Annual Variations in Norway Spruce Xylem Studied Using Infrared Micro-spectroscopy
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Site and Xylem Samples
2.2. Temperature and Drought Data
2.3. Silviscan Density Measurements and Infrared Microspectroscopy
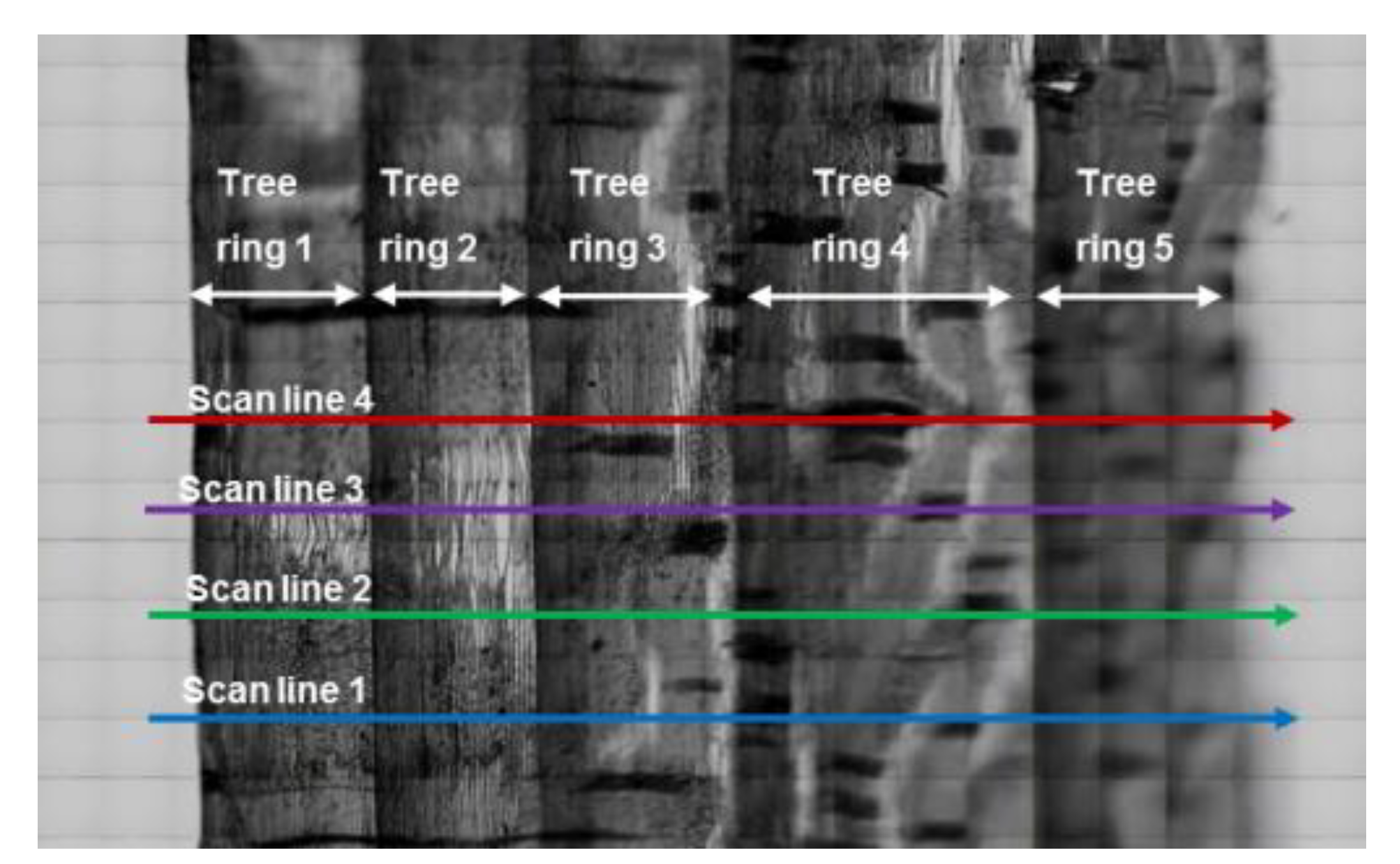
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water; Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. Working Group I: Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Van Mantgem, P.J.; Stephenson, N.L. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Lett. 2007, 10, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak decline in a southern Finnish forest as affected by a drought sequence. Geochronometria 2013, 41, 92–103. [Google Scholar] [CrossRef]
- Rosner, S.; Světlík, J.; Andreassen, K.; Børja, I.; Dalsgaard, L.; Evans, R.; Luss, S.; Tveito, O.E.; Solberg, S. Novel hydraulic vulnerability proxies for a boreal conifer species reveal that opportunists may have lower survival prospects under extreme climatic events. Front. Plant Sci. 2016, 7, 831. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Fonti, P.; Larsen, J.B.; Ræbild, A.; Callesen, I.; Pedersen, N.B.; Hansen, J.K. Projecting tree-growth responses into future climate: A study case from a Danish-wide common garden. Agric. For. Meteorol. 2017, 247, 240–251. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A.; Plourde, P.-Y. Predicting xylem phenology in black spruce under climate warming. Glob. Chang. Biol. 2011, 17, 614–625. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A. Causes and correlations in cambium phenology: Towards an integrated framework of xylogenesis. J. Exp. Bot. 2012, 63, 2117–2126. [Google Scholar] [CrossRef]
- Park, Y.I.; Spiecker, H. Variations in the tree-ring structure of Norway spruce (Picea abies) under contrasting climates. Dendrochronologia 2005, 23, 93–104. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Gyenge, J.E.; de Urquiza, M.M.; Varela, S. Adaptability to climate change in forestry species: Drought effects on growth and wood anatomy of ponderosa pines growing at different competition levels. For. Syst. 2012, 21, 162–173. [Google Scholar] [CrossRef]
- Bryukhanova, M.; Fonti, P. Xylem plasticity allows rapid hydraulic adjustment to annual climatic variability. Trees Struct. Funct. 2013, 27, 485–496. [Google Scholar] [CrossRef]
- Fonti, P.; Heller, O.; Cherubini, P.; Rigling, A.; Arend, M. Wood anatomical responses of oak saplings exposed to air warming and soil drought. Plant Biol. 2013, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.M.; Almería, I.; Eugenio, M.; von Arx, G. Under pressure: How a Mediterranean high-mountain forb coordinates growth and hydraulic xylem anatomy in response to temperature and water constraints. Funct. Ecol. 2013, 27, 1295–1303. [Google Scholar] [CrossRef]
- Xu, J.; Lu, J.; Bao, F.; Evans, R.; Downes, G.M. Climate response of cell characteristics in tree rings of Picea crassifolia. Holzforschung 2013, 67, 217–225. [Google Scholar] [CrossRef]
- Fonti, P.; Babushkina, E.A. Tracheid anatomical responses to climate in a forest-steppe in Southern Siberia. Dendrochronologia 2016, 39, 32–41. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K. Xylogenesis: Coniferous trees of temperate forests are listening to the climate tale during the growing season but only remember the last words! Plant Physiol. 2016, 171, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Rossi, S.; Thibeault-Martel, M.; Plourde, P-Y. Relationships of climate and cell features in stems and roots of black spruce and balsam fir. Ann. For. Sci. 2010, 67, 402. [Google Scholar] [CrossRef]
- Schmulsky, R.; Jones, P.D. Forest Products and Wood Science an Introduction, 6th ed.; Wiley-Blackwell: Chichester, UK, 2011. [Google Scholar]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Bioref. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Bertaud, F.; Holmbom, B. Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci. Technol. 2004, 38, 245–256. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Kessel Verlag: Remangen, Germany, 2003. [Google Scholar]
- Fackler, K.; Thygesen, L.G. Microspectroscopy as applied to the study of wood molecular structure. Wood Sci. Technol. 2013, 47, 203–222. [Google Scholar] [CrossRef]
- Tyutkova, E.A.; Loskutov, S.R.; Shestakov, N.P. FTIR spectroscopy of early and latewood of Larix gmelinii growing along the polar treeline: The correlation between absorption bands and climatic factors. Wood Mater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Gindl, W. Cell-wall lignin content related to tracheid dimensions in drought sensitive Austrian pine (Pinus nigra). IAWA J. 2001, 22, 113–120. [Google Scholar] [CrossRef]
- Fredriksson, M.; Pedersen, N.B.; Thygesen, L.G. The cell wall composition of Norway spruce earlywood and latewood revisited. Int. Wood Prod. J. 2018, 9, 80–85. [Google Scholar] [CrossRef]
- Huang, W.; Hansen, J.K.; Ræbild, A.; Thygesen, L.G.; Bilde, B.; Larsen, J.B. Hvordan påvirkes skovtræarternes vækst af klimaet? [How is the forest tree growth affected by climate?]. Skoven 2018, 10, 385–387. (In Danish) [Google Scholar]
- Holmsgaard, E.; Bang, C. Et træartsforsøg med nåletræer, bøg og eg; de første 10 år [A species trial with conifers, beech and oak; the first 10 years]. Forstl Forsøgsvæs Dan 1977, 35, 159–196. (In Danish) [Google Scholar]
- Callesen, I. Transfer Functions for Carbon Sequestration, Nitrogen Retention and Nutrient Release Capability in Forest Soils Based on Soil Texture Classifications. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2003. [Google Scholar]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Willmott, C.J.; Rowe, C.M.; Mintz, Y. Climatology of the terrestrial seasonal water cycle. J. Climatol. 1985, 5, 589–606. [Google Scholar] [CrossRef]
- Evans, R.; Ilic, J. Rapid prediction of wood stiffness from microfibril angle and density. For. Prod. J. 2001, 51, 53–57. [Google Scholar]
- Lundqvist, S.; Hansson, Å; Olsson, L. SilviScan Measurements on Maritime Pine: French Samples Cut Perpendicular to the Fibres. STFI-Packforsk Report No. 326. Available online: http://www.innventia.com/Documents/Rapporter/STFI-Packforsk%20report%20326.pdf (accessed on 14 February 2019).
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef] [Green Version]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. Localisation and characterisation of incipient brown-rot decay within spruce wood cell walls using FT-IR imaging microscopy. Enzyme Microb. Technol. 2010, 47, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.; Jameel, H. Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Marchessault, R.H. Application of infra-red spectroscopy to cellulose and wood polysaccharides. In Proceedings of the Pure and Applied Chemistry Wood chemistry Symposium, Montreal, QC, Canada, 9–11 August 1961; Volume 5, pp. 107–130. [Google Scholar] [CrossRef]
- SAS Institute Ins. SAS/STAT® 14.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Dawson, J.F. Moderation in management research: What, why, when and how. J. Bus. Psychol. 2014, 29, 1–9. [Google Scholar] [CrossRef]
- Gindl, W.; Grabner, M.; Wimmer, R. The influence of temperature on latewood lignin content in treeline Norway spruce compared with maximum density and ring width. Trees Struct. Funct. 2000, 14, 409–414. [Google Scholar] [CrossRef]
- Gindl, W.; Grabner, M. Characteristics of spruce [Picea abies (L.) Karst] latewood formed under abnormally low temperatures. Holzforschung 2000, 54, 9–11. [Google Scholar] [CrossRef]
- Kilpeläinen, A.; Peltola, H.; Ryyppö, A.; Sauvala, K.; Laitinen, K.; Kellomäki, S. Wood properties of Scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiol. 2003, 23, 889–897. [Google Scholar] [CrossRef]
- Kilpeläinen, A.; Peltola, H.; Ryyppö, A.; Kellomäki, S. Scots pine responses to elevated temperature and carbon dioxide concentration: Growth and wood properties. Tree Physiol. 2005, 25, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kostiainen, K.; Kaakinen, S.; Saranpää, P.; Sigurdsson, B.D.; Lundqvist, S.O.; Linder, S.; Vapaavuori, E. Stem wood properties of mature Norway spruce after 3 years of continuous exposure to elevated [CO2] and temperature. Glob. Chang. Biol. 2009, 15, 368–379. [Google Scholar] [CrossRef]
- Donaldson, L.A. Abnormal lignin distribution in wood from severely drought stressed pinus radiata trees. IAWA J. 2002, 23, 161–178. [Google Scholar] [CrossRef]
- Nanayakkara, B.; Lagane, F.; Hodgkiss, P.; Dibley, M.; Smaill, S.; Riddell, M.; Harrington, J.; Cown, D. Effects of induced drought and tilting on biomass allocation, wood properties, compression wood formation and chemical composition of young Pinus radiata genotypes (clones). Holzforschung 2014, 68, 455–465. [Google Scholar] [CrossRef]
- Cabane, M.; Afif, D.; Hawkins, S. Lignins and Abiotic Stresses. Adv. Bot. Res. 2012, 61, 219–262. [Google Scholar] [CrossRef]
- Girona, M.M.; Rossi, S.; Lussier, J.-M.; Walsh, D.; Morin, H. Understanding tree growth responses after partial cuttings: A new approach. PLoS ONE 2017, 12, e0172653. [Google Scholar] [CrossRef]
| Band Position [cm−1] | Wood Polymer | Assignment 1 |
|---|---|---|
| 1508 | Lignin | Aromatic skeletal vibrations |
| 1104 | Holocellulose | Ring asymmetric valence vibration in polysaccharides |
| 1056 | Cellulose | C-O valence vibration mainly from C3-OH in six membered pyranose rings (present in all cellulose monomers, lacking in some hemicellulose monomers) |
| 1732 | Hemicellulose | C=O stretch in unconjugated carbonyl groups of carbohydrate origin (side chain acetylation in mannan, carboxylic acid side chain in xylan, and ester groups in lignin-carbohydrate complexes) |
| Peak Ratio | Tree No. | Number of Years a | Prev. or Curr. Year b | Month(s) | Coefficient | p-Value | |
|---|---|---|---|---|---|---|---|
| Influence of temperature | |||||||
| Cellulose/lignin | 1056/1508 | N1 | 11 | curr. | Jun | 0.70 | 0.016 |
| N2 | 10 | prev. | Jul | −0.64 | 0.047 | ||
| N2 | 10 | curr. | Mar | −0.69 | 0.026 | ||
| N3 | 12 | prev. | Sep | 0.59 | 0.045 | ||
| all trees | 6 | ns | ns | ns | ns | ||
| Holocellulose/lignin | 1104/1508 | N1 | 11 | curr. | Jun | 0.74 | 0.009 |
| N2 | 10 | prev. | Jul | −0.64 | 0.048 | ||
| N3 | 12 | prev. | Sep | 0.64 | 0.024 | ||
| N3 | 12 | curr. | Jul | −0.59 | 0.042 | ||
| all trees | 6 | curr. | Jun | 0.84 | 0.037 | ||
| Holocellulose/hemicellulose | 1104/1732 | N1 | 11 | curr. | Jun | 0.64 | 0.036 |
| N2 | 10 | curr. | Mar | −0.67 | 0.034 | ||
| N3 | 12 | prev. | Sep | 0.68 | 0.015 | ||
| all trees | 6 | ns | ns | ns | ns | ||
| Hemicellulose/lignin | 1732/1508 | N1 | 11 | ns | ns | ns | ns |
| N2 | 10 | curr. | Mar | 0.63 | 0.049 | ||
| N3 | 12 | curr. | Feb | 0.64 | 0.026 | ||
| N3 | 12 | curr. | May | 0.59 | 0.043 | ||
| all trees | 6 | prev. | Jul | 0.86 | 0.027 | ||
| all trees | 6 | prev. | Sep | 0.84 | 0.036 | ||
| all trees | 6 | prev. | Nov | 0.82 | 0.043 | ||
| Influence of DI | |||||||
| Cellulose/lignin | 1056/1508 | N2 | 10 | curr. | Jun–Aug | −0.64 | 0.046 |
| N3 | 12 | prev. | Mar–Jun | 0.61 | 0.037 | ||
| all trees | 6 | ns | ns | ns | ns | ||
| Holocellulose/lignin | 1104/1508 | all trees | 6 | curr. | Mar–Jun | 0.82 | 0.046 |
| Hemicellulose/lignin | 1732/1508 | N3 | 12 | curr. | Apr–Jun | −0.67 | 0.016 |
| N3 | 12 | curr. | May–Jun | −0.69 | 0.013 | ||
| all trees | 6 | prev. | Mar–Jul | −0.96 | 0.003 | ||
| all trees | 6 | prev. | Apr–Jul | −0.95 | 0.004 | ||
| all trees | 6 | prev. | Jun–Jul | −0.88 | 0.021 | ||
| all trees | 6 | prev. | May–Jul | −0.86 | 0.028 |
| Peak Ratio | Tree No. | Number of Years a | EW | Density | LUMt | LUMr | CWT |
|---|---|---|---|---|---|---|---|
| 1056/1508 (Cellulose/lignin) | N1 | 11 | ns | ns | ns | ns | ns |
| N2 | 10 | ns | ns | ns | ns | ns | |
| N3 | 12 | ns | ns | ns | ns | ns | |
| all trees | 6 | ns | ns | ns | −0.82 * | ns | |
| 1104/1508 (holocellulose/lignin) | N1 | 11 | ns | ns | ns | ns | ns |
| N2 | 10 | 0.65 * | ns | ns | ns | ns | |
| N3 | 12 | ns | ns | ns | ns | ns | |
| all trees | 6 | 0.85 * | ns | ns | −0.85 * | ns | |
| 1104/1732 (Hollocellulose/hemicellulose) | N1 | 11 | ns | ns | ns | ns | ns |
| N2 | 10 | ns | ns | ns | ns | ns | |
| N3 | 12 | ns | ns | ns | ns | ns | |
| all trees | 6 | 0.97 ** | ns | ns | −0.83 * | ns | |
| 1732/1508 (Hemicellulose/lignin) | N1 | 11 | ns | ns | ns | 0.64 * | ns |
| N2 | 10 | ns | ns | ns | ns | ns | |
| N3 | 12 | ns | ns | ns | ns | 0.58 * | |
| all trees | 6 | ns | ns | ns | ns | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Pedersen, N.B.; Fredriksson, M.; Thygesen, L.G. Annual Variations in Norway Spruce Xylem Studied Using Infrared Micro-spectroscopy. Forests 2019, 10, 164. https://doi.org/10.3390/f10020164
Huang W, Pedersen NB, Fredriksson M, Thygesen LG. Annual Variations in Norway Spruce Xylem Studied Using Infrared Micro-spectroscopy. Forests. 2019; 10(2):164. https://doi.org/10.3390/f10020164
Chicago/Turabian StyleHuang, Weiwei, Nanna Bjerregaard Pedersen, Maria Fredriksson, and Lisbeth Garbrecht Thygesen. 2019. "Annual Variations in Norway Spruce Xylem Studied Using Infrared Micro-spectroscopy" Forests 10, no. 2: 164. https://doi.org/10.3390/f10020164





