Carbon Cycles of Forest Ecosystems in a Typical Climate Transition Zone under Future Climate Change: A Case Study of Shaanxi Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Modeling Forest NPP
2.3. Model Validation
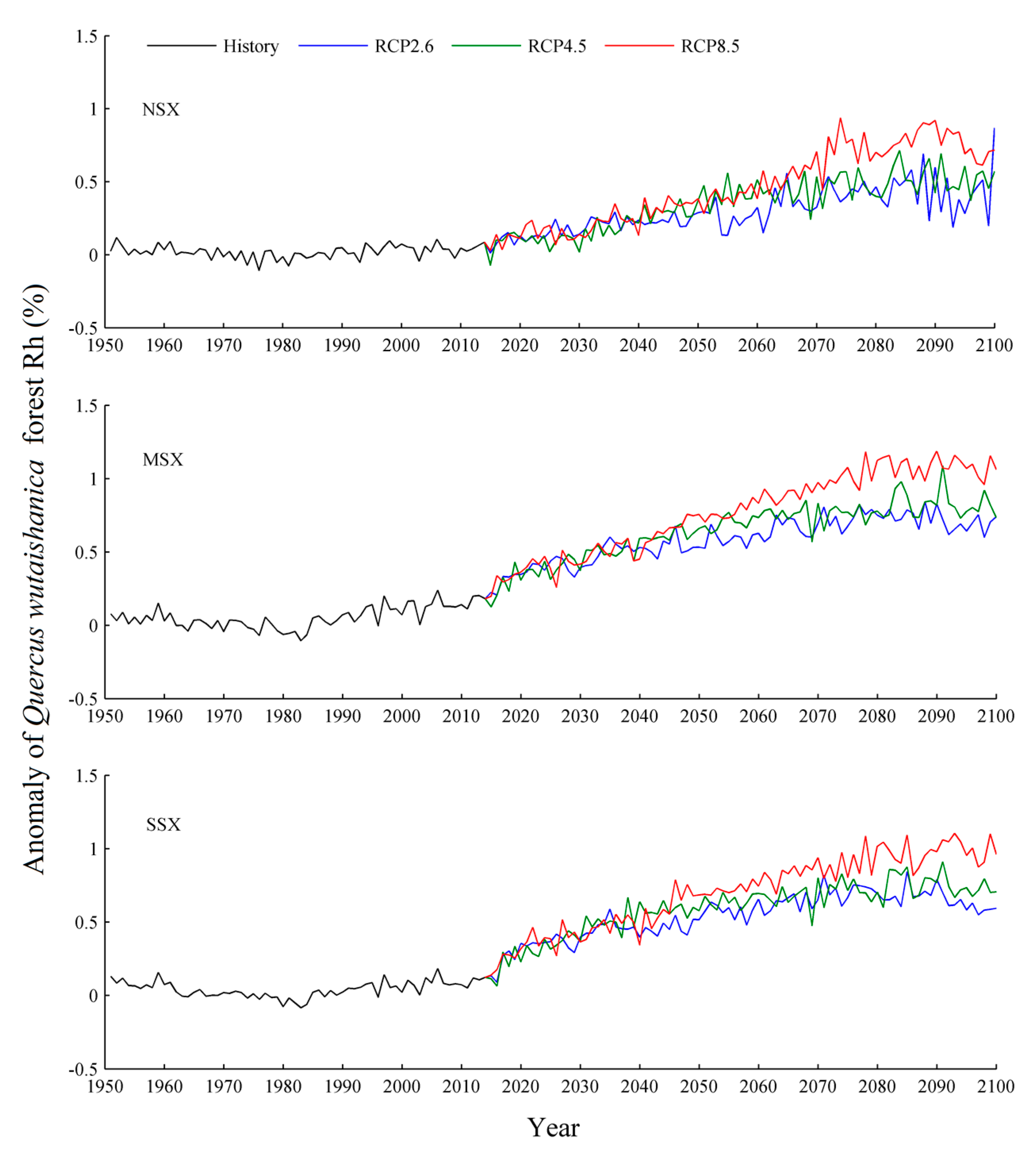
3. Results
3.1. Net Ecosystem Productivity
3.2. Soil Heterotrophic Respiration
3.3. Sensitivity of NPP to Temperature, Precipitation, and CO2 Concentration
4. Discussion
4.1. Sensitivity of Forests to Precipitation, Temperature, and CO2 Concentration
4.2. Soil Heterotrophic Respiration
4.3. Net Ecosystem Productivity
4.4. Uncertainty Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; p. 1535. [Google Scholar]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Zheng, J.L.; Mao, F.J.; Du, H.Q.; Li, X.J.; Zhou, G.M.; Dong, L.F.; Zhang, M.; Han, N.; Liu, T.Y.; Xing, L.Q. Spatiotemporal simulation of net ecosystem productivity and its response to climate change in subtropical forests. Forests 2019, 10, 708. [Google Scholar] [CrossRef]
- Poulter, B.; Hattermann, F.; Hawkins, E.D.; Zaehle, S.; Sitch, S.; Restrepo-Coupe, N.; Heyder, U.; Cramer, W. Robust dynamics of Amazon dieback to climate change with perturbed ecosystem model parameters. Glob. Chang. Biol. 2010, 16, 2476–2495. [Google Scholar] [CrossRef]
- Feng, X.; Uriarte, M.; Gonzalez, G.; Reed, S.; Thompson, J.; Zimmerman, J.K.; Murphy, L. Improving predictions of tropical forest response to climate change through integration of field studies and ecosystem modeling. Glob. Chang. Biol. 2018, 24, e213–e232. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Lomas, M.; Beer, C.; Liu, H.; Fang, J.; Friedlingstein, P.; Huang, Y.; Muraoka, H.; Son, Y.; et al. Contribution of climate change and rising CO2 to terrestrial carbon balance in East Asia: A multi-model analysis. Glob. Planet. Chang. 2011, 75, 133–142. [Google Scholar] [CrossRef]
- Morales, P.; Sykes, M.T.; Prentice, I.C.; Smith, P.; Smith, B.; Bugmann, H.; Zierl, B.; Friedlingstein, P.; Viovy, N.; Sabate, S.; et al. Comparing and evaluating process–based ecosystem model predictions of carbon and water fluxes in major European forest biomes. Glob. Chang. Biol. 2005, 11, 2211–2233. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Zhu, B.; Tan, K.; Tao, S. Changes in vegetation net primary productivity from 1982 to 1999 in China. Glob. Biogeochem. Cycles 2005, 19, GB2027. [Google Scholar] [CrossRef]
- Ren, G.; Ding, Y.; Zhao, Z.; Zheng, J.; Wu, T.; Tang, G.; Xu, Y. Recent progress in studies of climate change in China. Adv. Atmos. Sci. 2012, 29, 958–977. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, S.; Peng, S. Climate change affects forest productivity in a typical climate transition region of China. Sustainability 2019, 11, 2856. [Google Scholar] [CrossRef]
- Liu, W.; Sang, T. Potential productivity of the Miscanthus energy crop in the Loess Plateau of China under climate change. Environ. Res. Lett. 2013, 8, 044003. [Google Scholar] [CrossRef]
- Nayak, R.K.; Patel, N.R.; Dadhwal, V.K. Spatio-temporal variability of net ecosystem productivity over India and its relationship to climatic variables. Environ. Earth Sci. 2015, 74, 1743–1753. [Google Scholar] [CrossRef]
- Su, H.; Sang, W.; Wang, Y.; Ma, K. Simulating Picea schrenkiana forest productivity under climatic changes and atmospheric CO2 increase in Tianshan Mountains, Xinjiang Autonomous Region, China. For. Ecol. Manag. 2007, 246, 273–284. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, C.; Chen, Y.; Xu, Z. Simulating the productivity of a subalpine forest at high elevations under representative concentration pathway scenarios in the Qilian Mountains of northwest China. Scand. J. For. Res. 2017, 32, 166–173. [Google Scholar] [CrossRef]
- Keenan, T.F.; Baker, I.; Barr, A.; Ciais, P.; Davis, K.; Dietze, M.; Dragoni, D.; Gough, C.M.; Grant, R.; Hollinger, D.; et al. Terrestrial biosphere model performance for inter–annual variability of land–atmosphere CO2 exchange. Glob. Chang. Biol. 2012, 18, 1971–1987. [Google Scholar] [CrossRef]
- Law, B.E.; Turner, D.; Campbell, J.; Sun, O.J.; Van Tuyl, S.; Ritts, W.D.; Cohen, W.B. Disturbance and climate effects on carbon stocks and fluxes across Western Oregon USA. Glob. Chang. Biol. 2004, 10, 1429–1444. [Google Scholar] [CrossRef]
- Smith, B.; Warlind, D.; Arneth, A.; Hickler, T.; Leadley, P.; Siltberg, J.; Zaehle, S. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences 2014, 10, 18613–18685. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, S.; Yin, Y. Responses of terrestrial ecosystems’ net primary productivity to future regional climate change in China. PLoS ONE 2013, 8, e60849. [Google Scholar] [CrossRef]
- Ahlstrom, A.; Xia, J.; Arneth, A.; Luo, Y.; Smith, B. Importance of vegetation dynamics for future terrestrial carbon cycling. Environ. Res. Lett. 2015, 10, 054019. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, K.; Zhang, H. Carbon storage by forest vegetation and its spatial distribution in shaanxi. Resour. Sci. 2012, 34, 1781–1789. (In Chinese) [Google Scholar]
- Sitch, S.; Smith, B.; Prentice, I.C.; Arneth, A.; Bondeau, A.; Cramer, W.; Kaplan, J.O.; Levis, S.; Lucht, W.; Sykes, M.T.; et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Chang. Biol. 2003, 9, 161–185. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Peng, S.; Gang, C.; Cao, Y.; Chen, Y. Assessment of climate change trends over the Loess Plateau in China from 1901 to 2100. Int. J. Climatol. 2018, 38, 2250–2264. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef]
- Attaur, R.; Dawood, M. Spatio–statistical analysis of temperature fluctuation using Mann–Kendall and Sen’s slope approach. Clim. Dynam. 2017, 48, 783–797. [Google Scholar]
- Song, B.L. Study on Biomass, Carbon and Nitrogen Pool and Carbon Sequestration Characteristics of Two Typical Forest Ecosystems in Loess Hilly. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Liu, Y.; Wang, T.; Huang, M.; Yao, Y.; Ciais, P.; Piao, S. Changes in interannual climate sensitivities of terrestrial carbon fluxes during the 21st century predicted by CMIP5 Earth System Models. J. Geophys. Res. Biogeosci. 2016, 121, 903–918. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2012, 3, 52–58. [Google Scholar] [CrossRef]
- Schuur, E. Productivity and global climate revisited: The sensitivity of tropical forest growth to precipitation. Ecology 2003, 84, 1165–1170. [Google Scholar] [CrossRef]
- Schuur, E.A.; Matson, P.A. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 2001, 128, 431–442. [Google Scholar] [CrossRef]
- Peng, S.; Ding, Y.; Wen, Z.; Chen, Y.; Cao, Y.; Ren, J. Spatiotemporal change and trend analysis of potential evapotranspiration over the Loess Plateau of China during 2011–2100. Agric. For. Meteorol. 2017, 233, 183–194. [Google Scholar] [CrossRef]
- Digrado, A.; de la Motte, L.G.; Bachy, A.; Mozaffar, A.; Schoon, N.; Bussotti, F.; Amelynck, C.; Dalcq, A.C.; Fauconnier, M.L.; Aubinet, M.; et al. Decrease in the photosynthetic performance of temperate grassland species does not lead to a decline in the gross primary production of the ecosystem. Front. Plant Sci. 2018, 9, 67. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Medlyn, B.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.; Kirschbaum, M.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- Norby, R.; Warren, J.; Iversen, C.; Medlyn, B.; McMurtrie, R. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Piao, S.; Janssens, I.A.; Tang, J.; Liu, W.; Chi, Y.; Wang, J.; Xu, S. Soil respiration under climate warming: Differential response of heterotrophic and autotrophic respiration. Glob. Chang. Biol. 2014, 20, 3229–3237. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yang, Z.; Lin, C.; Xiong, D.; Lin, W.; Xu, C.; Chen, G.; Xie, J.; Li, Y.; et al. Will heterotrophic soil respiration be more sensitive to warming than autotrophic respiration in subtropical forests? Eur. J. Soil Sci. 2019, 70, 655–663. [Google Scholar] [CrossRef]
- Teramoto, M.; Liang, N.S.; Ishida, S.; Zeng, J.Y. Long-term stimulatory warming effect on soil heterotrophic respiration in a cool-temperate broad-leaved deciduous forest in Northern Japan. J. Geophys. Res. Biogeosci. 2018, 123, 1161–1177. [Google Scholar] [CrossRef]
- Liu, R.; Li, N.; Su, H.; Sang, W. Simulation and analysis on future carbon balance of three deciduous forests in Beijing mountain area, warm temperate zone of China. Chin. J. Plant Ecol. 2009, 33, 516–534. (In Chinese) [Google Scholar]
- Rey, A.; Pegoraro, E.; Tedeschi, V.; De Parri, I.; Jarvis, P.; Valentini, R. Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob. Chang. Biol. 2002, 8, 851–866. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Glob. Chang. Biol. 2004, 10, 161–169. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Huang, R.; Liao, Y.; Li, Y. Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant Soil 2014, 379, 361–371. [Google Scholar] [CrossRef]
- Chen, F.; Yan, G.; Xing, Y.; Zhang, J.; Wang, Q.; Wang, H.; Huang, B.; Hong, Z.; Dai, G.; Zheng, X.; et al. Effects of N addition and precipitation reduction on soil respiration and its components in a temperate forest. Agric. For. Meteorol. 2019, 271, 336–345. [Google Scholar] [CrossRef]
- Ji, J.; Huang, M.; Li, K. Prediction of carbon exchanges between China terrestrial ecosystem and atmosphere in 21st century. Sci. China Ser. D Earth Sci. 2008, 51, 885–898. (In Chinese) [Google Scholar] [CrossRef]
- Fei, X.; Song, Q.; Zhang, Y.; Liu, Y.; Sha, L.; Yu, G.; Zhang, L.; Duan, C.; Deng, Y.; Wu, C.; et al. Carbon exchanges and their responses to temperature and precipitation in forest ecosystems in Yunnan, Southwest China. Sci. Total Environ. 2018, 616–617, 824–840. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Li, S.; Wen, X.; Li, Q.; Wang, H. Effects of sky conditions on net ecosystem productivity of a subtropical coniferous plantation vary from half-hourly to daily timescales. Sci. Total Environ. 2019, 651, 3002–3014. [Google Scholar] [CrossRef]
- Jung, M.; Reichstein, M.; Schwalm, C.R.; Huntingford, C.; Sitch, S.; Ahlstrom, A.; Arneth, A.; Camps-Valls, G.; Ciais, P.; Friedlingstein, P.; et al. Compensatory water effects link yearly global land CO2 sink changes to temperature. Nature 2017, 541, 516–520. [Google Scholar] [CrossRef]
- Cox, P.M.; Pearson, D.; Booth, B.B.; Friedlingstein, P.; Huntingford, C.; Jones, C.D.; Luke, C.M. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature 2013, 494, 341–344. [Google Scholar] [CrossRef]
- Gatebe, C.; Kuznetsov, A.; Melnikova, I. Cloud optical parameters from airborne observation of diffuse solar radiation accomplished in USA and USSR in different geographical regions. Int. J. Remote Sens. 2014, 35, 5812–5829. [Google Scholar]
- van Gorsel, E.; Berni, J.A.J.; Briggs, P.; Cabello-Leblic, A.; Chasmer, L.; Cleugh, H.A.; Hacker, J.; Hantson, S.; Haverd, V.; Hughes, D.; et al. Primary and secondary effects of climate variability on net ecosystem carbon exchange in an evergreen Eucalyptus forest. Agric. For. Meteorol. 2013, 182–183, 248–256. [Google Scholar] [CrossRef]
- Knohl, A.; Baldocchi, D.D. Effects of diffuse radiation on canopy gas exchange processes in a forest ecosystem. J. Geophys. Res. Biogeosci. 2008, 113, G02023. [Google Scholar] [CrossRef]
- Cheng, S.J.; Bohrer, G.; Steiner, A.L.; Hollinger, D.Y.; Suyker, A.; Phillips, R.P.; Nadelhoffer, K.J. Variations in the influence of diffuse light on gross primary productivity in temperate ecosystems. Agric. For. Meteorol. 2015, 201, 98–110. [Google Scholar] [CrossRef]
- Moyano, F.E.; Kutsch, W.L.; Rebmann, C. Soil respiration fluxes in relation to photosynthetic activity in broad-leaf and needle-leaf forest stands. Agric For. Meteorol. 2008, 148, 135–143. [Google Scholar] [CrossRef]
- Seiler, C.; Hutjes, R.W.A.; Kruijt, B.; Hickler, T. The sensitivity of wet and dry tropical forests to climate change in Bolivia. J. Geophys. Res. Biogeosci. 2015, 120, 399–413. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
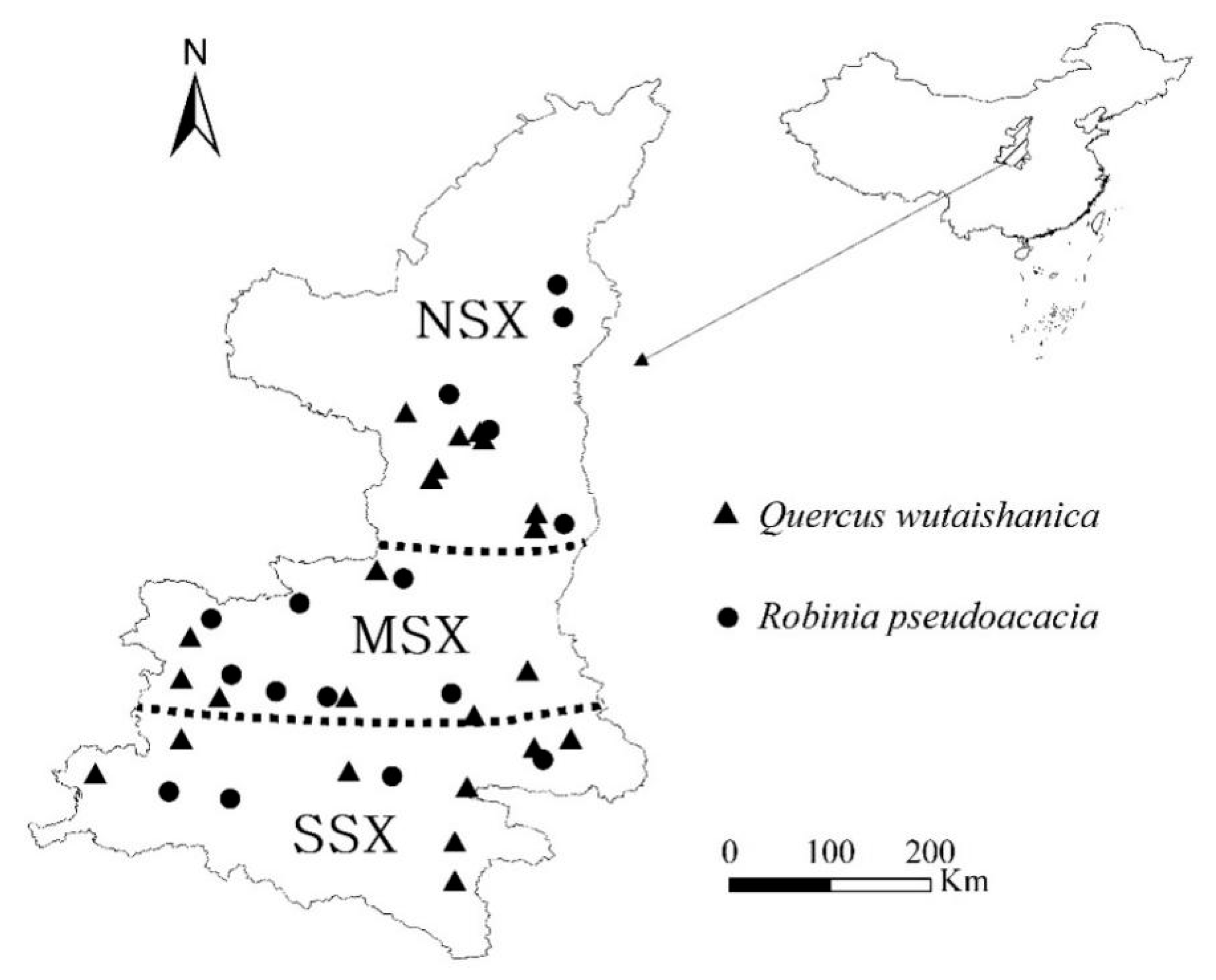

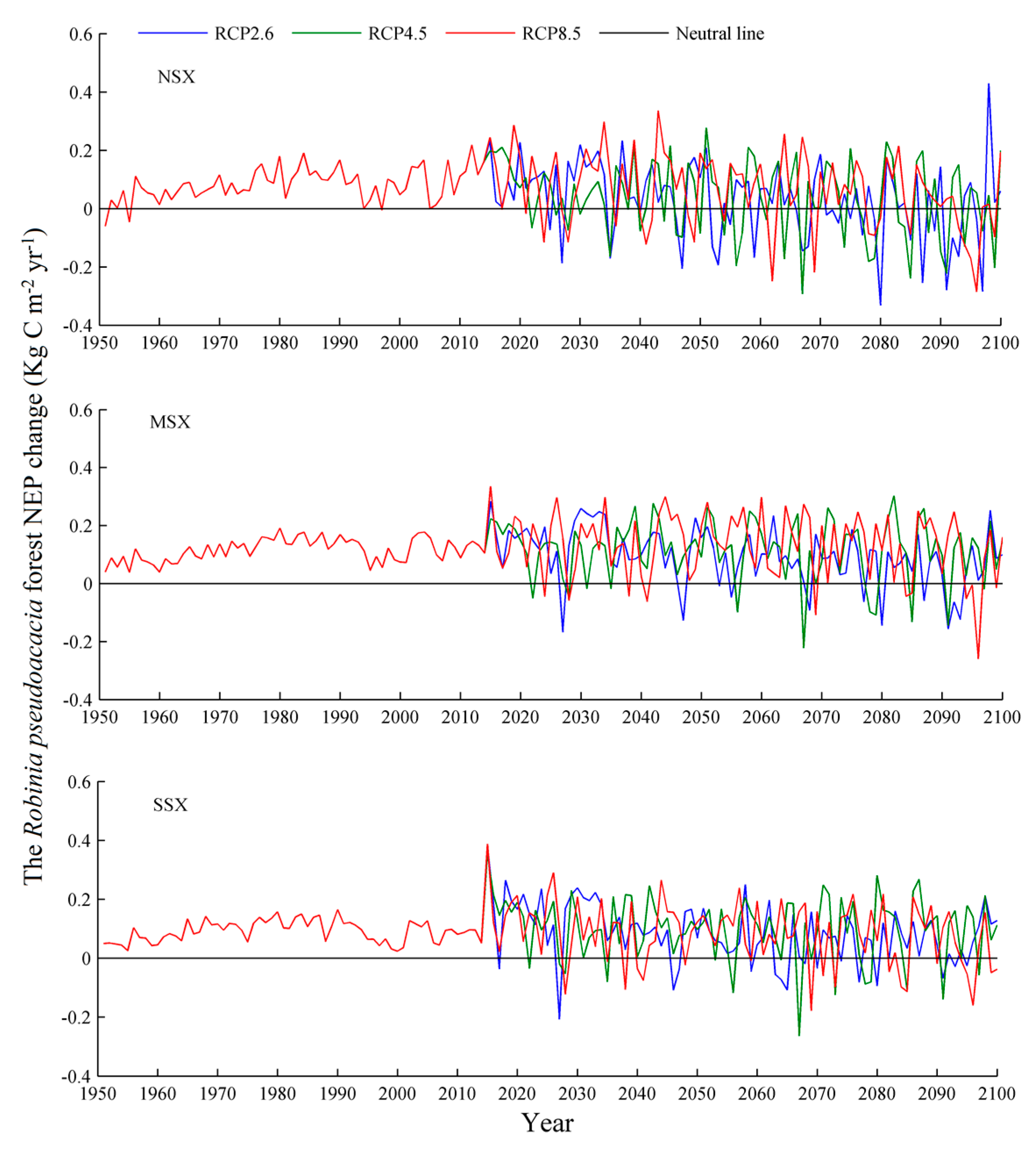
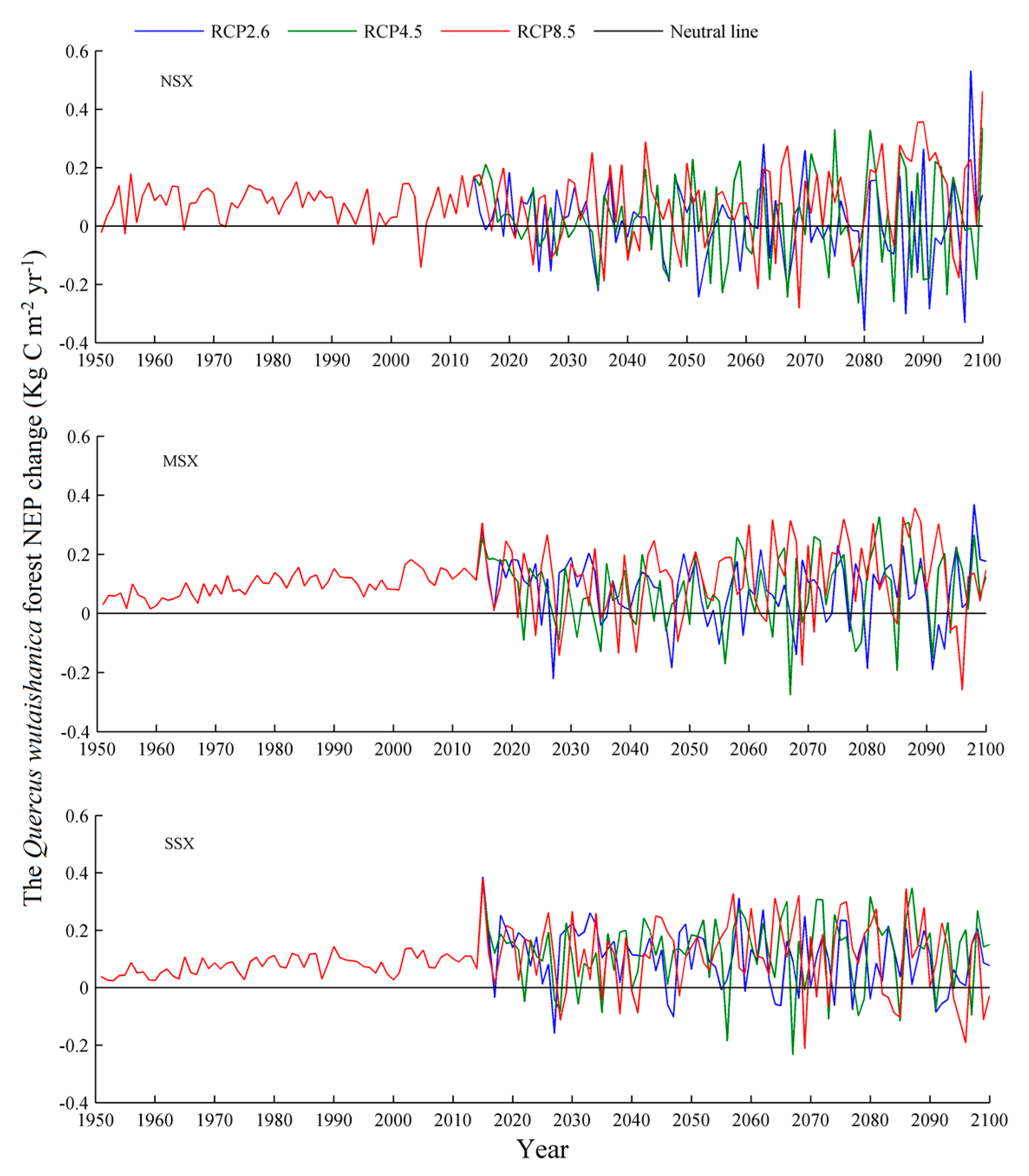
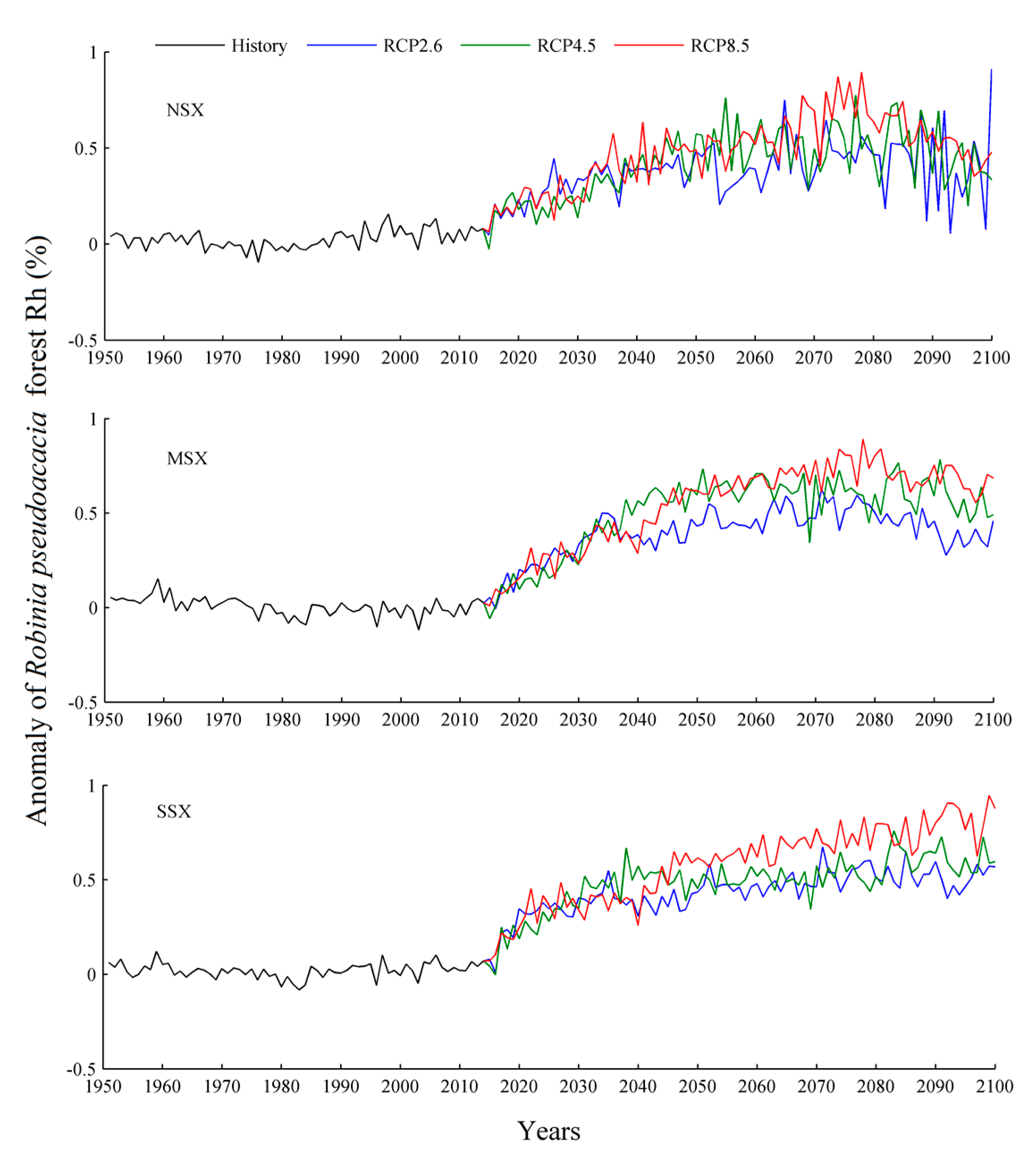
| Species | Years | NEP (g C m−2 yr−1) | Source |
|---|---|---|---|
| RP | 2010–2012 | 145.3 (109.8–206.2) | LPJ-GUESS model |
| 181.4 | Reference [26] | ||
| QW | 2010–2012 | 120.4 (81.1–145.7) | LPJ-GUESS model |
| 96.6 | Reference [26] |
| Site | Species | 1951–2014 | 2015–2100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCP2.6 | RCP4.5 | RCP8.5 | |||||||||||
| Av | T | Sd | Av | T | Sd | Av | T | Sd | Av | T | Sd | ||
| NSX | QW | 81.7 | 14.00 * | 55.5 | 30.2 | −16.00 * | 133.4 | 38.0 | −10.00 * | 129.9 | 63.1 | −15.00 * | 124.3 |
| RP | 85.8 | −1.70 | 60.7 | 5.8 | −6.30 | 141.1 | 19.9 | 0.70 | 148.1 | 78.3 | 6.00 | 142.4 | |
| MSX | QW | 115.1 | 9.00 * | 39.1 | 93.6 | −15.00 * | 97.8 | 116.2 | −3.90 | 103.7 | 131.2 | −6.30 * | 111.7 |
| RP | 96.5 | 15.00 * | 39.1 | 78.5 | −4.10 | 109.5 | 83.7 | 4.10 | 123.5 | 117.2 | 6.40 | 129.5 | |
| SSX | QW | 90.9 | 2.90 | 35.9 | 83.9 | −16.00 * | 99.6 | 101.4 | −3.70 | 108.6 | 85.5 | −11.00 * | 107.0 |
| RP | 97.4 | 9.70 * | 32.0 | 105.3 | −13.00 * | 105.4 | 124.9 | 3.90 | 122.7 | 112.6 | 4.80 | 129.3 | |
| Site | Species | 1951–2014 | 2015–2100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCP2.6 | RCP4.5 | RCP8.5 | |||||||||||
| Av | An | Sd | An | Av | Sd | Av | An | Sd | An | Av | Sd | ||
| NSX | QW | 434.4 | 2.0% | 19.0 | 553.5 | 30.0% | 65.4 | 574.4 | 34.9% | 76.2 | 622.9 | 46.2% | 109.9 |
| RP | 383.8 | 1.5% | 18.4 | 515.5 | 37.7% | 57.5 | 530.2 | 41.6% | 63.7 | 553.7 | 47.9% | 67.5 | |
| MSX | QW | 394.7 | 5.5% | 28.7 | 593.4 | 58.6% | 54.6 | 615.8 | 64.6% | 69.8 | 662.7 | 77.1% | 102.2 |
| RP | 378.0 | 2.4% | 17.9 | 526.3 | 39.8% | 47.3 | 565.3 | 50.2% | 73.3 | 582.1 | 54.6% | 80.3 | |
| SSX | QW | 380.5 | 4.2% | 20.1 | 562.9 | 54.2% | 55.0 | 582.1 | 59.4% | 64.8 | 621.8 | 70.3% | 91.9 |
| RP | 367.0 | 1.9% | 14.4 | 517.3 | 43.7% | 40.3 | 534.2 | 48.3% | 50.6 | 569.4 | 58.1% | 73.4 | |
| R. pseudoacacia Forest | C1 | C2 | C3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | ||
| NSX | P1 | 0% | −11.4% | −29.0% | 7.2% | 2.3% | −15.2% | 30.4% | 12.7% | −12.9% |
| P2 | 13.0% | 5.7% | −16.1% | 28.1% | 26.0% | −3.6% | 44.6% | 34.8% | 15.9% | |
| P3 | 18.4% | 21.8% | 1.1% | 39.3% | 25.6% | 20.8% | 51.9% | 36.6% | 32.1% | |
| MSX | P1 | 0% | −7.6% | −25.5% | 15.6% | 6.8% | −5.5% | 28.2% | 17.3% | 8.8% |
| P2 | 2.6% | −3.1% | −17.8% | 18.1% | 10.6% | −3.5% | 24.0% | 23.0% | 9.4% | |
| P3 | 7.9% | −5.1% | −16.1% | 20.6% | 12.9% | −5.9% | 29.2% | 23.7% | 10.7% | |
| SSX | P1 | 0% | −9.1% | −29.5% | 23.1% | 11.9% | −11.4% | 45.9% | 23.1% | 3.1% |
| P2 | 7.4% | −10.2% | −29.8% | 19.3% | 13.5% | −17.0% | 37.0% | 22.5% | 0.8% | |
| P3 | 1.2% | −7.1% | −27.5% | 25.4% | 12.7% | −13.9% | 40.0% | 20.1% | 2.7% | |
| Q. wutaishanica Forest | C1 | C2 | C3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | ||
| NSX | P1 | 0% | −2.7% | −20.7% | 18.0% | 1.7% | −6.8% | 31.3% | 17.6% | 10.2% |
| P2 | 20.5% | 10.3% | −5.0% | 38.7% | 25.7% | 5.6% | 44.8% | 34.0% | 19.9% | |
| P3 | 27.7% | 12.6% | 7.4% | 41.6% | 32.0% | 19.3% | 53.0% | 41.2% | 28.1% | |
| MSX | P1 | 0% | −12.0% | −20.4% | 9.6% | −0.7% | −10.1% | 18.4% | 8.6% | −4.2% |
| P2 | −0.1% | −5.6% | −18.8% | 12.4% | 1.7% | −6.3% | 20.4% | 10.4% | 0.8% | |
| P3 | 6.9% | −1.3% | −11.5% | 16.3% | 5.0% | −5.5% | 20.3% | 11.6% | 1.7% | |
| SSX | P1 | 0% | −19.2% | −33.2% | 4.0% | −10.2% | −19.1% | 11.7% | −0.7% | −15.7% |
| P2 | 1.9% | −13.2% | −29.5% | 7.8% | 7.5% | −19.5% | 15.9% | 0.5% | −13.4% | |
| P3 | −0.7% | −16.6% | −26.1% | 9.4% | −2.3% | −16.6% | 15.4% | 1.7% | −10.8% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Peng, S.; Chen, Y. Carbon Cycles of Forest Ecosystems in a Typical Climate Transition Zone under Future Climate Change: A Case Study of Shaanxi Province, China. Forests 2019, 10, 1150. https://doi.org/10.3390/f10121150
Liang S, Peng S, Chen Y. Carbon Cycles of Forest Ecosystems in a Typical Climate Transition Zone under Future Climate Change: A Case Study of Shaanxi Province, China. Forests. 2019; 10(12):1150. https://doi.org/10.3390/f10121150
Chicago/Turabian StyleLiang, Siqi, Shouzhang Peng, and Yunming Chen. 2019. "Carbon Cycles of Forest Ecosystems in a Typical Climate Transition Zone under Future Climate Change: A Case Study of Shaanxi Province, China" Forests 10, no. 12: 1150. https://doi.org/10.3390/f10121150
APA StyleLiang, S., Peng, S., & Chen, Y. (2019). Carbon Cycles of Forest Ecosystems in a Typical Climate Transition Zone under Future Climate Change: A Case Study of Shaanxi Province, China. Forests, 10(12), 1150. https://doi.org/10.3390/f10121150




