Retention Forestry Supports Bird Diversity in Managed, Temperate Hardwood Floodplain Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Forest Management Practice, and Study Plots
2.2. Bird Census in Study Plots
2.3. Data Analysis
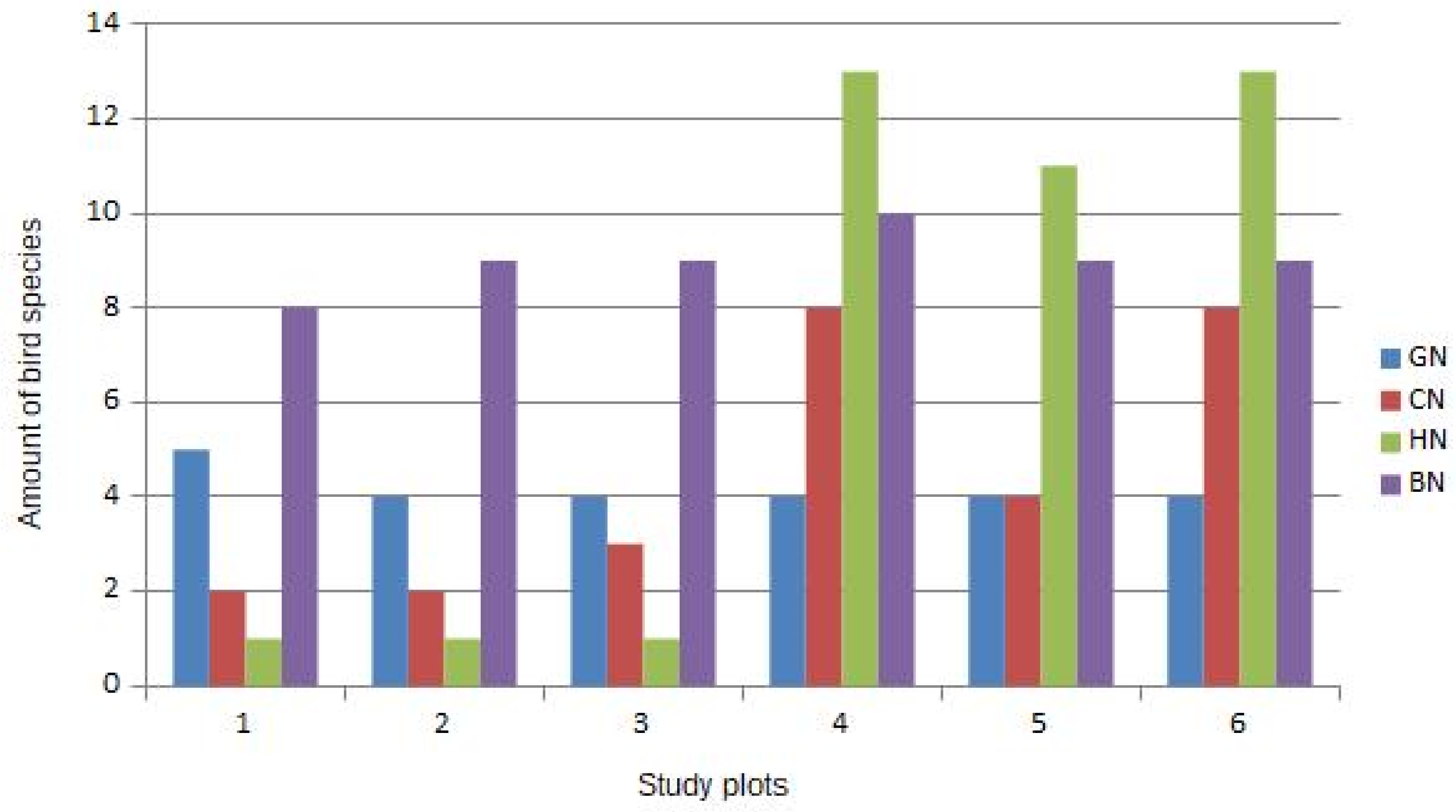
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keenan, R.J.; Kimmins, J.P. The ecological effects of clear-cutting. Environ. Rev. 1993, 1, 121–144. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F. Conserving Forest Biodiversity: A Comprehensive Multiscaled Approach; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Beese, W.J.; Dunsworth, B.G.; Zielke, K.; Bancroft, B. Maintaining attributes of old-growth forests in coastal B.C. through variable retention. For. Chron. 2003, 79, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Aubry, K.B.; Halpern, C.B.; Maguire, D.A. Ecological effects of variable-retention harvests in the northwestern United States: The DEMO study. For. Snow Landsc. Res. 2004, 78, 119–137. [Google Scholar]
- Vanha-Majamaa, I.; Jalonen, J. Green tree retention in Fennoscandian forestry. Scand. J. For. Res. 2001, 3, 79–90. [Google Scholar] [CrossRef]
- Luoma, D.L.; Eberhart, J.L.; Molina, R.; Amaranthus, M.P. Response of ectomycorrhizal fungus sporocarp production to varying levels and patterns of green-tree retention. For. Ecol. Manag. 2004, 202, 337–354. [Google Scholar] [CrossRef]
- Nelson, C.R.; Halpern, C.B. Edge-related responses of understory plants to aggregated retention harvest in the Pacific Northwest. Ecol. Appl. 2005, 15, 196–209. [Google Scholar] [CrossRef]
- Halaj, J.; Halpern, C.B.; Yi, H. Response of litter-dwelling spiders and carabid beetles to varying levels and patterns of green-tree retention. For. Ecol. Manag. 2008, 255, 887–900. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S. Green-tree retention and recovery of an old-forest specialist, the southern red-backed vole (Myodes gapperi), 20 years after harvest. Wildl. Res. 2018, 44, 669–680. [Google Scholar] [CrossRef]
- Franklin, J.F.; Berg, D.R.; Thornburgh, D.A.; Tappeiner, J.C. Alternative silvicultural approaches to timber harvesting: Variable retention harvest systems. In Creating Forestry for the 21st Century: The Science of Ecosystem Management; Kohm, K.A., Franklin, J.F., Eds.; Island Press: Washington, DC, USA, 1997; pp. 111–139. [Google Scholar]
- Rosenvald, R.; Lõhmus, A. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For. Ecol. Manag. 2008, 255, 1–15. [Google Scholar] [CrossRef]
- Otto, C.R.V.; Roloff, G.J. Songbird response to green-tree retention prescriptions in clearcut forests. Forest For. Ecol. Manag. 2012, 284, 241–250. [Google Scholar] [CrossRef]
- Machar, I. Attempt to summarize the problems: Is a sustainable management of floodplain forest geobiocenoses possible? In Biodiversity and Target Management of Floodplain Forests in the Morava River Basin (Czech Republic); Machar, I., Ed.; Palacky University: Olomouc, Czech Republic, 2010; pp. 189–226. [Google Scholar]
- Fuller, J.R. Avian responses to transitional habitats in temperate cultural landscapes. Woodland edges and young-growth. In Birds and Habitat. Relationships in Changing Landscapes; Fuller, R.J., Ed.; Cambridge Univ. Press: Cambridge, UK, 2012; pp. 125–149. [Google Scholar]
- Lance, A.; Phinney, M. Bird responses to partial retention timber harvesting in central interior British Columbia. For. Ecol. Manag. 2001, 142, 267–280. [Google Scholar] [CrossRef]
- Schieck, J.; Hobson, K.A. Bird communities associated with live residual tree patches within cut blocks and burned habitat in mixedwood boreal forests. Can. J. For. Res. 2000, 30, 1281–1295. [Google Scholar] [CrossRef]
- Poulin, J.F.; Villard, M.A.; Edman, M.; Goulet, P.J.; Eriksson, A.M. Thresholds in nesting habitat requirements of an old forest specialist, the Brown Creeper (Certhia americana), as conservation target. Biol. Conserv. 2008, 141, 1129–1137. [Google Scholar] [CrossRef]
- Franklin, J.F. Biological legacies: A critical management concept from Mount St. Helens. Trans. N. A. Wildlands Nat. Resour. Conf. 1990, 55, 216–219. [Google Scholar]
- Mazurek, M.J.; Zielinski, W.J. Individual legacy trees influence vertebrate wildlife diversity in commercial forests. For. Ecol. Manag. 2004, 193, 321–334. [Google Scholar] [CrossRef] [Green Version]
- Kilianova, H.; Pechanec, V.; Brus, J.; Kirchner, K.; Machar, I. Analysis of the development of land use in the Morava River floodplain, with special emphasis on the landscape matrix. Morav. Geogr. Rec. 2017, 25, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Machar, I. Conservation and Management of Floodplain Forests in the Protected Landscape Area Litovelske Pomoravi (Czech Republic) Introduction. In Conservation and Management of Floodplain Forests in the Protected Landscape Area Litovelske Pomoravi (Czech Republic); Machar, I., Ed.; Palacky University: Olomouc, Czech Republic, 2009; pp. 7–108. [Google Scholar]
- Miko, L. Nature and landscape protection in the European context. In Ochrana prirody a krajiny v Ceske republice, vols I and II; Machar, I., Drobilova, L., Eds.; Palacky University: Olomouc, Czech Republic, 2012; pp. 43–49. [Google Scholar]
- Machar, I.; Simon, J.; Rejsek, K.; Pechanec, V.; Brus, J.; Kilianova, H. Assessment of Forest Management in Protected Areas Based on Multidisciplinary Research. Forests 2016, 7, 285. [Google Scholar] [CrossRef]
- Salekl, L.; Sivacioglu, A.; Topacoglu, O.; Zahradnile, D.; Jerabkoval, L.; Machar, I. Crowns of old remnant oak standards. Fresenius Environ. Bull. 2017, 26, 4023–4032. [Google Scholar]
- Simon, J.; Machar, I.; Bucek, A. Linking the historical research with the growth simulation model of hardwood floodplain forests. Polish J. Ecol. 2014, 62, 273–288. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burges, N.D.; Hill, D.A.; Mustoe, S. Bird Census Techniques; Academic Press: London, UK, 2007; pp. 42–64. [Google Scholar]
- Alldredge, M.W.; Simons, T.R.; Pollock, K.H. A field evaluation of distance measurement error in auditory avian point count surveys. J. Wildl. Manag. 2007, 71, 2759–2766. [Google Scholar] [CrossRef]
- Kosinski, Z.; Kempa, M.; Hybsz, R. Accuracy and efficiency of different techniques for censusing territorial Middle Spotted Woodpeckers Dendrocopos medius. Acta Ornithol. 2004, 39, 29–34. [Google Scholar] [CrossRef]
- Wiens, J.A. The Ecology of Bird Communities, vol. 1, Foundation and Patterns; Cambridge University Press: Cambridge, UK, 1989; pp. 159–162. [Google Scholar]
- Stastny, K.; Hudec, K. Fauna of the Czech Republic. Birds 2 and 3, 2nd ed.; Academia: Prague, Czech Republic, 2011; pp. 1–1178. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 33–44. [Google Scholar]
- Jaccard, P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull. Soc. Vaudoise Sci. Nat. 1901, 37, 547–579. [Google Scholar]
- Rosner, B. Fundamentals of Biostatistics; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2010; pp. 1–360. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- Machar, I.; Kulhavy, A.; Sejak, J.; Pechanec, V. Conservation effectiveness and monetary value of floodplain forests habitats in the Czech Republic. Rep. For. Res.-Zpr. Lesn. Vyzk. 2018, 63, 206–213. [Google Scholar]
- James, F.C.; Warner, N.O. Relationships between temperate forest bird communities and vegetation structure. Ecology 1982, 63, 159–171. [Google Scholar] [CrossRef]
- Perry, R.W.; Jenkins, J.M.A.; Thill, R.E.; Thompson, F.R. Long-term effects of different forest regeneration methods on mature forest birds. For. Ecol. Manag. 2018, 408, 183–194. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F. Managing Stand Structure as Part of Ecologically Sustainable Forest Management in Australian Mountain Ash Forests. Conserv. Biol. 1997, 11, 1053–1068. [Google Scholar] [CrossRef]
- Skorupski, J.; Jankowiak, L.; Kiriaka, B.; Rek, T.; Wysocki, D. Beech forest structure and territory size of four songbird species in Puszcza Bukowa, NW Poland: Implications for bird-friendly silvicultural practices in a temperate forest. Ethol. Ecol. Evolut. 2017. [Google Scholar] [CrossRef]
- Uradnicek, L.; Sramek, M.; Dreslerova, J. Checklist of champion trees in the Czech Republic. J. Landsc. Ecol. 2017, 10, 109–120. [Google Scholar] [CrossRef]
- Kilianova, H.; Pechanec, V.; Svobodova, J.; Machar, I. Analysis of the evolution of the floodplain forests in the aluvium of the Morava river. In 12th International Multidisciplinary Scientific Geoconference, SGEM 2012, Albena, Bulgaria, 17–23 June 2012; SGEM: Albena, Bulgaria, 2012; Volume IV, pp. 1–8. [Google Scholar]
- Knutson, M.G.; McColl, L.E.; Suarez, S.A. Breeding bird assemblages associated with stages of forest succession in large river floodplains. Nat. Areas J. 2005, 25, 55–70. [Google Scholar]
- Miklin, J.; Hauck, D.; Konvicka, O.; Cizek, L. Veteran trees and saproxylic insects in the floodplains of Lower Morava and Dyje rivers, Czech Republic. J. Maps 2017, 13, 291–299. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. The importance of managing and conserving large old trees: A case study from Victorian mountain ash forests. Royal Soc. Vic. 2016, 128, 64–70. [Google Scholar] [CrossRef]
- Remm, J.; Lõhmus, A. Tree cavities in forests—the broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Leso, P. Breeding bird communities of two succession stages of young oak forests. Sylvia 2003, 39, 67–78. [Google Scholar]
- Hunter, J.E.; Mazurek, M.J. Characteristics of trees used by nesting and roosting Vaux´s swifts nest in northwestern California. West. Birds 2004, 34, 225–229. [Google Scholar]
- Brawn, J.D.; Balda, R.P. Population biology of cavity-nesters in northern Arizona: Do nest sites limit breeding densities? Condor 1988, 90, 61–71. [Google Scholar] [CrossRef]
- Irwin, L.L.; Rock, D.F.; Miller, G.P. Stand structures used by northern spotted owls in managed forests. J. Raptor Res. 2000, 34, 175–186. [Google Scholar]
- Rodewald, A.D.; Yahner, R.H. Bird communities associated with harvested hardwood stands containing residual trees. J. Wildlife Manag. 2000, 64, 924–932. [Google Scholar] [CrossRef]
- Söderström, B. Effects of different levels of green- and dead-tree retention on hemi-boreal forest bird communities in Sweden. For. Ecol. Manag. 2009, 257, 215–222. [Google Scholar] [CrossRef]
- Müller, J.; Stadler, J.; Brandl, R. Composition versus physiognomy of vegetation as predictors of bird assemblages: The role of lidar. Remote Sens. Environ. 2010, 114, 490–495. [Google Scholar] [CrossRef]
- Radford, J.Q.; Bennett, A.F.; Cheers, G.J. Landscape-level tresholds of habitat cover for woodland-dependent birds. Biol. Conserv. 2005, 124, 317–337. [Google Scholar] [CrossRef]
- Pierson, J.C.; Mortelliti, A.; Barton, P.S.; Lane, P.W.; Lindenmayer, D.B. Evaluating the effectiveness of overstory cover as a surrogate for bird community diversity and population trends. Ecol. Indic. 2016, 61, 790–798. [Google Scholar] [CrossRef]
- Montague-Drake, R.M.; Lindenmayer, D.B.; Cunningham, R.B. Factors affecting site occupancy by woodland bird species of conservation concern. Biol. Conserv. 2009, 142, 2896–2903. [Google Scholar] [CrossRef]
- Machar, I.; Poprach, K. Tanks and cisterns for fodder molasses on farms as ecological traps. Listy Cukrov. Repar. 2012, 128, 347–349. [Google Scholar]
- Tittler, R.; Hannon, S.J.; Norton, M.R. Residual tree retention ameliorates short-term effects of clear-cutting on some boreal songbirds. Ecol. Appl. 2001, 11, 1656–1666. [Google Scholar] [CrossRef]
- Venier, L.A.; Dalley, K.; Goulet, P.; Mills, S.; Pitt, D.; Cowcill, K. Benefits of aggregate green tree retention to boreal forest birds. For. Ecol. Manag. 2015, 343, 80–87. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Wood, J.; McBurney, L.; Blair, D.; Banks, S.C. Single large versus several small: The SLOSS debate in the context of bird responses to a variable retention logging experiment. For. Ecol. Manag. 2015, 339, 1–10. [Google Scholar] [CrossRef]
| Study Plots (Study Plot Number; Local Name and Coordinates; Size of the Study Plot Area; Total Amount of Individual Legacy Oak Trees (ILOT) in the Study Plot) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bird species | Nesting guilds 1 | No. 1 Pnovice 49.7100131N, 17.1330297E 2.8 ha ILOT | No. 2 Stepanov 49.6667017N, 17.1945703E 4.4 ha ILOT | No. 3 Horni les 49.7011319N, 17.0944058E 4.8 ha ILOT | No. 4 Hrube les 49.6821428N, 17.1744858E 3.4 ha 21 ILOT | No. 5 Brezova 49.6881956N, 17.1257342E 2.9 ha 19 ILOT | No. 6 Odchovna 49.6858078N, 17.1300256E 3.9 ha 26 ILOT | ||||||
| DE 2 | DO 3 | DE | DO | DE | DO | DE | DO | DE | DO | DE | DO | ||
| Aegithalos caudatus | BN | 1.7 | 3.0 | - | - | 1.9 | 3.2 | 0.4 | 0.5 | - | - | 0.6 | 0.7 |
| Anthus trivialis | GN | 0.8 | 1.4 | 2.2 | 4.1 | 0.5 | 0.9 | 0.4 | 0.5 | 0.2 | 0.2 | 0.3 | 0.4 |
| Carduelis carduelis | CN | 1.2 | 2.2 | - | - | - | - | 0.3 | 0.4 | - | - | 0.3 | 0.4 |
| Carduelis chloris | BN | 0.8 | 1.4 | 1.5 | 2.8 | 2.0 | 3.4 | 0.5 | 0.6 | 0.3 | 0.4 | - | - |
| Coccothraustes coccothraustes | BN | - | - | 0.3 | 0.6 | - | - | 0.3 | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 |
| Certhia familiaris | HN | - | - | - | - | - | - | 0.3 | 0.4 | 0.6 | 0.8 | 0.2 | 0.2 |
| Columba palumbus | CN | - | - | - | - | - | - | 0.9 | 1.1 | 0.6 | 0.8 | 0.3 | 0.4 |
| Cyanistes caeruleus | HN | - | - | 0.5 | 0.9 | - | - | 4.5 | 5.4 | 3.9 | 5.1 | 5.2 | 6.1 |
| Dendrocopos major | HN | - | - | - | - | - | - | 4.2 | 5.1 | 4.2 | 5.4 | 5.0 | 5.8 |
| Dryobates minor | HN | - | - | - | - | - | - | 0.3 | 0.4 | 0.6 | 0.8 | 0.3 | 0.4 |
| Emberiza citrinella | BN | 4.4 | 7.9 | 2.2 | 4.1 | 3.9 | 6.6 | 2.2 | 2.6 | 3.0 | 3.9 | 2.1 | 2.5 |
| Erithacus rubecula | GN | 9.1 | 16.4 | 11.8 | 21.8 | 11.1 | 18.9 | 9.4 | 11.3 | 8.9 | 11.5 | 7.7 | 9.0 |
| Ficedula albicollis | HN | - | - | - | - | - | - | 4.5 | 5.4 | 3.8 | 4.9 | 5.6 | 6.5 |
| Fringilla coelebs | CN | 4.2 | 7.6 | 3.5 | 6.5 | 3.8 | 6.5 | 3.1 | 3.7 | 2.0 | 2.6 | 2.6 | 3.0 |
| Garrulus glandarius | CN | - | - | - | - | 0.5 | 0.9 | - | - | 0.3 | 0.4 | 1.0 | 1.2 |
| Hipolais icterina | BN | - | - | - | - | 0.5 | 0.9 | 0.3 | 0.4 | - | - | - | - |
| Lanius collurio | BN | - | - | 0.5 | 0.9 | - | - | - | - | 0.2 | 0.3 | - | - |
| Luscinia megarhynchos | BN | 0.8 | 1.4 | - | - | - | - | - | - | - | - | 0.3 | 0.4 |
| Leiopicus medius | HN | - | - | - | - | - | - | 3.0 | 3.6 | 3.1 | 4.0 | 3.1 | 3.5 |
| Locustella fluviatilis | GN | 1.6 | 2.9 | - | - | - | - | - | - | 0.3 | 0.4 | - | - |
| Muscicapa striata | HN | - | - | - | - | - | - | 0.6 | 0.7 | - | - | 0.3 | 0.4 |
| Oriolus oriolus | CN | - | - | - | - | - | - | 0.3 | 0.4 | 0.3 | 0.4 | 0.6 | 0.7 |
| Parus major | HN | 0.8 | 1.4 | - | - | 0.6 | 1.0 | 9.1 | 10.9 | 9.0 | 11.6 | 6.4 | 7.3 |
| Phasianus colchicus | GN | - | - | 1.0 | 1.8 | 0.9 | 1.5 | 0.6 | 0.7 | - | - | 0.3 | 0.4 |
| Phylloscopus collybita | GN | 7.2 | 13 | 10.9 | 20.1 | 9.9 | 16.9 | 4.1 | 4.9 | 5.2 | 6.5 | 7.8 | 9.1 |
| Picus viridis | HN | - | - | - | - | - | - | 0.4 | 0.5 | 0.3 | 0.4 | 0.3 | 0.4 |
| Poecile palustris | HN | - | - | - | - | - | - | 3.5 | 4.2 | 3.9 | 5.0 | 0.5 | 0.6 |
| Prunella modularis | BN | 3.5 | 6.3 | 2.5 | 4.6 | 2.4 | 4.2 | 2.1 | 2.5 | 1.9 | 2.5 | 0.7 | 0.8 |
| Sitta europaea | HN | - | - | - | - | - | - | 5.0 | 6.0 | 4.2 | 5.4 | 5.6 | 6.5 |
| Steptopelia turtur | CN | - | - | - | - | - | - | 0.2 | 0.2 | - | - | 1.3 | 1.5 |
| Strix aluco | HN | - | - | - | - | - | - | 0.3 | 0.4 | - | - | 0.3 | 0.4 |
| Sturnus vulgaris | HN | - | - | - | - | - | - | 9.6 | 11.6 | 7.1 | 9.2 | 8.7 | 10.2 |
| Turdus merula | BN | 7.2 | 13.0 | 6.2 | 11.5 | 5.9 | 10.1 | 3.9 | 4.7 | 5.5 | 7.1 | 5.3 | 6.2 |
| Turdus philomelos | CN | - | - | 1.5 | 2.8 | 0.5 | 0.9 | 0.3 | 0.4 | - | - | 0.6 | 0.7 |
| Turdus pilaris | CN | - | - | - | - | - | - | 0.6 | 0.7 | - | - | 0.9 | 1.1 |
| Turdus viscivorus | CN | - | - | - | - | - | - | 0.3 | 0.4 | - | - | - | - |
| Troglodytes troglodytes | BN | 1.7 | 3.0 | 2.1 | 3.9 | 2.3 | 3.9 | 0.3 | 0.4 | 0.6 | 0.8 | 0.9 | 1.1 |
| Sylvia atricapilla | BN | 9.1 | 16.4 | 6.9 | 12.7 | 10.1 | 17.3 | 6.6 | 7.8 | 6.7 | 8.7 | 8.7 | 10.2 |
| Sylvia curruca | BN | 1.5 | 2.7 | 0.5 | 0.9 | 1.7 | 2.9 | 0.7 | 0.8 | 0.3 | 0.4 | 1.2 | 1.4 |
| Total of DE/study plot | 55.6 | 54.1 | 58.5 | 83.1 | 77.3 | 85.4 | |||||||
| Total of species/study plot | 16 | 16 | 17 | 35 | 28 | 34 | |||||||
| Jaccard index (%) | 52 | 59 | 41 | 62 | 67 | 39 | |||||||
| Study Plot Number (and Local Name) | Presence of ILOT | Mean Density | Median | Standard Deviation | Skewness |
|---|---|---|---|---|---|
| 1 (Pnovice) | No | 1.43 | 0 | 2.58 | 1.97 |
| 2 (Stepanov) | No | 1.39 | 0 | 2.85 | 2.56 |
| 3 (Horni les) | No | 1.50 | 0 | 2.92 | 2.30 |
| 4 (Hrube les) | Yes | 2.13 | 0.6 | 2.76 | 1.48 |
| 6 (Odchovna) | Yes | 1.98 | 0.3 | 2.66 | 1.27 |
| 5 (Brezova) | Yes | 2.19 | 0.6 | 2.80 | 1.19 |
| Study Plot Number (and Local Name) | 3 (Horni les) | 2 (Stepanov) | 1 (Pnovice) |
|---|---|---|---|
| 4 (Hrube les) | U = 496.5 0.007 * | U = 462.5 0.002 * | U = 495 0.007 * |
| 6 (Odchovna) | U = 502.5 0.008 * | U = 476.5 0.004 * | U = 505 0.009 * |
| 5 (Brezova) | U = 583.5 0.066 | U = 556.0 0.033 | U = 587.5 0.071 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machar, I.; Schlossarek, M.; Pechanec, V.; Uradnicek, L.; Praus, L.; Sıvacıoğlu, A. Retention Forestry Supports Bird Diversity in Managed, Temperate Hardwood Floodplain Forests. Forests 2019, 10, 300. https://doi.org/10.3390/f10040300
Machar I, Schlossarek M, Pechanec V, Uradnicek L, Praus L, Sıvacıoğlu A. Retention Forestry Supports Bird Diversity in Managed, Temperate Hardwood Floodplain Forests. Forests. 2019; 10(4):300. https://doi.org/10.3390/f10040300
Chicago/Turabian StyleMachar, Ivo, Martin Schlossarek, Vilem Pechanec, Lubos Uradnicek, Ludek Praus, and Ahmet Sıvacıoğlu. 2019. "Retention Forestry Supports Bird Diversity in Managed, Temperate Hardwood Floodplain Forests" Forests 10, no. 4: 300. https://doi.org/10.3390/f10040300
APA StyleMachar, I., Schlossarek, M., Pechanec, V., Uradnicek, L., Praus, L., & Sıvacıoğlu, A. (2019). Retention Forestry Supports Bird Diversity in Managed, Temperate Hardwood Floodplain Forests. Forests, 10(4), 300. https://doi.org/10.3390/f10040300






