson, M.D.; Degen, T. Biological Flora of the British Isles: Fagus sylvatica. J. Ecol. 2012, 100, 1557–1608. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Ferezliev, A. Relationship between particular dendrobiometrical indicators of natural European beech (Fagus sylvatica L.) dendrocenoses in Central Balkan Range. Folia Oecol. 2017, 44, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Zhiyanski, M.; Glushkova, M.; Kirova, L. Quantitative and qualitative features of soil humus in mountain treeline ecosystems. Silva Balc. 2017, 18, 5–23. [Google Scholar]
- Ferezliev, A.; Mavrevski, R.; Delkov, A. Correlation between average and dominant height of middle-aged Douglas fir plantations in the North-West Rhodopes. Silva Balc. 2018, 19, 13–26. [Google Scholar]
- Schuurman, J.J.; Goedewaagen, M.A.J. Methods for the Examination of Root Systems and Roots; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1971. [Google Scholar]
- Persson, H. Root Dynamics in a Young Scots Pine Stand in Central Sweden. Oikos 1978, 30, 508. [Google Scholar] [CrossRef]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2013, 362, 357–372. [Google Scholar] [CrossRef]
- Fairley, R.; Alexander, I. Methods of calculating fine root production in forests. In Ecological Interactions in Soil: Plants, Microbes and Animals; Special Publication Number 4 of the British Ecological Society; Fiter, A.H., Ed.; Blackwell Scientific: Oxford, UK, 1985; pp. 37–42. ISBN 0632013869. [Google Scholar]
- McClaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Kucbel, S.; Jaloviar, P.; Špišák, J. Quantity, vertical distribution and morphology of fine roots in Norway spruce stands with different stem density. Plant Root 2011, 5, 46–55. [Google Scholar] [CrossRef]
- Finér, L.; Helmisaari, H.S.; Lõhmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konôpka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root seasonal pattern, production and turnover rate of European beech (Fagus sylvatica L.) stands in Italy Prealps: Possible implications of coppice conversion to high forest. Plant Biosyst. 2012, 146, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- Montagnoli, A.; Di Iorio, A.; Terzaghi, M.; Trupiano, D.; Scippa, G.S.; Chiatante, D. Influence of soil temperature and water content on fine-root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 2014, 133, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Wu, K. Fine Root Production and Turnover and Its Contribution to Nutrient Cycling in Two Beech (Fagus sylvatica L.) Forest Ecosystems. Ber. Forsch. Zent. Waldökosyst. Reihe A 2000, 170, 1–130. [Google Scholar]
- Claus, A.; George, E. Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can. J. For. Res. 2005, 35, 1617–1625. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For. Ecol. Manag. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Hermann, R.; Lavender, D. Pseudotsuga menziesii (Mirb.) Franco Douglas-fir. In Silvics of North America; United States Department of Agriculture: Washington, DC, USA, 1990; Volume 1, pp. 527–554. [Google Scholar]
- Olsthoorn, A. Fine root density and root biomass of two Douglas-fir stands on sandy soils in The Netherlands. 1. Root biomass in early summer. Neth. J. Agric. Sci. 1991, 39, 49–60. [Google Scholar]
- Kurz, W.A. Net Primary Production, Production Allocation, and Foliage Efficiency in Second Growth Douglas-Fir Stands with Differing Site Quality. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1989. [Google Scholar]
- Keyes, M.R.; Grier, C.C. Above- and below-ground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can. J. For. Res. 1981, 11, 599–605. [Google Scholar] [CrossRef]
- Hendriks, C.; Bianchi, F. Root density and root biomass in pure and mixed forest stands of Douglas-fir and Beech. Neth. J. Agric. Sci. 1995, 43, 321–331. [Google Scholar]
- Leuschner, C.; Hertel, D.; Coners, H.; Büttner, V. Root competition between beech and oak: A hypothesis. Oecologia 2001, 126, 276–284. [Google Scholar] [CrossRef]
- Curt, T.; Prévosto, B. Root biomass and rooting profile of naturally regenerated beech in mid-elevation Scots pine woodlands. Plant Ecol. 2003, 167, 269–282. [Google Scholar] [CrossRef]
- Konôpka, B. Differences in fine root traits between Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica L.)—A case study in the Kysucké Beskydy Mts. J. For. Sci. 2009, 55, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Bolte, A.; Villanueva, I. Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur. J. For. Res. 2006, 125, 15–26. [Google Scholar] [CrossRef]
- Rust, S.; Savill, P.S. The root systems of Fraxinus excelsior and Fagus sylvatica and their competitive relationships. Forestry 2000, 73, 499–508. [Google Scholar] [CrossRef]
- Tingey, D.T.; Phillips, D.L.; Johnson, M.G.; Rygiewicz, P.T.; Beedlow, P.A.; Hogsett, W.E. Estimates of Douglas-fir fine root production and mortality from minirhizotrons. For. Ecol. Manag. 2005, 204, 359–370. [Google Scholar] [CrossRef]
- Moser, B.; Bachofen, C.; Müller, J.D.; Metslaid, M.; Wohlgemuth, T. Root architecture might account for contrasting establishment success of Pseudotsuga menziesii var. menziesii and Pinus sylvestris in Central Europe under dry conditions. Ann. For. Sci. 2016, 73, 959–970. [Google Scholar]
- Hertel, D.; Strecker, T.; Müller-Haubold, H.; Leuschner, C. Fine root biomass and dynamics in beech forests across a precipitation gradient—Is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Grossman, J.D.; Rice, K.J. Evolution of root plasticity responses to variation in soil nutrient distribution and concentration. Evol. Appl. 2012, 5, 850–857. [Google Scholar] [CrossRef]
- Nisbet, T. Water Use by Trees. Forestry Commission Information Note 65; Forestry Commission: Edinburgh, UK, 2005.
- Thomas, F.; Bögelein, R.; Werner, W. Interaction between Douglas fir and European beech: Investigations in pure and mixed stands. Forstarch. Forstwiss. Fachz. 2015, 86, 83–91. [Google Scholar]
- Kubisch, P.; Hertel, D.; Leuschner, C. Fine root productivity and turnover of ectomycorrhizal and arbuscular mycorrhizal tree species in a temperate broad-leaved mixed forest. Front. Plant Sci. 2016, 7, 1233. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.K. Fine Root Growth and Vitality of European Beech in Acid Forest Soils with a Low Base Saturation. Ph.D. Thesis, Swiss Federal Institute Of Technology Zurich, Zurich, Switzerland, 2007. [Google Scholar]
- Harris, W.; Santantonio, D.; McGinty, D. The dynamic belowground ecosystem. In Forests: Fresh Perspectives from Ecosystem Analysis, Proceedings of the 40th Annual Biology Colloquium, Corvallis, OR, USA, 27–28 April 1979; Richard, H.W., Ed.; Oregon State University: Corvallis, OR, USA, 1980; pp. 119–129. [Google Scholar]
- Burton, A.J.; Pregitzer, K.S.; Hendrick, R.L. Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 2000, 125, 389–399. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Sabaté, S.; Gracia, C.A. Annual and seasonal changes in fine root biomass of a Quercus ilex L. forest. Plant Soil 2001, 230, 125–134. [Google Scholar] [CrossRef]
- Santantonio, D.; Hermann, R.K. Standing crop, production, and turnover of fine roots on dry, moderate, and wet sites of mature Douglas-fir in western Oregon. Ann. Des. Sci. For. 1985, 42, 113–142. [Google Scholar] [CrossRef] [Green Version]
- Joslin, J.; Wolfe, M.; Hanson, P. Effects of altered water regimes on forest root systems. New Phytol. 2000, 147, 117–129. [Google Scholar] [CrossRef]
- Stober, C.; George, E.; Persson, H. Root Growth and Response to Nitrogen. In Carbon and Nitrogen Cycling in European Forest Ecosystems. Ecological Studies Analysis and Synthesis; Schulze, E.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 99–121. [Google Scholar]
- Vanguelova, E.I.; Nortcliff, S.; Moffat, A.J.; Kennedy, F. Morphology, biomass and nutrient status of fine roots of Scots pine (Pinus sylvestris) as influenced by seasonal fluctuations in soil moisture and soil solution chemistry. Plant Soil 2005, 270, 233–247. [Google Scholar] [CrossRef]
- Zhiyanski, M. Fine-root production and turnover based on 2-yr of sequential coring in four forest species in Bulgaria. Silva Balc. 2015, 16, 34–49. [Google Scholar]
- Solly, E.F.; Brunner, I.; Helmisaari, H.S.; Herzog, C.; Leppälammi-Kujansuu, J.; Schöning, I.; Schrumpf, M.; Schweingruber, F.H.; Trumbore, S.E.; Hagedorn, F. Unravelling the age of fine roots of temperate and boreal forests. Nat. Commun. 2018, 9, 3006. [Google Scholar] [CrossRef]

| Tree Species | Fagus sylvatica | Pseudotsuga menziezii | ||
|---|---|---|---|---|
| Sampling plot abbreviation | Fs 1 | Fs 2 | Pm 1 | Pm 2 |
| Geographical region | Central Balkan Mountains | Rhodope Mountains | ||
| GPS coordinates | 42°47′ 4.47″ N 24°36′ 53.16″ E | 42°47′ 5.21″ N 24°36′ 33.39″ E | 42°02′ 28.19″ N 23°56′ 20.76″ E | 42°02′ 30.32″ N 23°56′ 27.05″ E |
| Mean altitude (m) | 1330 | 1325 | 1100 | 1050 |
| Mean annual temperature (°C) | 6.7 | 7.5 | ||
| Annual precipitation (mm) | 1240 | 677 | ||
| Forests composition | Beech 100% | Douglas fir 87% Scots pine 13% | ||
| Mean stand age in 2015 (years) | 64 | 47 | 37 | 47 |
| Stand density (trees/ha) | 2090 | 2700 | 661 | 1700 |
| Mean tree height (m) | 17.2 | 16.1 | 25.1 | 30.8 |
| Mean diameter at breast height (Dbh in cm) | 14.6 | 9.1 | 24.1 | 23.0 |
| Soil type (WRB, 2014) | Dystric Cambisols | Dystric Cambisols | ||
| pH H2O 0–45 cm | 4.57 ± 0.14 | 4.41 ± 0.08 | 5.15 ± 0.10 | 5.22 ± 0.15 |
| pH CaCl2 0–45 cm | 4.07 ± 0.10 | 3.91 ± 0.07 | 4.45 ± 0.14 | 4.48 ± 0.14 |
| Total nitrogen % 0–45 cm | 0.47 ± 0.12 | 0.52 ± 0.09 | 0.08 ± 0.03 | 0.05 ± 0.01 |
| Total carbon % 0–45 cm | 5.42 ± 1.73 | 6.00 ± 1.17 | 1.47 ± 0.53 | 0.80 ± 0.15 |
| Soil bulk density (g/cm3) | 0.78 ± 0.08 | 0.71 ± 0.06 | 1.08 ± 0.05 | 1.22 ± 0.04 |
| Tree Species/Sampling Plot | Depth | Fine Roots (0–2 mm) | Small Roots (2–5 mm) | Coarse Roots (5–10 mm) | |||
|---|---|---|---|---|---|---|---|
| Biomass | Necromass | Biomass | Necromass | Biomass | Necromass | ||
| Beech | |||||||
| Fs1 | 0–45 | 165.2 ± 5.5 | 10.8 ± 3.2 | 282.1 ± 18.4 | 32.9 ± 10.1 | 153.2 ± 16.7 | 14.2 ± 5.1 |
| 0–15 | 62.5 ± 3.0 | 5.2 ± 1.5 | 92.2 ± 7.6 | 20.4 ± 5.2 | 47.6 ± 8.4 | 1.7 ± 5.1 | |
| 15–30 | 55.0 ± 3.8 | 2.5 ± 0.7 | 96.0 ± 12.2 | 8.2 ± 1.9 | 64.4 ± 24.6 | 0.9 ± 0.9 | |
| 30–45 | 47.7 ± 3.2 | 3.1 ± 1.6 | 93.9 ± 10.0 | 4.2 ± 0.7 | 41.3 ± 14.2 | 1.5 ± 1.5 | |
| Fs2 | 0–45 | 127.5 ± 5.3 | 8.2 ± 1.9 | 171.6 ± 10.5 | 16.0 ± 5.5 | 118.6 ± 29.9 | 7.7 ± 3.2 |
| 0–15 | 47.2 ± 2.7 | 4.0 ± 1.1 | 58.7 ± 4.3 | 5.9 ± 1.6 | 24.0 ± 9.6 | 5.2 ± 2.8 | |
| 15–30 | 45.7 ± 3.4 | 2.2 ± 0.7 | 57.8 ± 5.3 | 7.2 ± 1.9 | 54.7 ± 18.9 | 1.2 ± 0.8 | |
| 30–45 | 34.6 ± 4.3 | 2.0 ± 0.6 | 55.0 ± 7.1 | 2.9 ± 0.7 | 39.9 ± 7.8 | 1.3 ± 1.0 | |
| Douglas fir | |||||||
| Pm1 | 0–45 | 145.5 ± 6.6 | 41.3 ± 6.9 | 164.1 ± 28.2 | 57.8 ± 23.3 | 132.5 ± 31.5 | 37.7 ± 9.0 |
| 0–15 | 82.9 ± 6.0 | 20.7 ± 3.2 | 90.4 ± 7.3 | 20.9 ± 2.1 | 42.0 ± 11.2 | 9.9 ± 3.9 | |
| 15–30 | 37.5 ± 3.5 | 10.8 ± 1.8 | 49.9 ± 7.8 | 17.0 ± 3.8 | 44.9 ± 9.1 | 18.4 ± 5.7 | |
| 30–45 | 25.1 ± 1.6 | 9.8 ± 2.5 | 23.8 ± 3.9 | 9.9 ± 5.4 | 45.5 ± 17.6 | 9.4 ± 4.6 | |
| Pm2 | 0–45 | 124.3 ± 5.0 | 13.1 ± 3.7 | 125.2 ± 13.3 | 11.1 ± 6.8 | 126.0 ± 25.0 | 3.2 ± 2.1 |
| 0–15 | 75.8 ± 3.8 | 8.3 ± 2.2 | 61.9 ± 9.4 | 5.0 ± 1.6 | 46.0 ± 21.9 | 1.4 ± 1.4 | |
| 15–30 | 31.3 ± 1.6 | 3.8 ± 1.6 | 38.2 ± 6.1 | 3.2 ± 0.7 | 33.7 ± 9.3 | na | |
| 30–45 | 17.1 ± 1.1 | 1.0 ± 0.3 | 25.1 ± 3.4 | 2.8 ± 0.6 | 46.3 ± 14.7 | 1.8 ± 1.8 | |
| Fine Roots (≤2 mm) | Small Roots (2–5 mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tree Species/Sampling Plot | DM | MM | DM | MM | |||||
| Pa | TR | Pa | TR | Pa | TR | ||||
| Depth | Bmean | Bmax | Bmean | Bmax | |||||
| (cm) | (g m−2 yr−1) | (yr−1) | (yr−1) | (g m−2 yr−1) | (yr−1) | (yr−1) | (g m−2 yr-1) | (yr−1) | |
| Beech | |||||||||
| Fs1 | 0–45 | 170 | 1.07 | 0.78 | 114 | 0.71 | 0.52 | 478 | 339 |
| 0–15 | 54 | 0.81 | 0.63 | 37 | 0.58 | 0.45 | 166 | 131 | |
| 15–30 | 59 | 1.05 | 0.77 | 43 | 0.77 | 0.57 | 144 | 93 | |
| 30–45 | 57 | 1.34 | 0.94 | 34 | 0.79 | 0.53 | 169 | 116 | |
| Fs2 | 0–45 | 155 | 1.28 | 0.81 | 106 | 0.90 | 0.56 | 307 | 230 |
| 0–15 | 63 | 1.32 | 0.94 | 30 | 0.66 | 0.47 | 84 | 53 | |
| 15–30 | 38 | 0.90 | 0.55 | 39 | 0.96 | 0.56 | 121 | 99 | |
| 30–45 | 54 | 1.62 | 0.95 | 36 | 1.09 | 0.65 | 101 | 78 | |
| Douglas fir | |||||||||
| Pm1 | 0–45 | 134 | 1.10 | 0.77 | 113 | 0.85 | 0.59 | 345 | 208 |
| 0–15 | 63 | 0.89 | 0.65 | 55 | 0.71 | 0.52 | 146 | 85 | |
| 15–30 | 40 | 1.09 | 0.73 | 37 | 0.99 | 0.63 | 137 | 84 | |
| 30–45 | 31 | 1.31 | 0.92 | 21 | 0.87 | 0.62 | 61 | 38 | |
| Pm2 | 0–45 | 101 | 0.98 | 0.68 | 99 | 0.91 | 0.62 | 297 | 198 |
| 0–15 | 48 | 0.64 | 0.51 | 50 | 0.68 | 0.54 | 135 | 86 | |
| 15–30 | 32 | 1.12 | 0.71 | 31 | 1.00 | 0.62 | 103 | 68 | |
| 30–45 | 20 | 1.17 | 0.81 | 17 | 1.05 | 0.71 | 59 | 44 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozanova, L.; Zhiyanski, M.; Vanguelova, E.; Doncheva, S.; Marinov, M.P.; Lazarova, S. Dynamics and Vertical Distribution of Roots in European Beech Forests and Douglas Fir Plantations in Bulgaria. Forests 2019, 10, 1123. https://doi.org/10.3390/f10121123
Lozanova L, Zhiyanski M, Vanguelova E, Doncheva S, Marinov MP, Lazarova S. Dynamics and Vertical Distribution of Roots in European Beech Forests and Douglas Fir Plantations in Bulgaria. Forests. 2019; 10(12):1123. https://doi.org/10.3390/f10121123
Chicago/Turabian StyleLozanova, Lyudmila, Miglena Zhiyanski, Elena Vanguelova, Svetla Doncheva, Martin P. Marinov, and Stela Lazarova. 2019. "Dynamics and Vertical Distribution of Roots in European Beech Forests and Douglas Fir Plantations in Bulgaria" Forests 10, no. 12: 1123. https://doi.org/10.3390/f10121123





