Effect of Long-Term vs. Short-Term Ambient Ozone Exposure on Radial Stem Growth, Sap Flux and Xylem Morphology of O3-Sensitive Poplar Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Treatment Description
2.2. Environmental Variables
2.3. Stem Radius Growth
2.4. Sap Flow
2.5. Tree Harvest, Xylem Morphology and Huber Value
2.6. Data Analyses
2.7. Statistical Analyses
3. Results
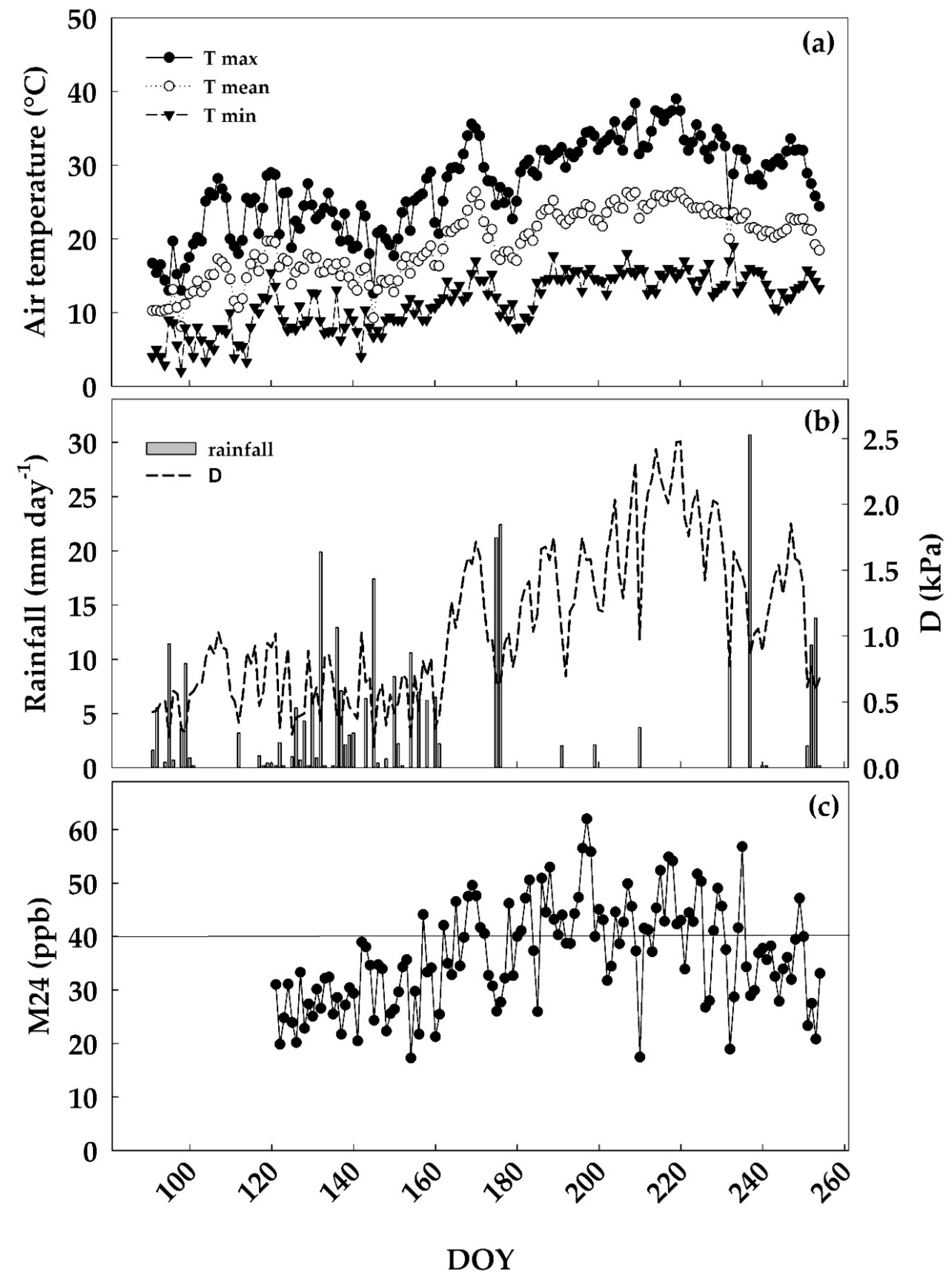
3.1. Environmental Conditions
3.2. Effect of Long-term O3 Exposure on Intra-annual Radial Stem Growth and Seasonal Sap Flux Density
3.3. Effect of Short-term O3 Exposure under High Evaporative Demand on Radial Stem Growth, Stem Water Deficit and Sap Flow
4. Discussion
4.1. Effect of Long-term O3 Exposure on Intra-annual Radial Stem Growth and Phenology
4.2. Effect of Long-term O3 Exposure on Sap Flow and Hydraulic Traits
4.3. Effect of Short-term O3 Exposure under High Evaporative Demand on Radial Stem Growth, Stem Water Deficit and Sap Flow
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paoletti, E. Ozone impacts on forests. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Resour. 2007, 2, 13. [Google Scholar] [CrossRef]
- Mills, G.; Pleijel, H.; Malley, C.; Sinha, B.; Cooper, O.; Schultz, M.; Neufeld, H.; Simpson, D.; Sharps, K.; Feng, Z.; et al. Tropospheric ozone assessment report: Present-day tropospheric ozone distribution and trends relevant to vegetation. Elementa-Sci. Anthrop. 2018, 6. [Google Scholar] [CrossRef]
- Pellegrini, E.; Hoshika, Y.; Dusart, N.; Cotrozzi, L.; Gérard, J.; Nali, C.; Vaultier, M.-N.; Jolivet, Y.; Lorenzini, G.; Paoletti, E. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019, 647, 390–399. [Google Scholar] [CrossRef]
- Zhang, L.; Hoshika, Y.; Carrari, E.; Cotrozzi, L.; Pellegrini, E.; Paoletti, E. Effects of nitrogen and phosphorus imbalance on photosynthetic traits of poplar Oxford clone under ozone pollution. J. Plant Res. 2018, 131, 915–924. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, B.; Wu, T.; Wen, M.-X.; Fan, L.-X.; Feng, Z.-Z.; Paoletti, E. Transcriptomic analysis of Pak Choi under acute ozone exposure revealed regulatory mechanism against ozone stress. BMC Plant Boil. 2017, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Carrari, E.; Zhang, L.; Carriero, G.; Pignatelli, S.; Fasano, G.; Materassi, A.; Paoletti, E. Testing a ratio of photosynthesis to O3 uptake as an index for assessing O3-induced foliar visible injury in poplar trees. Environ. Sci. Pollut. R. 2018, 25, 8113–8124. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, Z.; Catalayud, V.; Yuan, X.; Xu, Y.; Paoletti, E. A meta-analysis on growth, physiological, and biochemical responses of woody species to ground-level ozone highlights the role of plant functional types. Plant Cell Environ. 2017, 40, 2369–2380. [Google Scholar] [CrossRef]
- Manning, W.J. Establishing a cause and effect relationship for ambient ozone exposure and tree growth in the forest: Progress and an experimental approach. Environ. Pollut. 2005, 137, 443–454. [Google Scholar] [CrossRef]
- Pretzsch, H.; Dieler, J.; Matyssek, R.; Wipfler, P. Tree and stand growth of mature Norway spruce and European beech under long-term ozone fumigation. Environ. Pollut. 2010, 158, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Talhelm, A.F.; Pregitzer, K.S.; Kubiske, M.E.; Zak, D.R.; Campany, C.E.; Burton, A.J.; Dickson, R.E.; Hendrey, G.R.; Isebrands, J.G.; Lewin, K.F.; et al. Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests. Chang. Boil. 2014, 20, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Karnosky, D.; Wieser, G.; Percy, K.; Oksanen, E.; Grams, T.; Kubiske, M.; Hanke, D.; Pretzsch, H.; Grams, T. Advances in understanding ozone impact on forest trees: Messages from novel phytotron and free-air fumigation studies. Environ. Pollut. 2010, 158, 1990–2006. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S.; Kubiske, M.E.; Burton, A.J. Forest productivity under elevated CO2 and O3: positive feedbacks to soil N cycling sustain decade-long net primary productivity enhancement by CO2. Ecol. Lett. 2011, 14, 1220–1226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubiske, M.E.; Quinn, V.S.; Marquardt, P.E.; Karnosky, D.F. Effects of Elevated Atmospheric CO2 and/or O3 on Intra- and Interspecific Competitive Ability of Aspen. Plant Boil. 2007, 9, 342–355. [Google Scholar] [CrossRef]
- Oksanen, E.; Riikonen, J.; Kaakinen, S.; Holopainen, T.; Vapaavuori, E. Structural characteristics and chemical composition of birch (Betula pendula) leaves are modified by increasing CO2 and ozone. Chang. Boil. 2005, 11, 732–748. [Google Scholar] [CrossRef]
- Hoshika, Y.; Pecori, F.; Conese, I.; Bardelli, T.; Marchi, E.; Manning, W.J.; Badea, O.; Paoletti, E. Effects of a three-year exposure to ambient ozone on biomass allocation in poplar using ethylenediurea. Environ. Pollut. 2013, 180, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Contran, N.; Manning, W.J.; Ferrara, A.M. Use of the antiozonant ethylenediurea (EDU) in Italy: Verification of the effects of ambient ozone on crop plants and trees and investigation of EDU’s mode of action. Environ. Pollut. 2009, 157, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. Perspectives for elucidating the ethylenediurea (EDU) mode of action for protection against O3 phytotoxicity. Ecotoxicol. Environ. Saf. 2017, 142, 530–537. [Google Scholar] [CrossRef]
- Agathokleous, E.; Koike, T.; Watanabe, M.; Hoshika, Y.; Saitanis, C. Ethylenediurea (EDU), an effective phytoproctectant against O3 deleterious effects and a valuable research tool. J. Agric. Meteorol. 2015, 71, 185–195. [Google Scholar] [CrossRef]
- Agathokleous, E.; Paoletti, E.; Manning, W.J.; Kitao, M.; Saitanis, C.J.; Koike, T. High doses of ethylenediurea (EDU) as soil drenches did not increase leaf N content or cause phytotoxicity in willow grown in fertile soil. Ecotoxicol. Environ. Saf. 2018, 147, 574–584. [Google Scholar] [CrossRef]
- Carriero, G.; Emiliani, G.; Giovannelli, A.; Hoshika, Y.; Manning, W.; Traversi, M.; Paoletti, E. Effects of long-term ambient ozone exposure on biomass and wood traits in poplar treated with ethylenediurea (EDU). Environ. Pollut. 2015, 206, 575–581. [Google Scholar] [CrossRef]
- Hoshika, Y.; Omasa, K.; Paoletti, E. Whole-Tree Water Use Efficiency Is Decreased by Ambient Ozone and Not Affected by O3-Induced Stomatal Sluggishness. PLoS ONE 2012, 7, e39270. [Google Scholar] [CrossRef] [PubMed]
- Katanic, M.; Paoletti, E.; Orlovic, S.; Grebenc, T.; Kraigher, H. Mycorrhizal status of an ozone-sensitive poplar clone treated with the antiozonant ethylene diurea. Eur. J. Forest Res. 2014, 133, 735–743. [Google Scholar] [CrossRef]
- Bowman, D.M.; Brienen, R.J.; Gloor, E.; Phillips, O.L.; Prior, L.D. Detecting trends in tree growth: Not so simple. Trends Plant Sci. 2013, 18, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Cocozza, C.; Palombo, C.; Tognetti, R.; La Porta, N.; Anichini, M.; Giovannelli, A.; Emiliani, G. Monitoring intra-annual dynamics of wood formation with microcores and dendrometers in Picea abies at two different altitudes. Tree Physiol. 2016, 36, 832–846. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Newbery, D.M. Modeling tree water deficit from microclimate: An approach to quantifying drought stress. Tree Physiol. 2005, 25, 147–156. [Google Scholar] [CrossRef]
- Cocozza, C.; Giovannelli, A.; Lasserre, B.; Cantini, C.; Lombardi, F.; Tognetti, R. A novel mathematical procedure to interpret the stem radius variation in olive trees. Agric. Meteorol. 2012, 161, 80–93. [Google Scholar] [CrossRef]
- Van Der Maaten, E.; Van Der Maaten-Theunissen, M.; Smiljanić, M.; Rossi, S.; Simard, S.; Wilmking, M.; DesLauriers, A.; Fonti, P.; Von Arx, G.; Bouriaud, O. dendrometeR: Analyzing the pulse of trees in R. Dendrochronologia 2016, 40, 12–16. [Google Scholar] [CrossRef]
- Rossi, S.; DesLauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Woo, S.Y.; Hinckley, T.M. The effects of ozone on growth and stomatal response in the F2 generation of hybrid poplar (Populus trichocarpa × Populus deltoides). Boil. Plant. 2005, 49, 395–404. [Google Scholar] [CrossRef]
- Zweifel, R.; Drew, D.M.; Schweingruber, F.; Downes, G.M. Xylem as the main origin of stem radius changes in Eucalyptus. Funct. Plant Boil. 2014, 41, 520–534. [Google Scholar] [CrossRef]
- DesLauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef]
- Berta, M.; Giovannelli, A.; Sebastiani, F.; Camussi, A.; Racchi, M.L. Transcriptome changes in the cambial region of poplar (Populus alba L.) in response to water deficit. Plant Boil. 2010, 12, 341–354. [Google Scholar] [CrossRef]
- Zweifel, R.; Eugster, W.; Etzold, S.; Dobbertin, M.; Buchmann, N.; Hasler, R. Link between continuous stem radius changes and net ecosystem productivity of a subalpine Norway spruce forest in the Swiss Alps. New Phytol. 2010, 187, 819–830. [Google Scholar] [CrossRef]
- Traversari, S.; Francini, A.; Traversi, M.L.; Emiliani, G.; Sorce, C.; Sebastiani, L.; Giovannelli, A. Can sugar metabolism in the cambial region explain the water deficit tolerance in poplar? J. Exp. Bot. 2018, 69, 4083–4097. [Google Scholar] [CrossRef]
- Cocozza, C.; Lasserre, B.; Giovannelli, A.; Castro, G.; Fragnelli, G.; Tognetti, R. Low temperature induces different cold sensitivity in two poplar clones (Populus × canadensis Monch ’I-214’ and P. deltoides Marsh. ’Dvina’). J. Exp. Bot. 2009, 60, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Balducci, L.; DesLauriers, A.; Giovannelli, A.; Rossi, S.; Rathgeber, C.B. Effects of temperature and water deficit on cambial activity and woody ring features in Picea mariana saplings. Tree Physiol. 2013, 33, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- DesLauriers, A.; Beaulieu, M.; Balducci, L.; Giovannelli, A.; Gagnon, M.J.; Rossi, S. Impact of warming and drought on carbon balance related to wood formation in black spruce. Ann. Bot. 2014, 114, 335–345. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.B.; Nosal, M.; Wullschleger, S.D.; Sun, G. Interactive effects of ozone and climate on tree growth and water use in a southern Appalachian forest in the USA. New Phytol. 2007, 174, 109–124. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Wullschleger, S.D.; Nosal, M.; Cate, T.M.; Perkins, T.D. Diurnal and seasonal changes in stem increment and water use by yellow poplar trees in response to environmental stress. Tree Physiol. 2003, 23, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Allen, S. Measurement of sap flow in plant stems. J. Exp. Bot. 1996, 47, 1833–1844. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Leuzinger, S. Use of sap flow measurements to validate stomatal functions for mature beech (Fagus sylvatica) in view of ozone uptake calculations. Environ. Pollut. 2010, 158, 2954–2963. [Google Scholar] [CrossRef]
- Wieser, G.; Matyssek, R.; Köstner, B.; Oberhuber, W. Quantifying ozone uptake at the canopy level of spruce, pine and larch trees at the alpine timberline: An approach based on sap flow measurement. Environ. Pollut. 2003, 126, 5–8. [Google Scholar] [CrossRef]
- Wang, B.; Shugart, H.H.; Shuman, J.K.; Lerdau, M.T. Forests and ozone: Productivity, carbon storage and feedbacks. Sci. Rep. 2016, 6, 22133. [Google Scholar] [CrossRef]
- Zweifel, R.; Häsler, R. Dynamics of water storage in mature subalpine Picea abies: Temporal and spatial patterns of change in stem radius. Tree Physiol. 2001, 21, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; Nikinmaa, E.; Riikonen, A.; Daley, M.; Pettijohn, J.C.; Mikkelsen, T.N.; Phillips, N.; Holbrook, N.M. Linking xylem diameter variations with sap flow measurements. Plant Soil 2008, 305, 77–90. [Google Scholar] [CrossRef]
- Richet, N.; Afif, D.; Huber, F.; Pollet, B.; Banvoy, J.; El Zein, R.; Lapierre, C.; Dizengremel, P.; Perré, P.; Cabané, M. Cellulose and lignin biosynthesis is altered by ozone in wood of hybrid poplar (Populus tremula × alba). J. Exp. Bot. 2011, 62, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- Kostiainen, K.; Jalkanen, H.; Kaakinen, S.; Saranpää, P.; Vapaavuori, E. Wood properties of two silver birch clones exposed to elevated CO2 and O3. Chang. Boil. 2006, 12, 1230–1240. [Google Scholar] [CrossRef]
- Hoshika, Y.; Katata, G.; Deushi, M.; Watanabe, M.; Koike, T.; Paoletti, E. Ozone-induced stomatal sluggishness changes carbon and water balance of temperate deciduous forests. Sci. Rep. 2015, 5, 9871. [Google Scholar] [CrossRef]
- Goff, J.A.; Gratch, S. Low-pressure Properties of water from −160 to 212 F. In Proceedings of the 52nd Annual Meeting of the American Society of Heating and Ventilating Engineers, New York, NY, USA, 10–13 June 1946; pp. 95–122. [Google Scholar]
- UNECE-ICP-Forest. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forest. Part II Visual Assessment of Frown Condition; UNECE-ICP-Forest: Hamburg, Germany, 2010. [Google Scholar]
- Hoshika, Y.; Moura, B.; Paoletti, E. Ozone risk assessment in three oak species as affected by soil water availability. Environ. Sci. Pollut. R. 2018, 25, 8125–8136. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, A.; Deslauriers, A.; Fragnelli, G.; Scaletti, L.; Castro, G.; Rossi, S.; Crivellaro, A. Evaluation of drought response of two poplar clones (Populus x canadensis Monch ’I-214’ and P. deltoides Marsh. ’Dvina’) through high resolution analysis of stem growth. J. Exp. Bot. 2007, 58, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Tognetti, R.; Giovannelli, A. High-Resolution Analytical Approach to Describe the Sensitivity of Tree–Environment Dependences through Stem Radial Variation. Forests 2018, 9, 134. [Google Scholar] [CrossRef]
- Granier, A. A new method of sap flow measurement in tree stems. Ann. Des. Sci. For. 1985, 42, 193–200. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Sperry, J.S.; Saliendra, N.Z. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant Cell Environ. 1994, 17, 1233–1241. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H.; Tyree, P.M.T. Xylem Structure and the Ascent of Sap; Springer: New York, NK, USA, 2002. [Google Scholar]
- Santiago, L.; Machado, K.; Woodruff, D.; Meinzer, F.; Santiago, L.S.; Goldstein, G.; Fisher, J.B.; Jones, T. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef] [PubMed]
- DesLauriers, A.; Giovannelli, A.; Rossi, S.; Castro, G.; Fragnelli, G.; Traversi, L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol. 2009, 29, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Bouriaud, O.; Deleuze, C.; Leban, J.-M.; Bert, D. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef]
- Rossi, S.; DesLauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef]
- DesLauriers, A.; Urbinati, C.; Carrer, M.; Morin, H. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Vollenweider, P.; Woodcock, H.; Kelty, M.; Hofer, R.-M. Reduction of stem growth and site dependency of leaf injury in Massachusetts black cherries exhibiting ozone symptoms. Environ. Pollut. 2003, 125, 467–480. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Kahle, H.-P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Radial growth variation of Norway spruce (Picea abies (L.) Karst.) across latitudinal and altitudinal gradients in central and northern Europe. Ecol. Manag. 2002, 171, 243–259. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Chang. Boil. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Zhai, L.; Bergeron, Y.; Huang, J.-G.; Berninger, F. Variation in intra-annual wood formation, and foliage and shoot development of three major Canadian boreal tree species. Am. J. Bot. 2012, 99, 827–837. [Google Scholar] [CrossRef]
- De Luis, M.; Novak, K.; Raventós, J.; Gričar, J.; Prislan, P.; Čufar, K. Cambial activity, wood formation and sapling survival of Pinus halepensis exposed to different irrigation regimes. Ecol. Manag. 2011, 262, 1630–1638. [Google Scholar] [CrossRef]
- Oberhuber, W. Soil water availability and evaporative demand affect seasonal growth dynamics and use of stored water in co-occurring saplings and mature conifers under drought. Trees 2017, 31, 467–478. [Google Scholar] [CrossRef]
- Hinckley, T.M.; Brooks, J.R.; Čermák, J.; Ceulemans, R.; Kučera, J.; Meinzer, F.; Roberts, D.A. Water flux in a hybrid poplar stand. Tree Physiol. 1994, 14, 1005–1018. [Google Scholar] [CrossRef]
- Uddling, J.; Teclaw, R.M.; Kubiske, M.E.; Pregitzer, K.S.; Ellsworth, D.S. Sap flux in pure aspen and mixed aspen-birch forests exposed to elevated concentrations of carbon dioxide and ozone. Tree Physiol. 2008, 28, 1231–1243. [Google Scholar] [CrossRef]
- Pääkkönen, E.; Váhala, J.; Holopainen, T.; Karjalainen, R.; Kärenlampi, L. Growth responses and related biochemical and ultrastructural changes of the photosynthetic apparatus in birch (Betula pendula) saplings exposed to low concentrations of ozone. Tree Physiol. 1996, 16, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Bytnerowicz, A.; Karlsson, P.-E.; Paoletti, E.; Sanz, M.; Schaub, M.; Wieser, G. Promoting the O3 flux concept for European forest trees. Environ. Pollut. 2007, 146, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, J.; Unsworth, M.H.; Wright, C.J. Seasonal changes in water use of ash trees exposed to ozone episodes. New Phytol. 1994, 127, 349–354. [Google Scholar] [CrossRef]
- Hoshika, Y.; Carriero, G.; Feng, Z.; Zhang, Y.; Paoletti, E. Determinants of stomatal sluggishness in ozone-exposed deciduous tree species. Sci. Total Environ. 2014, 481, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Leuschner, C.; Hertel, D.; Delzon, S.; Schuldt, B. Trade-offs between xylem hydraulic properties, wood anatomy and yield in Populus. Tree Physiol. 2014, 34, 744–756. [Google Scholar] [CrossRef]
- Cruiziat, P.; Cochard, H.; Améglio, T. Hydraulic architecture of trees: main concepts and results. Ann. Sci. 2002, 59, 723–752. [Google Scholar] [CrossRef]
- Haworth, M.; Centritto, M.; Giovannelli, A.; Marino, G.; Proietti, N.; Capitani, D.; De Carlo, A.; Loreto, F. Xylem morphology determines the drought response of two Arundo donax ecotypes from contrasting habitats. GCB Bioenergy 2017, 9, 119–131. [Google Scholar] [CrossRef]
- Carter, J.L.; White, D.A. Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth. Tree Physiol. 2009, 29, 1407–1418. [Google Scholar] [CrossRef]
- Schuldt, B.; Leuschner, C.; Horna, V.; Moser, G.; Kohler, M.; Van Straaten, O.; Barus, H. Change in hydraulic properties and leaf traits in a tall rainforest tree species subjected to long-term throughfall exclusion in the perhumid tropics. Biogeosciences 2011, 8, 2179–2194. [Google Scholar] [CrossRef]
- Kern, K.A.; Ewers, F.W.; Telewski, F.W.; Koehler, L. Mechanical perturbation affects conductivity, mechanical properties and aboveground biomass of hybrid poplars. Tree Physiol. 2005, 25, 1243–1251. [Google Scholar] [CrossRef]
- Magnani, F.; Grace, J.; Borghetti, M. Adjustment of tree structure in response to the environment under hydraulic constraints. Funct. Ecol. 2002, 16, 385–393. [Google Scholar] [CrossRef]
- Devi, M.J.; Reddy, V.R. Transpiration Response of Cotton to Vapor Pressure Deficit and Its Relationship With Stomatal Traits. Front. Plant Sci. 2018, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Pollastrini, M.; Desotgiu, R.; Camin, F.; Ziller, L.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Severe drought events increase the sensitivity to ozone on poplar clones. Environ. Exp. Bot. 2014, 100, 94–104. [Google Scholar] [CrossRef]
- Bloemen, J.; Fichot, R.; Horemans, J.; Broeckx, L.; Verlinden, M.; Zenone, T.; Ceulemans, R. Water use of a multigenotype poplar short-rotation coppice from tree to stand scale. GCB Bioenergy 2017, 9, 370–384. [Google Scholar] [CrossRef]
- Čermák, J.; Kučera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef]
- Balducci, L.; Cuny, H.E.; Rathgeber, C.B.K.; DesLauriers, A.; Giovannelli, A.; Rossi, S. Compensatory mechanisms mitigate the effect of warming and drought on wood formation. Plant Cell Environ. 2016, 39, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, C.; Marino, G.; Giovannelli, A.; Cantini, C.; Centritto, M.; Tognetti, R. Simultaneous measurements of stem radius variation and sap flux density reveal synchronisation of water storage and transpiration dynamics in olive trees. Ecohydrology 2015, 8, 33–45. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Crop load affects maximum daily trunk shrinkage of plum trees. Tree Physiol. 2007, 27, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Cochard, H.; Lacointe, A.; Ameglio, T. Could rapid diameter changes be facilitated by a variable hydraulic conductance? Plant Cell Environ. 2012, 35, 150–157. [Google Scholar] [CrossRef]
- Verstraeten, W.W.; Veroustraete, F.; Feyen, J. Estimating evapotranspiration of European forests from NOAA-imagery at satellite overpass time: Towards an operational processing chain for integrated optical and thermal sensor data products. Remote Sens. Environ. 2005, 96, 256–276. [Google Scholar] [CrossRef]
- Serengil, Y.; Augustaitis, A.; Bytnerowicz, A.; Grulke, N.; Kozovitz, A.R.; Matyssek, R.; Müller-Starck, G.; Schaub, M.; Wieser, G.; Coskun, A.A.; Paoletti, E. Adaptation of forest ecosystems to air pollution and climate change: A global assessment on research priorities. iForest 2010, 4, 44–48. [Google Scholar] [CrossRef]






| Treatment | Parameters | ||
|---|---|---|---|
| ti (DOY) | k (10−2) | R2 | |
| EDU | 144.1 ± 6.8 | 0.025 ± 0.005 | 0.99 |
| WAT | 122.4 ± 4.1 | 0.032 ± 0.007 | 0.97 |
| t-test (p-value) | 0.0002 | 0.11 | |
| Treatment | Xylem Morphology | Hydraulic Traits | |||
|---|---|---|---|---|---|
| Vd (n mm−2) | dm (µm) | DH (µm) | Kst (kg s−1m−1MPa−1) | Hv (10−4) | |
| WAT | 90.5 ± 6.3 | 26.3 ± 2.5 | 38.6 ± 3.3 | 2.2 ± 0.7 | 1.2 ± 0.3 |
| EDU | 90.6 ± 4.3 | 28.0 ± 1.9 | 37.6 ± 2.7 | 2.5 ± 0.6 | 1.5 ± 0.2 |
| t-test (p-value) | 0.98 | 0.31 | 0.67 | 0.45 | 0.02* |
| F = y0 + ax | y0 | a | R2 |
|---|---|---|---|
| WAT | −0.0062 ± 0.0007 | −0.0232 ± 0.001 | 0.988 |
| EDU | −0.0037 ± 0.0008 | −0.0112 ± 0.0007 | 0.975 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannelli, A.; Traversi, M.L.; Anichini, M.; Hoshika, Y.; Fares, S.; Paoletti, E. Effect of Long-Term vs. Short-Term Ambient Ozone Exposure on Radial Stem Growth, Sap Flux and Xylem Morphology of O3-Sensitive Poplar Trees. Forests 2019, 10, 396. https://doi.org/10.3390/f10050396
Giovannelli A, Traversi ML, Anichini M, Hoshika Y, Fares S, Paoletti E. Effect of Long-Term vs. Short-Term Ambient Ozone Exposure on Radial Stem Growth, Sap Flux and Xylem Morphology of O3-Sensitive Poplar Trees. Forests. 2019; 10(5):396. https://doi.org/10.3390/f10050396
Chicago/Turabian StyleGiovannelli, Alessio, Maria Laura Traversi, Monica Anichini, Yasutomo Hoshika, Silvano Fares, and Elena Paoletti. 2019. "Effect of Long-Term vs. Short-Term Ambient Ozone Exposure on Radial Stem Growth, Sap Flux and Xylem Morphology of O3-Sensitive Poplar Trees" Forests 10, no. 5: 396. https://doi.org/10.3390/f10050396
APA StyleGiovannelli, A., Traversi, M. L., Anichini, M., Hoshika, Y., Fares, S., & Paoletti, E. (2019). Effect of Long-Term vs. Short-Term Ambient Ozone Exposure on Radial Stem Growth, Sap Flux and Xylem Morphology of O3-Sensitive Poplar Trees. Forests, 10(5), 396. https://doi.org/10.3390/f10050396








