Expression Patterns of MYB (V-myb Myeloblastosis Viral Oncogene Homolog) Gene Family in Resistant and Susceptible Tung Trees Responding to Fusarium Wilt Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Pathogen Materials
2.2. Pathogen Inoculation
2.3. RNA Extraction and Expression Analysis
2.4. Selection and Identification of MYB Sequences in V. fordii and V. montana
2.5. Multiple Sequence Alignment and Phylogenetic Analysis
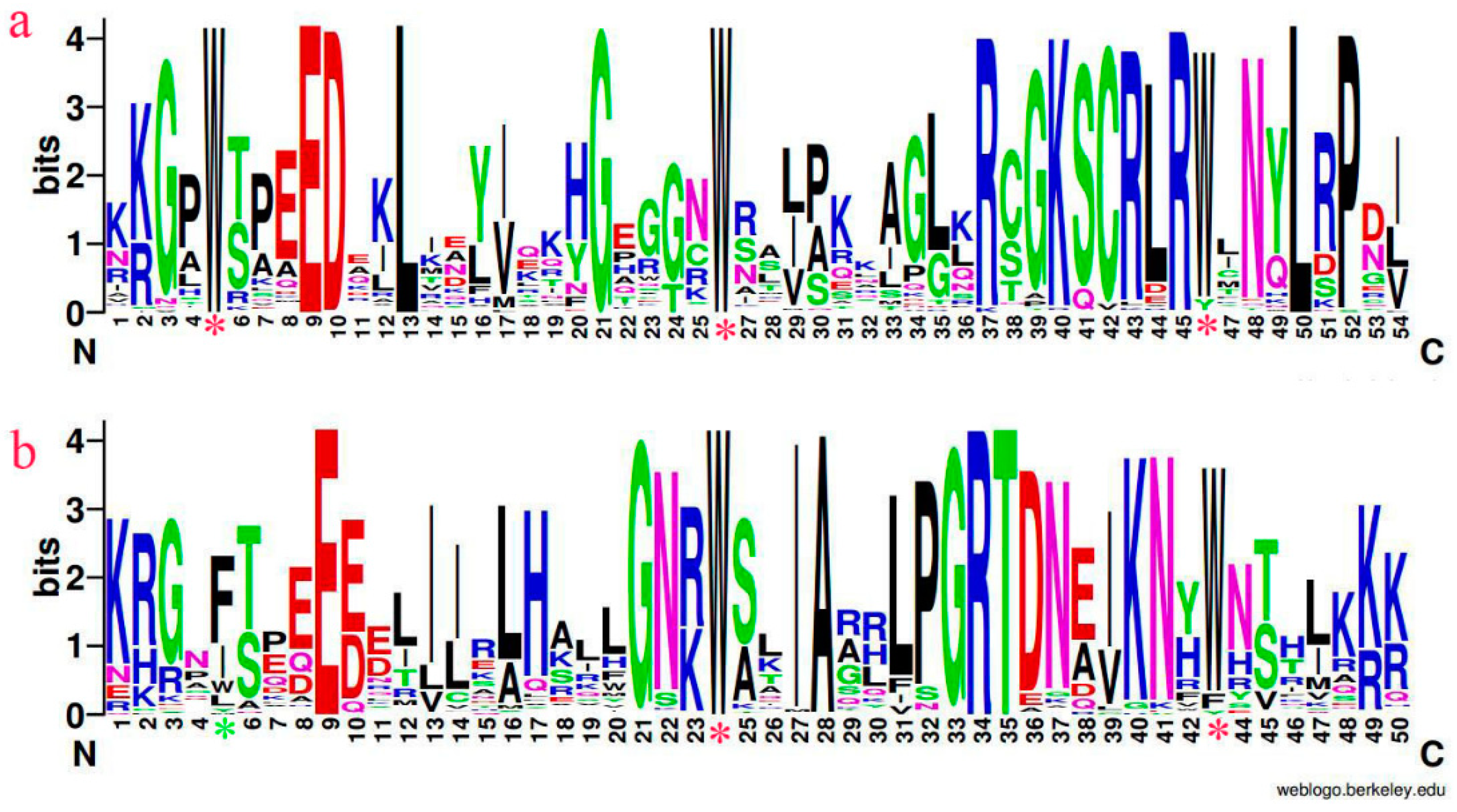
2.6. Conserved Motifs Analyses of the MYB Family
2.7. Evolution and Selection Analysis of the MYB Family
2.8. Expression Patterns of MYB Family during Four Infection Processes in V. fordii and V. montana
2.9. Network Analysis of VmMYB Family Genes Responding to Fof-1
2.10. Quantitative Real-Time PCR (RT-qPCR)
3. Results
3.1. Multiple Sequence Alignment and Phylogenetic Analysis in AtMYBs, VfMYBs, VmMYBs
3.2. Distribution of Conserved Motifs Outside of the MYBs Domain
3.3. The Analysis of Evolution and Selection in MYB Family by Ka/Ks Ratios Calculation
3.4. Expression Patterns of MYB Family in V. Fordii and V. montana during Infection
3.5. Network Analysis Based on Four Hub Genes Affording Resistance to Fof-1 in V. montana
3.6. Tissue-Specific Expression Pattern of Differentially Expressed MYB Genes in Response to Fof-1
4. Discussion
4.1. The R2R3 MYB Subfamily Plays a Crucial Role in Vernicia and the Systematic Evolution Gradually Tend to Diversity
4.2. Four Pairs of Vf/VmMYBs Had Negatively Correlated Expression between Two Species in Response to Fof-1
4.3. Functional Speculation of Four Hub Genes in Resistant V. montana
4.4. Tissue-Specific Expression Pattern of Different MYB Homologous Genes in Response to Fof-1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.H.; Chen, J.H.; Chang, C.Y.; Chang, C.C. Biodiesel production from tung (Vernicia montana) oil and its blending properties in different fatty acid compositions. Bioresour. Technol. 2010, 101, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, M.; Wu, L.; Wang, Y.; Chen, Y. Divergent expression patterns in two Vernicia species revealed the potential role of the hub gene VmAP2/ERF036 in resistance to Fusarium oxysporum in Vernicia montana. Genes 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yin, H.F.; Gao, M.; Zhu, H.P.; Zhang, Q.Y.; Wang, Y.D. Comparative transcriptomics atlases reveals different gene expression pattern related to Fusarium wilt disease resistance and susceptibility in two Vernicia Species. Front. Plant Sci. 2016, 7, 1974. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.C.; Dombrecht, B.; Manners, J.M.; Schenk, P.M.; Edgar, C.I.; Maclean, D.J.; Scheible, W.R.; Udvardi, M.K.; Kazan, K. Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol. 2005, 139, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Piqueras, R.; Sanchez-Serrano, J.J.; Solano, R. Ethylene response factor1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [PubMed]
- Mengiste, T.; Chen, X.; Salmeron, J.; Dietrich, R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 2003, 15, 2551–2565. [Google Scholar] [CrossRef]
- Vailleau, F.; Daniel, X.; Tronchet, M.; Montillet, J.-L.; Triantaphylides, C.; Roby, D. A R2R3-MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proc. Natl. Acad. Sci. USA 2002, 99, 10179–10184. [Google Scholar] [CrossRef]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Delessert, C.; Kazan, K.; Wilson, I.W.; Straeten, D.V.; Manners, J.; Dennis, E.S.; Dolferus, R. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. Plant J. 2005, 43, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Chico, J.M.; Sanchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef]
- Desveaux, D.; Subramaniam, R.; Despres, C.; Mess, J.N.; Levesque, C.; Fobert, P.R.; Dangl, J.L.; Brisson, N. A “whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev. Cell 2004, 6, 229–240. [Google Scholar] [CrossRef]
- He, Q.L.; Jones, D.C.; Li, W.; Xie, F.L.; Ma, J.; Sun, R.; Wang, Q.L.; Zhu, S.J.; Zhang, B.H. Genome-wide identification of R2R3-MYB genes and expression analyses during abiotic stress in Gossypium raimondii. Sci. Rep. 2016, 6, 22980. [Google Scholar] [CrossRef]
- Ogata, K.; Kanei-Ishii, C.; Sasaki, M.; Hatanaka, H.; Nagadoi, A.; Enari, M.; Nakamura, H.; Nishimura, Y.; Ishii, S.; Sarai, A. The cavity in the hydrophobic core of Myb DNA binding domain is reserved for DNA recognition and transactivation. Nat. Struct. Biol. 1996, 3, 178–818. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. Myb transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants. 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Li, X.L.; Xue, C.; Li, J.M.; Qiao, X.; Li, L.T.; Yu, L.; Huang, Y.H.; Wu, J. Genome-wide identification, evolution and functional divergence of MYB transcription factors in Chinese white pear (Pyrus bretschneideri). Plant Cell Physiol. 2016, 57, 824–847. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Jin, H.; Martin, C. Multifunctionality and diversity within the plant MYB-gene family. Plant Mol. Biol. 1999, 41, 577–585. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a grapevine R3R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Zhong, R.; Richardson, E.A.; Ye, Z.-H. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 2007, 19, 2776–2792. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, H.X.; Yao, H.P.; Li, C.L.; Chen, H.; Wang, A.H.; Park, S.U.; Wu, Q. Identification, isolation and expression analysis of eight stress-related R2R3-MYB genes in tartary buckwheat (Fagopyrum tataricum). Plant Cell Rep. 2016, 35, 1385–1396. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Ran, L.; Tian, Q.; Fan, D.; Luo, K. PtoMYB92 is a transcriptional activator of the lignin biosynthetic pathway during secondary cell wall formation in Populus tomentosa. Plant Cell Physiol. 2015, 56, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Yang, X.Y.; He, K.; Liu, M.H.; Li, J.G.; Gao, Z.F.; Lin, Z.Q.; Zhang, Y.F.; Wang, X.X.; Qiu, X.M.; et al. The MYB transcription factor superfamily of Arabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar]
- Hall, T. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Ali, H.; Liu, Y.; Azam, S.M.; Rahman, Z.U.; Priyadarshani, S.V.G.N.; Li, W.; Huang, X.; Hu, B.; Xiong, J.; Ali, U.; et al. Genomic survey, characterization and expression profile analysis of the SBP genes in pineapple (Ananas comosus L.). Int. J. Genom. 2017, 1032846. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Howe, E.; Holton, K.; Nair, S.; Schlauch, D.; Sinha, R.; Quackenbush, J. MeV: Multi Experiment Viewer. In Biomedical Informatics for Cancer Research; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ralf, S.; Martin, W.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar]
- Swanson, W.J.; Wong, A.; Wolfner, M.F.; Aquadro, C.F. Evolutionary expressed sequence tag analysis of Drosophila female reproductive tracts identifies genes subjected to positive selection. Genetics 2004, 168, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Le, G.J.; Baudry, A.; Huep, G.; Lanet, E.; Debeaujon, I.; Routaboul, J.M.; Alboresi, A.; Weisshaar, B.; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2010, 55, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2014, 19, 6150–6161. [Google Scholar] [CrossRef]
- Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2010, 40, 979–995. [Google Scholar] [CrossRef]
- Jiang, C.; Gu, X.; Peterson, T. Identification of conserved gene structures and carboxy-terminal motifs in the Myb gene family of Arabidopsis and Oryza sativa L. ssp. indica. Genome Biol. 2004, 5, R46. [Google Scholar] [CrossRef]
- Peng, X.J.; Liu, H.; Wang, D.; Shen, S.H. Genome-wide identification of the Jatropha curcas MYB family and functional analysis of the abiotic stress responsive gene JcMYB2. BMC Genomics 2016, 17, 251. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Madhuvanthi, R.; Karthikeyan, A.S.; Raghothama, K.G. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant 2009, 2, 43–58. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; González, E.; Bustos, R.; Linhares, F.; Leyva, A.; Paz-Ares, J. The transcriptional control of plant responses to phosphate limitation. J. Exp. Bot. 2004, 55, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Bates, T.R.; Lynch, J.P. Stimulation of root hair elongation in Arabidopsis thaliana, by low phosphorus availability. Plant Cell Environ. 1996, 19, 529–538. [Google Scholar] [CrossRef]
- Jiao, J.I.A.; Xing, J.H.; Dong, J.G.; Han, J.M.; Liu, J.S. Functional analysis of MYB73 of Arabidopsis thaliana against Bipolaris oryzae. Agric. Sci. China 2011, 10, 721–727. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Wojnar, W.; Rosikiewicz, M.; Szarejko, I.; Maluszynski, M.; Szweykowska-Kulinska, Z.; Jarmolowski, A. Arabidopsis suppressor mutant of abh1 shows a new face of the already known players: ABH1 (CBP80) and ABI4 in response to ABA and abiotic stresses during seed germination. Plant Mol. Biol. 2013, 81, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Abuqamar, S.; Chen, X.; Dhawan, R.; Bluhm, B.; Salmeron, J.; Lam, S.; Dietrich, R.A.; Mengiste, T. Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to botrytis infection. Plant J. 2010, 48, 28. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Koshiba, T.; Hattori, T.; Yamaguchi, M.; Suzuki, S.; Umezawa, T. The expression of a rice secondary wall-specific cellulose synthase gene, OsCesA7, is directly regulated by a rice transcription factor, OsMYB58/63. Planta 2015, 242, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.H. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Q.; Han, L.B.; Yang, C.L.; Wu, X.M.; Zhong, N.Q.; Wu, J.H.; Wang, F.X.; Wang, H.Y.; Xia, G.X. The cotton MYB108 forms a positive feedback regulation loop with CML11 and participates in the defense response against Verticillium dahliae infection. J. Exp. Bot. 2016, 67, 1935–1950. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Bernhardt, A.; Lee, J.H.; Hellmann, H. Identification of Arabidopsis MYB56 as a novel substrate for CRL3BPM E3 ligases. Mol. Plant 2015, 8, 242–250. [Google Scholar] [CrossRef] [PubMed]





| MYB Protein Classes | V. fordii | V. montana | A. thaliana | J. curcas |
|---|---|---|---|---|
| R2R3 | 64 | 69 | 126 | 123 |
| 1R | 6 | 4 | 64 | n.d. |
| 3R | 4 | 3 | 5 | 4 |
| 4R | 1 | 1 | 1 | 1 |
| Total | 75 | 77 | 196 | 128 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Q.; Gao, M.; Wu, L.; Wang, Y.; Chen, Y. Expression Patterns of MYB (V-myb Myeloblastosis Viral Oncogene Homolog) Gene Family in Resistant and Susceptible Tung Trees Responding to Fusarium Wilt Disease. Forests 2019, 10, 193. https://doi.org/10.3390/f10020193
Wang X, Zhang Q, Gao M, Wu L, Wang Y, Chen Y. Expression Patterns of MYB (V-myb Myeloblastosis Viral Oncogene Homolog) Gene Family in Resistant and Susceptible Tung Trees Responding to Fusarium Wilt Disease. Forests. 2019; 10(2):193. https://doi.org/10.3390/f10020193
Chicago/Turabian StyleWang, Xue, Qiyan Zhang, Ming Gao, Liwen Wu, Yangdong Wang, and Yicun Chen. 2019. "Expression Patterns of MYB (V-myb Myeloblastosis Viral Oncogene Homolog) Gene Family in Resistant and Susceptible Tung Trees Responding to Fusarium Wilt Disease" Forests 10, no. 2: 193. https://doi.org/10.3390/f10020193
APA StyleWang, X., Zhang, Q., Gao, M., Wu, L., Wang, Y., & Chen, Y. (2019). Expression Patterns of MYB (V-myb Myeloblastosis Viral Oncogene Homolog) Gene Family in Resistant and Susceptible Tung Trees Responding to Fusarium Wilt Disease. Forests, 10(2), 193. https://doi.org/10.3390/f10020193




