Carbon Storage Dynamics of Secondary Forest Succession in the Central Loess Plateau of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Plot Selection
2.2. Vegetation Biomass
2.3. Fine Root Biomass, Litter and CWD Biomass
2.4. Soil Sampling
2.5. Organic C Analysis and C Stock Calculation
2.6. Statistical Analysis
3. Results
3.1. Vegetation Biomass Carbon Stocks
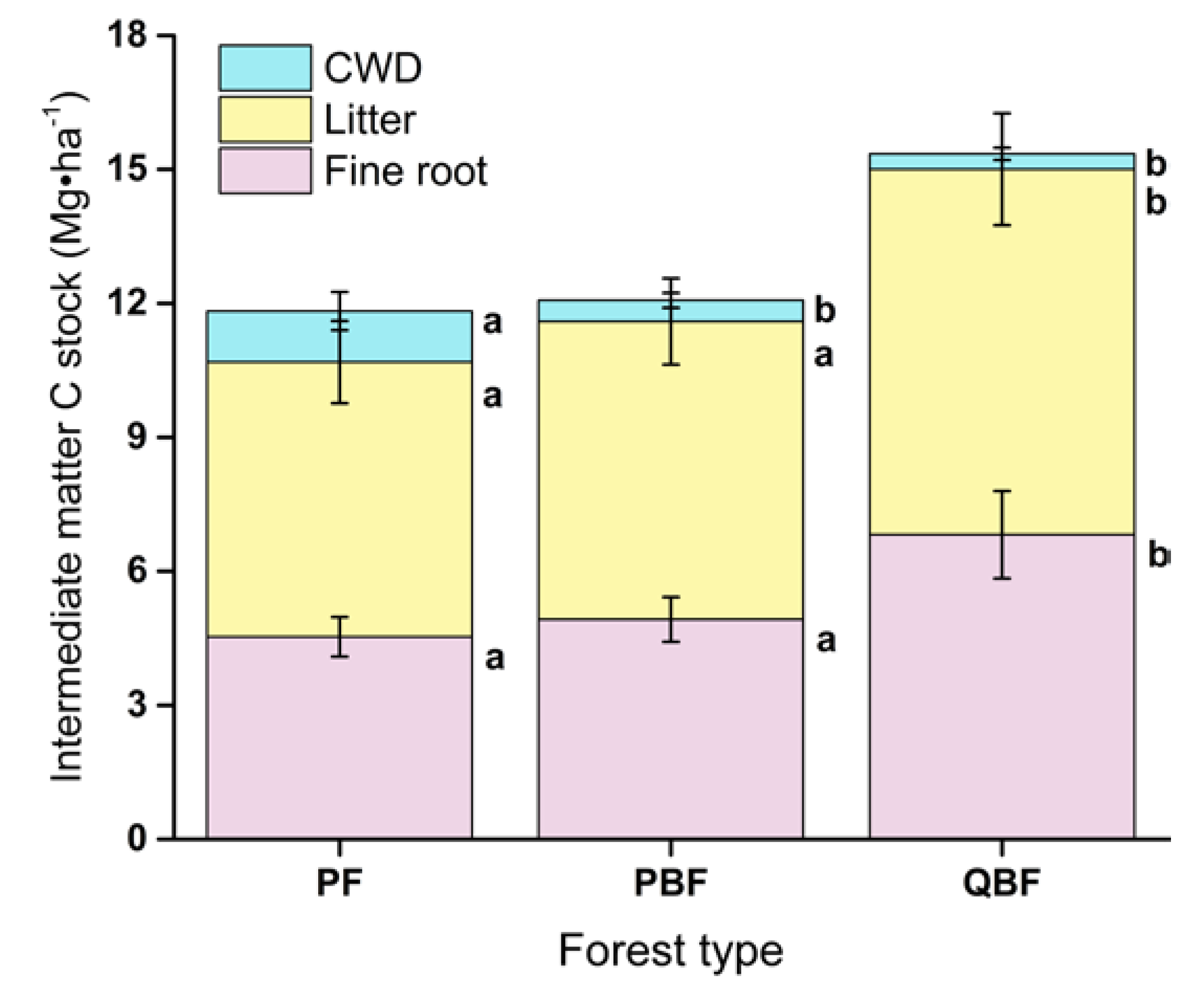
3.2. Fine Roots, Litter and CWD Carbon Stocks
3.3. Soil Carbon Stocks
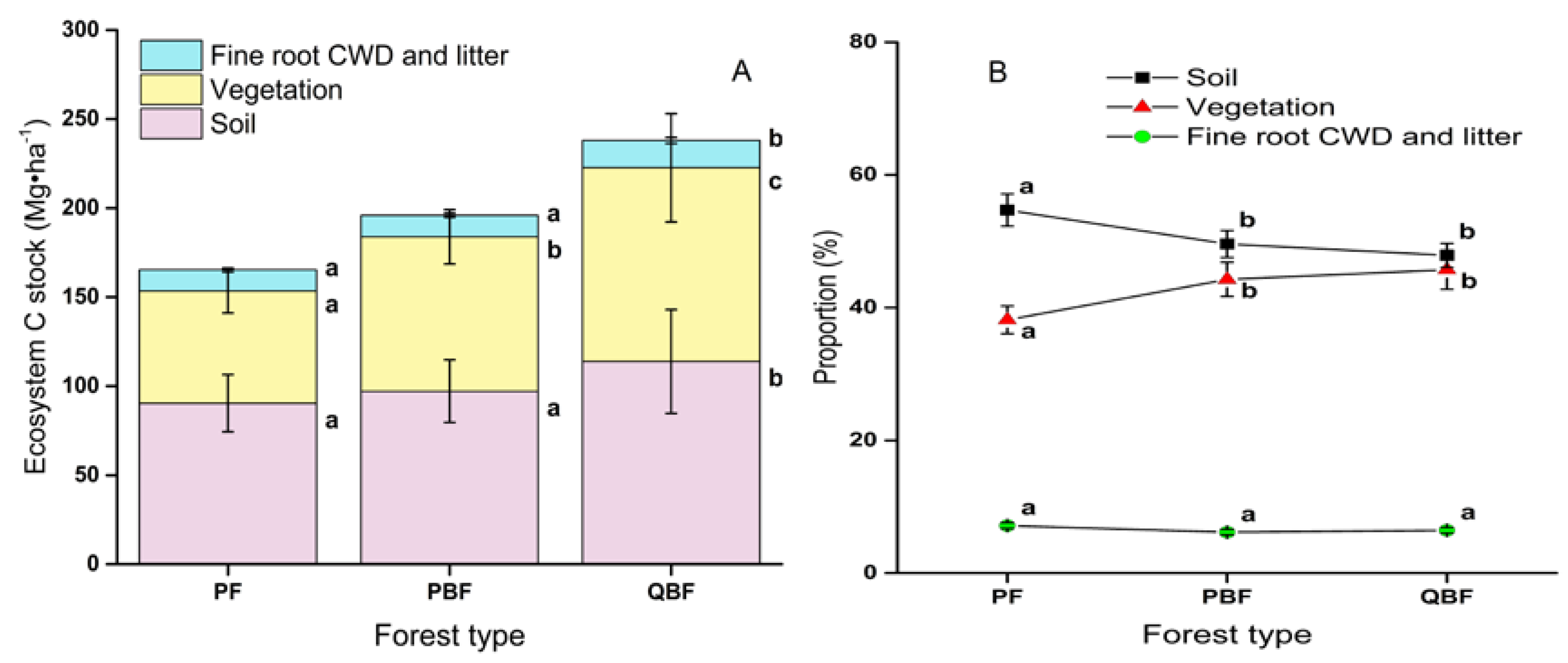
3.4. Ecosystem Carbon Stocks
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, S.L.; Schroeder, P.; Kern, J.S. Spatial distribution of biomass in forests of the eastern USA. Forest Ecol. Manag. 1999, 123, 81–90. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Gong, C.; Wang, S.L.; Zeng, Z.Q.; Deng, S.J.; Chen, J.P.; Long, K.S. Carbon storage and its distribution pattern of evergreen broad-leaved forests at different succession stages in mid-subtropical China. Chin. J. Ecol. 2011, 30, 1935–1941. [Google Scholar]
- Houghton, R.A.; Lawrence, K.T.; Hackler, J.L.; Brown, S. The spatial distribution of forest biomass in the Brazilian Amazon: A comparison of estimates. Glob. Chang. Biol. 2001, 7, 731–746. [Google Scholar] [CrossRef]
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.H.; Verardo, D.J.; Dokken, D.J.; Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.H. Land Use, Land-Use Change and Forestry: A Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Chinabaogao. Carbon Industry Analysis Report for 2018 in China. Website of China Report 2019. Available online: http://tuozi.chinabaogao.com/nengyuan/05S3534H018.html (accessed on 13 April 2019).
- Zhang, X.P.; Wang, M.B.; Liang, X.M. Quantitative classification and carbon density of the forest vegetation in Luliang Mountains of China. Plant Ecol. 2009, 201, 1–9. [Google Scholar] [CrossRef]
- Finegan, B. Forest succession. Nature 1984, 312, 109–114. [Google Scholar] [CrossRef]
- Asner, G.P. Painting the world REDD: Addressing scientific barriers to monitoring emissions from tropical forests. Environ. Res. Lett. 2011, 6, 1–3. [Google Scholar] [CrossRef]
- Edwards, D.P.; Fisher, B.; Boyd, E. Protecting degraded rainforests: Enhancement of forest carbon stocks under REDD. Conserv. Lett. 2010, 3, 313–316. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, A.P.; Peng, C.H.; Zhao, S.Q.; Ci, L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Aryal, D.R.; De Jong, B.H.J.; Ochoa-Gaona, S.; Esparza-Olguin, L.; Mendoza-Vega, J. Carbon stocks and changes in tropical secondary forests of southern Mexico. Agric. Ecosyst. Environ. 2014, 195, 220–230. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Chen, M.L.; Shangguan, Z.P.; Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, J.M.; Ma, Z.R.; Zhao, Y.; Su, J.S. Carbon storage in biomass, litter, and soil of different plantations in a semiarid temperate region of northwest China. Ann. Forest Sci. 2014, 71, 427–435. [Google Scholar] [CrossRef]
- Zhao, S.W.; Zhao, Y.G.; Wu, J.S. Quantitative analysis of soil pores under natural vegetation successions on the Loess Plateau. Sci. China Earth Sci. 2010, 53, 617–625. [Google Scholar] [CrossRef]
- Cheng, J.M.; Cheng, J.; Shao, H.B.; Zhao, L.P.; Yang, X.M. Soil Seed Banks and Forest Succession Direction Reflect Soil Quality in Ziwuling Mountain, Loess Plateau, China. Clean Soil Air Water 2012, 40, 140–147. [Google Scholar] [CrossRef]
- Fu, X.L.; Shao, M.A.; Wei, X.R.; Horton, R. Soil organic carbon and total nitrogen as affected by vegetation types in Northern Loess Plateau of China. Geoderma 2010, 155, 31–35. [Google Scholar] [CrossRef]
- Busse, M.D.; Sanchez, F.G.; Ratcliff, A.W.; Butnor, J.R.; Carter, E.A.; Powers, R.F. Soil carbon sequestration and changes in fungal and bacterial biomass following incorporation of forest residues. Soil Biol. Biochem. 2009, 41, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.B.; Halliday, M.J.; Siakimotu, S.J.M.; Gifford, R.M. Fine root production and litter input: Its effects on soil carbon. Plant Soil. 2005, 272, 1–10. [Google Scholar] [CrossRef]
- Sauer, T.J.; Cambardella, C.A.; Brandle, J.R. Soil carbon and tree litter dynamics in a red cedar-scotch pine shelterbelt. Agroforest Syst. 2007, 71, 163–174. [Google Scholar] [CrossRef]
- Sun, B.W.; Yang, X.D.; Zhang, Z.H.; Wen-Ji, M.A.; Arshad, A.; Huang, H.X.; Yan, E.R. Relationships between soil carbon pool and vegetation carbon return through succession of evergreen broad-leaved forests in Tiantong region, Zhejiang Province, Eastern China. Chin. J. Plant Ecol. 2013, 37, 803–810. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Raich, J.W. Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 1992, 73, 1139–1147. [Google Scholar] [CrossRef]
- Eaton, J.M.; Lawrence, D. Woody debris stocks and fluxes during succession. Forest Ecol. Manag. 2006, 232, 46–55. [Google Scholar] [CrossRef]
- Schmid, A.V.; Vogel, C.S.; Liebman, E.; Curtis, P.S.; Gough, C.M. Coarse woody debris and the carbon balance of a moderately disturbed forest. Forest Ecol. Manag. 2016, 361, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.L.; Green, M.B.; Yanai, R.D.; Woodall, C.W.; Fraver, S.; Harmon, M.E.; Hatfield, M.A.; Barnett, C.J.; See, C.R.; Domke, G.M. Estimating uncertainty in the volume and carbon storage of downed coarse woody debris. Ecol. Appl. 2019, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. Biogeosci. 2010, 115, 1–13. [Google Scholar] [CrossRef]
- Food and agriculture organization (FAO). United Nations forest issues-world forest situation. United Nations Forum on Forests 2019. Available online: https://www.un.org/zh/development/forest/africa.shtml (accessed on 13 April 2019).
- Zhang, X.W.; Li, G.X.; Yan, L.H. 2 Natural secondary forest management status and strategy. Forestry Sci. Tec. 2003, 28, 13 e0184415. [Google Scholar]
- Yang, Z.Q.; Dong, J.W.; Xu, X.L. Spatiotemporal pattern of forest fragmentation in the Loess Plateau. Resour. Sci. 2018, 40, 1246–1255. [Google Scholar]
- Zhu, J.J. A review on fundamental studies of secondary forest management. Chin. J. Appl. Ecol. 2002, 13, 1689–1694. [Google Scholar]
- State Forestry and Grassland Administration. Main results of the 8th National Forest Inventory (2009–2013). Available online: http://www.forestry.gov.cn/main/65/content-659670.html (accessed on 13 April 2019).
- Kang, D.; Guo, Y.X.; Ren, C.J.; Zhao, F.Z.; Feng, Y.Z.; Han, X.H.; Yang, G.H. Population structure and spatial pattern of main tree species in secondary Betula platyphylla forest in Ziwuling Mountains, China. Sci. Rep. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Shao, X. Reconstruction of seasonal temperature and precipitation of past 100 years from tree ring width and stable carbon isotope in Huangling, Shannxi. Sci. China 1997, 27, 271–276. [Google Scholar]
- Liu, S.M. Climatic characteristics in the Ziwuling Nature Reserve, Shaanxi Province. Arid. Zone Res. 2004, 21, 466–469. [Google Scholar]
- An, H.; Shangguan, Z.P. Photosynthetic characteristics of dominant plant species at different succession stages of vegetation on Loess Plateau. Ying Yong Sheng Tai Xue Bao 2007, 18, 1175. [Google Scholar] [PubMed]
- Zhu, Z. Recovering succession of vegetation in forest region of North Shaanxi Loess Plateau. J. Northw. Forestry Univ. 1993, 8, 87–494. [Google Scholar]
- Chinese Standard GB/T 33027-2016. In Methodology for Field Long-Term Observation of Forest Ecosystem; Standards Press of China: Beijing, China, 2016.
- Zhou, G.Y. Evaluation on the carbon pools of China’s forest ecosystems―Current status, capacities and sinks and studies on the mechanisms. Chin. J. Plant Ecol. 2016, 40, 279–281. [Google Scholar]
- Yang, B.; Xue, W.; Yu, S.; Zhou, J.; Zhang, W. Effects of stand age on biomass allocation and allometry of Quercus Acutissima in the Central Loess Plateau of China. Forests 2019, 10, 41. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.H.; Wu, M.; Xue, Y.Q.; Ma, L.W.; Zhou, J.Y. Effect of aboveground intervention on fine root mass, production, and turnover rate in a Chinese cork oak (Quercus variabilis Blume) forest. Plant Soil 2013, 368, 201–214. [Google Scholar] [CrossRef]
- Risch, A.C.; Jurgensen, M.F.; Page-Dumroese, D.S.; Wildi, O.; Schutz, M. Long-term development of above- and below-ground carbon stocks following land-use change in subalpine ecosystems of the Swiss National Park. Can. J. Forest Res. 2008, 38, 1590–1602. [Google Scholar] [CrossRef]
- Wang, J.; Epstein, H.E. Estimating carbon source-sink transition during secondary succession in a Virginia valley. Plant Soil 2013, 362, 135–147. [Google Scholar] [CrossRef]
- Fang, Y.T.; Mo, J.M.; Peng, S.L.; Li, D.J. Role of forest succession on carbon sequestration of forest ecosystems in lower subtropical China. Acta Ecol. Sin. 2003, 23, 1685–1694. [Google Scholar]
- Zeng, Z.Q.; Wang, S.L.; Zhang, C.M.; Gong, C.; Hu, Q. Carbon storage in evergreen broad-leaf forests in mid-subtropical region of China at four succession stages. J. For. Res. 2013, 24, 677–682. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Chang, J.; Jiang, B.; Jiang, H.; Peng, C.H.; Zhu, J.R.; Yuan, W.G.; Qi, L.Z.; Yu, S.Q. Carbon storage by ecological service forests in Zhejiang Province, subtropical China. Forest Ecol. Manag. 2007, 245, 64–75. [Google Scholar] [CrossRef]
- Lan, S.A.; Du, H.; Zeng, F.P.; Song, T.Q.; Peng, W.X.; Han, C.; Chen, L.; Su, L. Carbon storage and allocation in Cunninghamia lanceolata plantations with different stand ages. Ying Yong Sheng Tai Xue Bao 2016, 27, 1125–1134. [Google Scholar]
- Zhou, Y.R.; Zhen-Liang, Y.U.; Zhao, S.D. Carbon storage and budget of major chinese forest types. Acta Phytoecol. Sin. 2000, 24, 518–522. [Google Scholar]
- Fang, J.; Liu, G.; Xu, S. Biomass and net production of forest vegetation in China. Acta Ecol. Sin. 1996, 16, 497–508. [Google Scholar]
- Chen, L.C.; Wang, S.L.; Wang, Q.K. Ecosystem carbon stocks in a forest chronosequence in Hunan Province, South China. Plant Soil 2016, 409, 217–228. [Google Scholar] [CrossRef]
- Abdallah, F.; Chaieb, M. The influence of trees on nutrients, water, light availability and understorey vegetation in an arid environment. Appl. Veg. Sci. 2012, 15, 501–512. [Google Scholar] [CrossRef]
- Von Arx, G.; Dobbertin, M.; Rebetez, M. Spatio-temporal effects of forest canopy on understory microclimate in a long-term experiment in Switzerland. Agric. Forest Meteorol. 2012, 166, 144–155. [Google Scholar] [CrossRef]
- Holst, T.; Mayer, H.; Schindler, D. Microclimate within beech stands—Part II: Thermal conditions. Eur. J. Forest Res. 2004, 123, 13–28. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Mansilla-Salinero, P.; Rodríguez-Soalleiro, R.; Merino, A. Influence of tree species on carbon sequestration in afforested pastures in a humid temperate region. Plant Soil 2012, 353, 333–353. [Google Scholar] [CrossRef]
- Li, Z.P.; Han, F.X.; Su, Y.; Zhang, T.L. Assessment of soil organic and carbonate carbon storage in China. Geoderma 2007, 138, 119–126. [Google Scholar] [CrossRef]
- Novara, A.; Gristina, L.; La Mantia, T.; Ruhl, J. Carbon dynamics of soil organic matter in bulk soil and aggregate fraction during secondary succession in a Mediterranean environment. Geoderma 2013, 193, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. Forest Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Tang, G.; Li, K. Tree species controls on soil carbon sequestration and carbon stability following 20 years of afforestation in a valley-type savanna. Forest Ecol. Manag. 2013, 291, 13–19. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Nabulars, G.J.; Verburg, P.H.; de Waal, R.W. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. Forest Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Martius, C.; Höfer, H.; Garcia, M.V.B.; Römbke, J.; Förster, B.; Hanagarth, W. Microclimate in agroforestry systems in central Amazonia: Does canopy closure matter to soil organisms? Agroforest Syst. 2004, 60, 291–304. [Google Scholar] [CrossRef]



| Forest Type | Pinus tabuliformis Forests (PF) | Pine-Broadleaved Mixed Forest (PBF) | Quercus-Broadleaved Mixed Forest (QBF) |
|---|---|---|---|
| Successional stage | I | II | III |
| Stand age (a) | 20–30 | 20–60 | 30–100 |
| Canopy density (%) | 65–70 | 65–75 | 75–85 |
| Stand density (trees· ha-1) | 1755±83 | 1729±66 | 1580±61 |
| Elevation (m) | 1100–1200 | 1100–1200 | 1100–1200 |
| Slope (°) | 10–20 | 10–25 | 10–25 |
| Mean tree height (m) | 13.3 | 15.1 | 16.5 |
| Mean DBH (cm) | 14.6 | 15.3 | 17.2 |
| Soil type | Gray cinnamon soil | Gray cinnamon soil | Gray cinnamon soil |
| Dominant species | |||
| Trees | P. tabuliformis | P. tabulaeformis Populus davidiana | Q. acutissima Q. wutaishanica Betula platyphylla Malus baccata (L.) Borkh. |
| Shrubs | Spiraea salicifolia L. Lonicera japonica Thunb. | Sophora davidii (Franch.) Skeels Lespedeza bicolor Turcz. Smilax china L. | Sophora davidii (Franch.) Skeels. Rosa rubus Lévl. et Vant. Rosa xanthina Lindl. Celastrus orbiculatus Thunb. Euonymus alatus (Thunb.) Sieb. Viburnum dilatatum Thunb. |
| Herbs | Carex uda Maxim. | Elymus dahuricus Turcz. Rubia cordifolia L. | Carex uda Maxim. Imperata cylindrica (L.) Beauv. Tripolium vulgare Nees. Patrinia scabiosaefolia Fisch. ex Trev. Cephalanthera erecta (Thunb. ex A. Murray) Bl. |
| Tree species | Components | Equations | R2 | Sources |
|---|---|---|---|---|
| Pinus tabulaeformis | Stem (with bark) | 0.9652 | Zhou [39] | |
| Branch | 0.8672 | |||
| Foliage | 0.7543 | |||
| Root (≥ 0.2 cm) | 0.8761 | |||
| Populus davidiana | Stem (with bark) | 0.9782 | Zhou [39] | |
| Branch | 0.8594 | |||
| Foliage | 0.8740 | |||
| Root (≥ 0.2 cm) | 0.7795 | |||
| Betula platyphylla | Stem (with bark) | 0.9169 | Zhou [39] | |
| Branch | 0.8647 | |||
| Foliage | 0.7616 | |||
| Root (≥ 0.2 cm) | 0.8371 | |||
| Quercus wutaishanica | Stem (with bark) | 0.9578 | Zhou [39] | |
| Branch | 0.8564 | |||
| Foliage | 0.8169 | |||
| Root (≥ 0.2 cm) | 0.8863 | |||
| Quercus acutissima | Stem (with bark) | 0.9306 | Yang et al. [40] | |
| Branch | 0.8403 | |||
| Foliage | 0.7597 | |||
| Root (≥ 0.2 cm) | 0.8606 | |||
| Other hardwood tree species | Stem (with bark) | 0.9970 | Zhou [39] | |
| Branch | 0.9460 | |||
| Foliage | 0.9670 | |||
| Root (≥ 0.2 cm) | 0.9900 | |||
| Other conifer tree species | Stem (with bark) | 0.9350 | Zhou [39] | |
| Branch | 0.9860 | |||
| Foliage | 0.8780 | |||
| Root (≥ 0.2 cm) | 0.8400 |
| Characteristic | Decay Class | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Bark | Recently downed, intact | Beginning to peel or crack | Trace to absent | Absent | Absent |
| Sapwood | Present, intact | Mostly intact, partly soft | Soft and crushes under foot | Mostly absent | Absent |
| Heartwood (if present) | Not visible | Not visible | Visible in places | Beginning to decay | Showing signs of substantial decay |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhang, W.; Lu, Y.; Zhang, W.; Wang, Y. Carbon Storage Dynamics of Secondary Forest Succession in the Central Loess Plateau of China. Forests 2019, 10, 342. https://doi.org/10.3390/f10040342
Yang B, Zhang W, Lu Y, Zhang W, Wang Y. Carbon Storage Dynamics of Secondary Forest Succession in the Central Loess Plateau of China. Forests. 2019; 10(4):342. https://doi.org/10.3390/f10040342
Chicago/Turabian StyleYang, Bin, Wenhui Zhang, Yanlei Lu, Weiwei Zhang, and Yanan Wang. 2019. "Carbon Storage Dynamics of Secondary Forest Succession in the Central Loess Plateau of China" Forests 10, no. 4: 342. https://doi.org/10.3390/f10040342
APA StyleYang, B., Zhang, W., Lu, Y., Zhang, W., & Wang, Y. (2019). Carbon Storage Dynamics of Secondary Forest Succession in the Central Loess Plateau of China. Forests, 10(4), 342. https://doi.org/10.3390/f10040342




