Short-Term Effects of Different Forest Management Methods on Soil Microbial Communities of a Natural Quercus aliena var. acuteserrata Forest in Xiaolongshan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Soil Sampling Collection
2.3. Soil Sample Chemical Analysisd
2.4. DNA Extraction and PCR Amplification
2.5. PCR Amplification and Illumina MiSeq Sequencing
2.6. Processing of Sequencing Data
2.7. Statistical Analysis
3. Results
3.1. Soil Properties
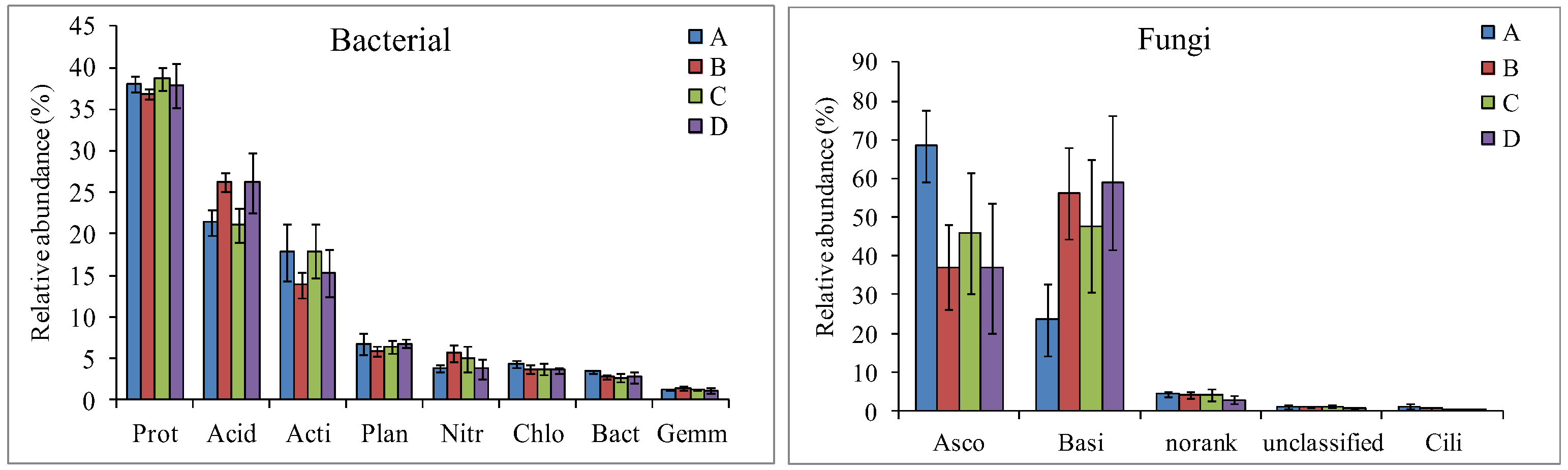
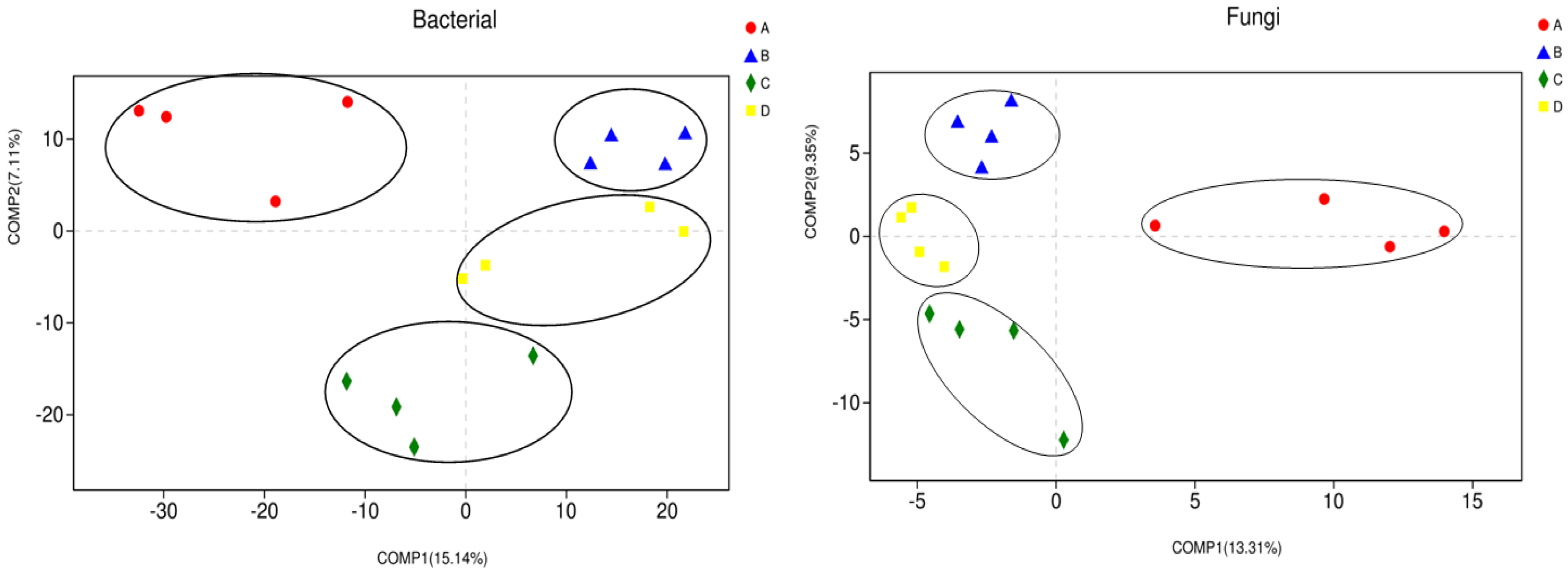
3.2. Microbial Community Diversity and Composition Analysis
3.3. Correlations between Soil Properties and Microbial Communities
3.4. Effect of Soil Properties on Microbial Communities at the Genus Level
4. Discussion
4.1. Forest Management Effects on Soil Nutrients
4.2. Changes in Soil Microbial Community Composition and Diversity
4.3. The Relationship between Soil Nutrients and Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wall, D.H.; Moore, J.C. Interactions underground: Soil biodiversity, mutualism, and ecosystem process. Bioscience 1999, 49, 109–117. [Google Scholar] [CrossRef]
- Allsion, V.J.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Turner, B.L. Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Joset chronosequence New Zealand. Soil Biol. Biochem. 2007, 39, 1770–1781. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bardgett, R.D.; von Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Comerford, N.B.; Franzluebbers, A.J.; Stromberger, M.E.; Morris, L.; Markewitz, D.; Moore, R. Assessment and evaluation of soil ecosystem services. Soil Horiz. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Saetere, P.; Bååth, E. Spatial variation and patterns of soil microbial community structure in a mixed spruce-birch stand. Soil Boil. Biochem. 2000, 32, 909–917. [Google Scholar] [CrossRef]
- Ruess, L.; Ferris, H. Decomposition pathways and successional changes. Nematol. Monogr. Perspect. 2004, 2, 547–556. [Google Scholar]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Danh, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Romaní, A.M.; Fischer, H.; Mille-Lindblom, C.; Tranvik, L.J. Interactions of bacteria and fungi on decomposing litter: Differential extracellular enzyme activities. Ecology 2006, 87, 2559–2569. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef]
- Richter, A.; Schöning, I.; Kahl, T.; Bauhus, J.; Ruess, L. Regional environmental conditions shape microbial community structure stronger than local forest management intensity. For. Ecol. Manag. 2018, 409, 250–259. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Barg, A.K.; Edmonds, R.L. Influence of partial cutting on site microclimate, soil nitrogen dynamics and microbial biomsass in Douglas-fir stands in western Washington. Can. J. For. Res. 1999, 29, 705–713. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unravelling rhizosphere microbial interactions: opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the loess plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Huang, Y.M.; Michel, K.; An, S.S.; Zechmeister-Boltenstern, S. Changes in microbial-community structure with depth and time in a chronosequence of restored grassland soils on the Loess Plateau in northwest China. J. Plant Nutr. Soil Sci. 2011, 174, 765–774. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef]
- Lewandowski, T.E.; Forrester, J.A.; Mladenoff, D.J.; D’Amato, A.W.; Fassnacht, D.S.; Padley, E.; Martin, K.J. Do biological legacies moderate the effects of forest harvesting on soil microbial community composition and soil respiration. For. Ecol. Manag. 2019, 432, 298–308. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N: Metaanalysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Foote, J.A.; Boutton, T.W.; Scott, D.A. Soil C and N storage and microbial biomass in US southern pine forests: Influence of forest management. For. Ecol. Manag. 2015, 355, 48–57. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Zifcakova, L.; Snajdr, J.; Ridl, J.; Vlcek, C.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Nacke, H.; Thürmer, A.; Wollherr, A.; Will, C.; Hodac, L.; Herold, N.; Schoning, I.; Schrumpf, M.; Daniel, R. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef] [PubMed]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links. Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Kennedy, N.; Brodie, E.; Connolly, J.; Clipson, N. Impact of lime, nitrogen and plant species on bacterial community structure in grassland microcosms. Environ. Microbiol. 2004, 6, 1070–1080. [Google Scholar] [CrossRef]
- Johnson, D.; Booth, R.E.; Whiteley, A.S.; Bailey, M.J.; Read, D.J.; Grime, J.P.; Leake, J.R. Plant community composition affects the biomass, activity and diversity of microorganisms in limestone grassland soil. Eur. J. Soil Sci. 2003, 54, 671–678. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Zhang, H. High throughput sequencing analysis of bacterial communities in soils of a typical Poyang Lake wetland. Acta Ecol. Sin. 2017, 37, 1650–1658, (In Chinese with English abstract). [Google Scholar]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.B.; Wang, G. Highthroughputsequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Wang, M.; Wan, P.; Chai, Y.; Guo, Y.; Zhang, X.; Yue, M. Adaptative Strategy of Leaf Traits to Drought Conditions: Quercus aliena var. acuteserrata Forest (the Qinling Mts. China). Pol. J. Ecol. 2015, 63, 77–87. [Google Scholar] [CrossRef]
- Hui, G.Y.; Hu, Y.B.; Zhao, Z.H. Research progress of structure-based forest management. For. Res. 2018, 31, 85–93, (In Chinese with English abstract). [Google Scholar]
- Hui, G.Y.; Hu, Y.B.; Zhao, Z.H. Further discussion on “Structure-based forest management”. World For. Res. 2009, 22, 14–19, (In Chinese with English abstract). [Google Scholar]
- O’Hara, K.L. The silviculture of transformation—A commentary. For. Ecol. Manag. 2001, 151, 81–86. [Google Scholar] [CrossRef]
- Li, Y.; Ye, S.; Hui, G.; Hu, Y.; Zhao, Z. Spatial structure of timber harvested according to structure-based forest management. For. Ecol. Manag. 2014, 322, 106–116. [Google Scholar] [CrossRef]
- Yuan, S.Y. Evaluation of Current Forest Management Models in Xiaolongshan Gansu Province. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2010. (In Chinese with English abstract). [Google Scholar]
- Chen, C.G.; Gong, L.Q.; Peng, H.; Liu, X.Z. Biomass and productivity of the sharptooth Oka forests. J. Northwest For. Coll. 1996, 11, 103–114, (In Chinese with English abstract). [Google Scholar]
- Wu, P.H.; Bai, G.P.; Dang, K.L.; Chang, W.; Li, M.Y. Thinning effects on growth of Pinus tabulaeformis middle-age forest on southern slope of Qinling Mountains. J. Cent. South Univ. For. Technol. 2017, 37, 20–26, (In Chinese with English abstract). [Google Scholar]
- Zhao, Z.H.; Liu, W.Z.; Shi, X.L.; Li, A.M.; Guo, X.L.; Zhang, G.Q.; Hui, G.Y. Structure Dynamic of Quercus aliena var. acuteserrata Natural Forest on Xiaolongshan. For. Res. 2015, 28, 759–766, (In Chinese with English abstract). [Google Scholar]
- Hou, H.; Zhang, S.Z.; Guan, J.H.; Du, S. Accumulation of soil organic carbon and total nitrogen in Quercus aliena var. acuteserrata forests at different age stages in the Xiaolongshan Mountains, Gansu Province. Acta Ecol. Sin. 2016, 36, 8025–8033, (In Chinese with English abstract). [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Zhang, D.H.; Ye, Z.F.; Fan, B.Y.; Wei, T.L. Influence of thinning on soil fertility in artificial forests. Chin. J. Appl. Ecol. 2001, 12, 672–676, (In Chinese with English abstract). [Google Scholar]
- Zhang, J.P.; Yu, L.Z.; Liu, L.F.; Zhang, J.X.; Gao, S.L.; Zhang, W.R.; Liu, C.Y. Effects of different silvicultural ways on soil nutrients and enzyme activity in larch plantation. Chin. J. Ecol. 2016, 35, 1403–1410, (In Chinese with English abstract). [Google Scholar]
- Yang, Y.; Geng, Y.; Zhou, H.; Zhao, G.; Wang, L. Effects of gaps in the forest canopyon soil microbial communities and enzyme activity in a Chinese pine forest. Pedobiologia 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Fazi, S.; Amalfitano, S.; Pernthaler, J.; Puddu, A. Bacterial communities associated with benthic organic matter in headwater stream microhabitats. Environ. Microbiol. 2005, 7, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, D.L. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Yu, Z.; Yao, Q.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol. Biochem. 2016, 95, 212–222. [Google Scholar] [CrossRef]
- Grayston, S.; Germida, J. Influence of crop rhizospheres on populations and activity of heterotrophic sulfur-oxidizing microorganisms. Soil Biol. Biochem. 1990, 22, 457–463. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Tikkanen, O.P.; Predtechenskaya, O.; Ruokolainen, A.; Heikkilä, R. Recovery of functional groups of fungi and wood-decaying species of conservation concern after variable intensity forest utilization. Eur. J. For. Res. 2017, 136, 1–11. [Google Scholar] [CrossRef]
- Hartmann, M.; Howes, C.G.; VanInsberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Nillson, R.H.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in northern coniferous forests. ISME J. 2012, 6, 2199. [Google Scholar] [CrossRef]
- Liu, J.; Ha, V.N.; Shen, Z.; Zhu, H.; Zhao, F.; Zhao, Z. Characteristics of bulk and rhizosphere soil microbial community in an ancient Platycladus orientalis forest. Appl. Soil Ecol. 2018, 132, 91–98. [Google Scholar] [CrossRef]
- Albertsen, M.; Hansen, L.B.S.; Saunders, A.M.; Nielsen, P.H.; Nielsen, K.L. A metagenome of a full-scale microbial community carrying out enhanced biological phosphorus removal. ISME J. 2012, 6, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jing, G.; Wei, L.; Jing, Z. Long-term grazing exclusion effects on vegetation characteristics, soil properties and bacterial communities in the semi-arid grasslands of China. Ecol. Eng. 2016, 97, 170–178. [Google Scholar] [CrossRef]
- Cheng, F.; Wei, X.; Hou, L.; Shang, Z.; Peng, X.; Zhao, P.; Fei, Z.; Zhang, S. Soil fungal communities of montane natural secondary forest types in China. J. Microbiol. 2015, 53, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Grantina, L.; Seile, E.; Kenigsvalde, K.; Kasparinskis, R.; Tabors, G.; Nikolajeva, V.; Jungerius, P.; Muiznieks, I. The influence of the land use on abundance and diversity of soil fungi: Comparison of conventional and molecular methods of analysis. Environ. Exp. Biol. 2011, 9, 9–21. [Google Scholar]
- Zhang, T.; Wang, N.F.; Liu, H.Y.; Zhang, Y.Q.; Yu, L.Y. Soil pH is a Key Determinant of Soil Fungal Community Composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Wang, L.M.; Huang, D.F.; Fang, Y.; Wang, F.; Li, F.L.; Liao, M. Soil fungal communities in tea plantation after 10 years of chemical vs. integrated fertilization. Chil. J. Agric. Res. 2017, 77, 355–364. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Osler, G.H.; Campbell, C.D.; Burslem, D.F.; van der Wal, R. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. J. Biogeogr. 2010, 37, 1317–1328. [Google Scholar] [CrossRef]
- Zumsteg, A.; Luster, J.; Göransson, H.; Smittenberg, R.H.; Brunner, I.; Bernasconi, S.M.; Zeyer, J.; Frey, B. Bacterial, archaeal and fungal succession in the fore field of a receding glacier. Microb. Ecol. 2012, 63, 552–564. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Ridgway, K.P.; Edwards, E.J.; Benham, D.G.; Young, J.P.W.; Fitter, A.H. Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Glob. Chang. Biol. 2004, 10, 52–64. [Google Scholar] [CrossRef]


| Plot | Elevation (m) | Slope (°) | Aspect | DBH (cm) | Height (m) | Canopy Cover |
|---|---|---|---|---|---|---|
| A1 | 1749 | 27 | East | 16.60 | 12.24 | 0.80 |
| A2 | 1727 | 34 | East | 13.10 | 12.27 | 0.80 |
| A3 | 1699 | 35 | East | 12.72 | 12.88 | 0.80 |
| A4 | 1709 | 35 | East | 12.48 | 12.70 | 0.80 |
| B1 | 1664 | 36 | East | 15.54 | 12.49 | 0.80 |
| B2 | 1727 | 37 | East | 13.37 | 12.17 | 0.80 |
| B3 | 1686 | 37 | East | 13.69 | 12.96 | 0.80 |
| B4 | 1680 | 35 | East | 15.00 | 12.73 | 0.80 |
| C1 | 1663 | 36 | East | 14.00 | 12.48 | 0.90 |
| C2 | 1717 | 35 | East | 14.42 | 11.94 | 0.90 |
| C3 | 1760 | 36 | East | 14.96 | 12.04 | 0.90 |
| C4 | 1735 | 34 | East | 14.50 | 12.88 | 0.90 |
| D1 | 1711 | 36 | East | 14.36 | 13.16 | 0.80 |
| D2 | 1700 | 36 | East | 13.91 | 12.03 | 0.80 |
| D3 | 1728 | 37 | East | 12.30 | 12.58 | 0.80 |
| D4 | 1683 | 37 | East | 13.22 | 12.24 | 0.80 |
| Modes | SOM (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | pH |
|---|---|---|---|---|---|---|---|---|
| A | 60.57 ± 14.18 | 2.70 ± 0.72 | 0.40 ± 0.10 | 18.63 ± 1.30 | 205.22 ± 39.57 | 5.82 ± 0.72b | 133.62 ± 31.76 | 6.65 ± 0.15 |
| B | 61.28 ± 8.48 | 2.77 ± 0.31 | 0.40 ± 0.04 | 17.40 ± 1.97 | 207.76 ± 23.13 | 6.90 ± 1.78bc | 132.50 ± 54.11 | 6.20 ± 0.27 |
| C | 64.78 ± 11.75 | 2.86 ± 0.53 | 0.38 ± 0.06 | 18.55 ± 2.10 | 205.13 ± 33.38 | 9.61 ± 2.22c | 123.75 ± 32.32 | 5.99 ± 0.64 |
| D | 56.76 ± 17.02 | 2.49 ± 0.76 | 0.38 ± 0.10 | 20.72 ± 1.53 | 200.06 ± 53.97 | 13.46 ± 3.43a | 226.87 ± 123.95 | 6.24 ± 0.06 |
| F | 0.246 | 0.269 | 0.042 | 2.472 | 0.026 | 9.043 | 1.862 | 2.321 |
| P | 0.862 (NS) | 0.847 (NS) | 0.988 (NS) | 0.112 (NS) | 0.994 (NS) | 0.002 | 0.190 (NS) | 0.127 (NS) |
| Plot | Bacteria | Fungi | ||
|---|---|---|---|---|
| Shannon | Chao | Shannon | Chao | |
| A | 6.19 ± 0.09a | 1966.60 ± 91.57a | 3.11 ± 0.24a | 230.21 ± 8.51a |
| B | 6.11 ± 0.07a | 1914.90 ± 50.37a | 2.95 ± 0.21a | 220.50 ± 10.11a |
| C | 6.08 ± 0.08a | 1910.80 ± 103.69a | 2.92 ± 0.21a | 213.59 ± 21.69a |
| D | 6.03 ± 0.17a | 1953.70 ± 22.67a | 2.64 ± 0.55a | 216.69 ± 25.10a |
| Bacteria | Fungi | |||||||
|---|---|---|---|---|---|---|---|---|
| RDA1 | RDA2 | r2 | p | RDA1 | RDA2 | r2 | p | |
| SOM | 0.757 | −0.653 | 0.421 | 0.031 * | 0.947 | −0.320 | 0.094 | 0.529 |
| TN | 0.766 | −0.642 | 0.432 | 0.030 * | 0.907 | −0.421 | 0.131 | 0.383 |
| TP | 0.961 | 0.276 | 0.024 | 0.859 | 0.802 | 0.596 | 0.253 | 0.141 |
| TK | −0.901 | −0.432 | 0.102 | 0.449 | 0.978 | 0.205 | 0.008 | 0.942 |
| AN | 0.848 | −0.53 | 0.428 | 0.031 * | 0.954 | −0.299 | 0.204 | 0.207 |
| AP | −0.906 | −0.421 | 0.013 | 0.909 | 0.999 | 0.014 | 0.048 | 0.725 |
| AK | 0.892 | 0.451 | 0.114 | 0.472 | 0.996 | 0.086 | 0.452 | 0.035 * |
| pH | −0.767 | −0.641 | 0.221 | 0.200 | −0.914 | 0.403 | 0.135 | 0.397 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, P.; Zhang, G.; Zhao, Z.; Hu, Y.; Liu, W.; Hui, G. Short-Term Effects of Different Forest Management Methods on Soil Microbial Communities of a Natural Quercus aliena var. acuteserrata Forest in Xiaolongshan, China. Forests 2019, 10, 161. https://doi.org/10.3390/f10020161
Wan P, Zhang G, Zhao Z, Hu Y, Liu W, Hui G. Short-Term Effects of Different Forest Management Methods on Soil Microbial Communities of a Natural Quercus aliena var. acuteserrata Forest in Xiaolongshan, China. Forests. 2019; 10(2):161. https://doi.org/10.3390/f10020161
Chicago/Turabian StyleWan, Pan, Gongqiao Zhang, Zhonghua Zhao, Yanbo Hu, Wenzhen Liu, and Gangying Hui. 2019. "Short-Term Effects of Different Forest Management Methods on Soil Microbial Communities of a Natural Quercus aliena var. acuteserrata Forest in Xiaolongshan, China" Forests 10, no. 2: 161. https://doi.org/10.3390/f10020161
APA StyleWan, P., Zhang, G., Zhao, Z., Hu, Y., Liu, W., & Hui, G. (2019). Short-Term Effects of Different Forest Management Methods on Soil Microbial Communities of a Natural Quercus aliena var. acuteserrata Forest in Xiaolongshan, China. Forests, 10(2), 161. https://doi.org/10.3390/f10020161




