Effects of Initial Soil Properties on Three-Year Performance of Six Tree Species in Tropical Dry Forest Restoration Plantings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
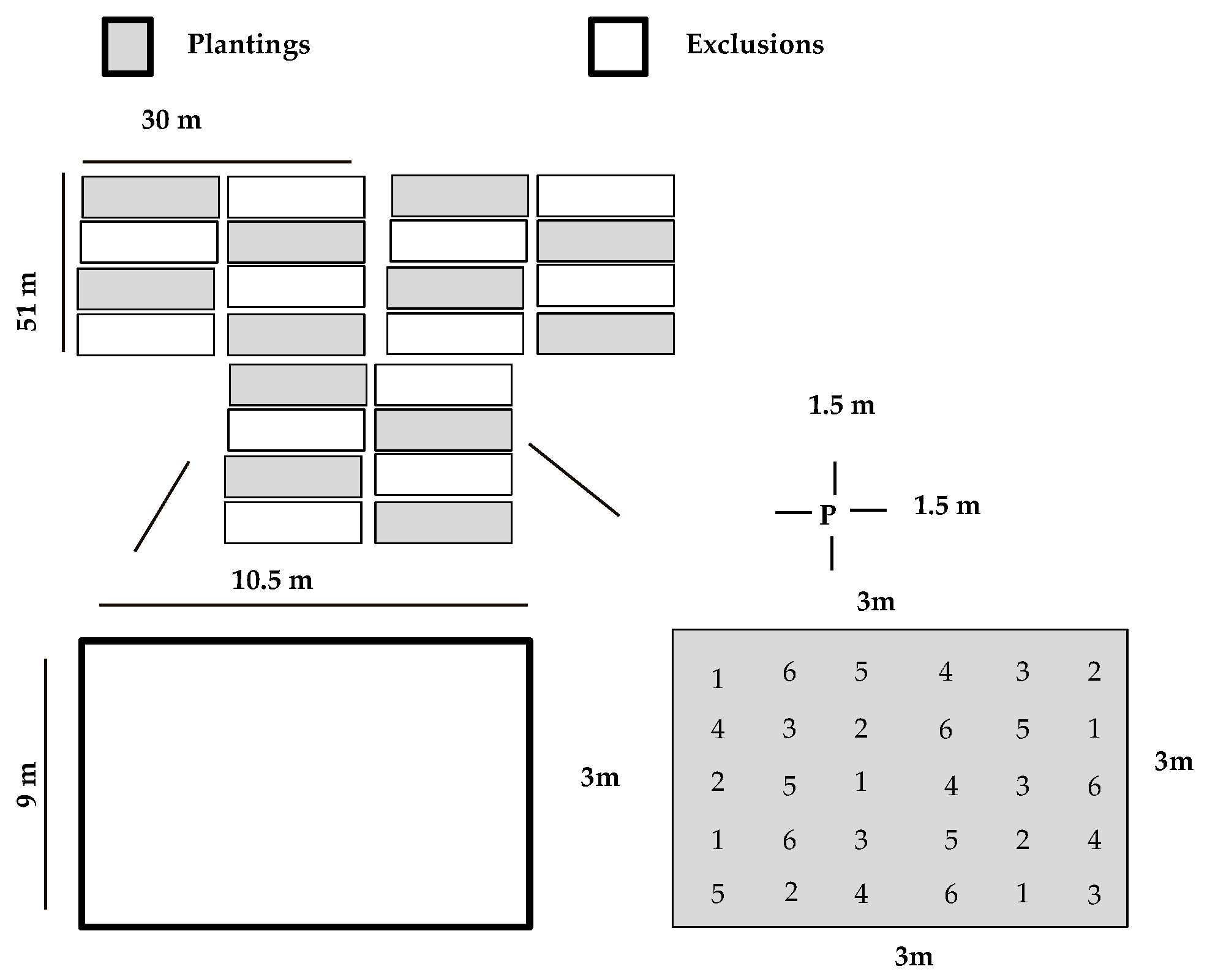
2.2. Plantings Design
2.3. Soil Conditions: Carbon, Nitrogen, and Phosphorus Contents
2.4. Statistical Analysis
3. Results
3.1. Soil Characteristics
3.1.1. Time
3.1.2. Restoration Intervention
3.2. Tree Performance
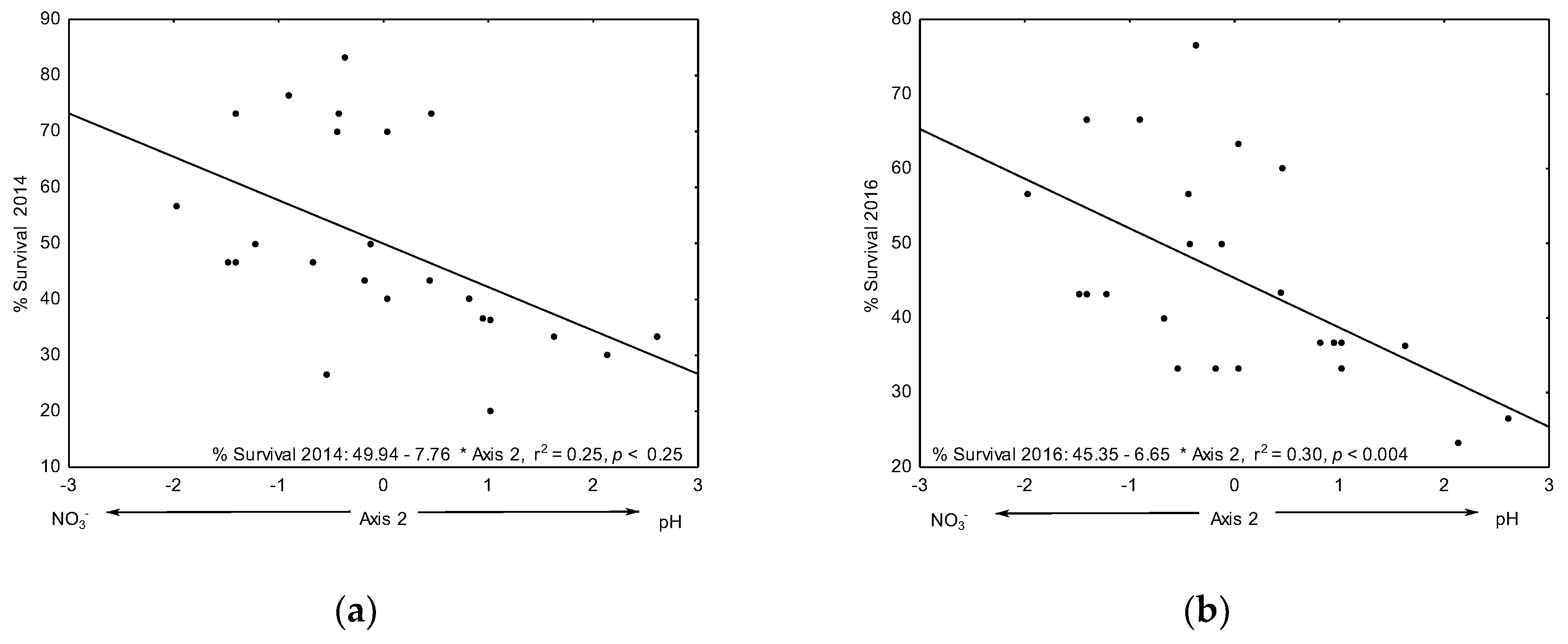
3.2.1. Survival
3.2.2. Height Relative Growth Rate
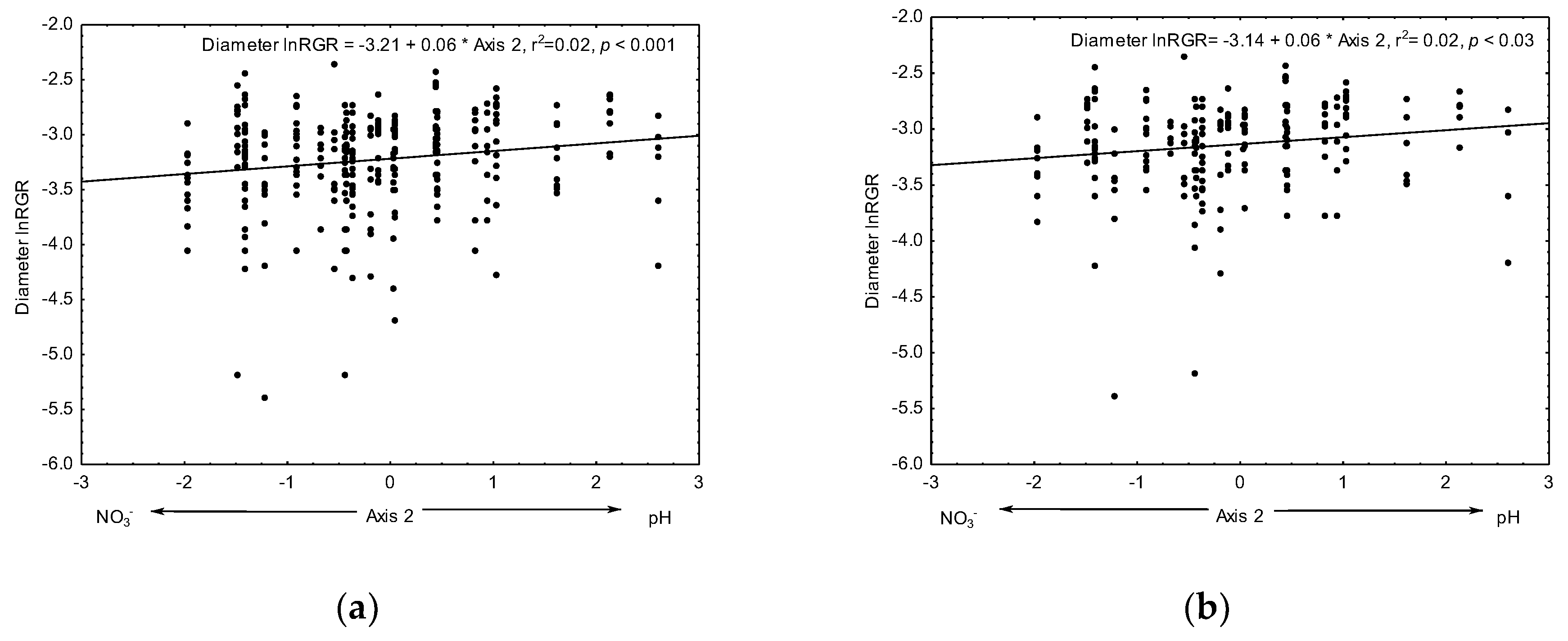
3.2.3. Diameter Relative Growth Rate
3.3. Effect of Initial Soil Properties on Tree Performance
3.3.1. Survival
3.3.2. Relative Growth
4. Discussion
4.1. Soil Conditions
4.2. Restoration Intervention
4.3. Performance of Fast- and Slow-Growing Species
4.4. Effects of Initial Soil Nutrients on Tree Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; E Gordon, J. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Portillo-Quintero, C. Extent and Drivers of Change of Neotropical Seasonally Dry Tropical Forests. In Seasonally Dry Tropical Forests; Island Press: Washington, DC, USA, 2011; pp. 45–57. [Google Scholar]
- Trejo, I.; Dirzo, R. Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biol. Conserv. 2000, 49, 133–142. [Google Scholar] [CrossRef]
- CONABIO. Capital Natural de México, Vol. I: Conocimiento Actual de la Biodiversidad; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, Mexico, 2008. Available online: https://www.biodiversidad.gob.mx/pais/conocimientoActual.html (accessed on 1 April 2016).
- Murphy, P.G.; E Lugo, A. Ecology of Tropical Dry Forest. Annu. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef] [Green Version]
- Ceccon, E.; Huante, P.; Campo, J. Effects of nitrogen and phosphorus fertilization on the survival and recruitment of seedlings of dominant tree species in two abandoned tropical dry forests in Yucatán, Mexico. Ecol. Manag. 2003, 182, 387–402. [Google Scholar] [CrossRef]
- Maass, J.M. Conversion of tropical dry forest to pasture and agriculture. In Seasonally Dry Tropical Forests; Bullock, S.H., Mooney, H.A., Medina, E., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 399–422. [Google Scholar]
- Campo, J. Shift from ecosystem P to N limitation at precipitation gradient in tropical dry forests at Yucatan, Mexico. Environ. Lett. 2016, 11, 95006. [Google Scholar] [CrossRef]
- Kennard, D.K. Secondary forest succession in a tropical dry forest: patterns of development across a 50-year chronosequence in lowland Bolivia. J. Trop. Ecol. 2002, 18, 53–66. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Meave, J.A.; Poorter, L.; Pérez-García, E.A.; Bongers, F. Pathways, mechanisms and predictability of vegetation change during tropical dry forest succession. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Pascarella, J.B.; Marcano-Vega, H.; Aide, T.M.; Zimmerman, J.K.; Rivera, L. Forest Regeneration in a Chronosequence of Tropical Abandoned Pastures: Implications for Restoration Ecology. Restor. Ecol. 2000, 8, 328–338. [Google Scholar]
- Saynes, V.; Hidalgo, C.; Etchevers, J.D.; Campo, J.E. Soil C and N dynamics in primary and secondary seasonally dry tropical forests in Mexico. Appl. Soil Ecol. 2005, 29, 282–289. [Google Scholar] [CrossRef]
- Campo, J.; Maass, M.; Jaramillo, V.J.; Martínez-Yrizar, A.; Sarukhán, J. Phosphorus cycling in a Mexican tropical dry forest ecosystem. Biogeochemistry 2001, 53, 161–179. [Google Scholar] [CrossRef]
- Roa-Fuentes, L.L.; Campo, J.; Parra-Tabla, V. Plant Biomass Allocation across a Precipitation Gradient: An Approach to Seasonally Dry Tropical Forest at Yucatán, Mexico. Ecosystems 2012, 15, 1234–1244. [Google Scholar] [CrossRef]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Martínez-Garza, C.; Osorio-Beristain, M.; Alcala, R.; Valenzuela-Galván, D.; Mariano, N. Ocho años de restauración experimental en las selvas estacionales de México. In Experiencias Mexicanas en la Restauración de los Ecosistemas; Ceccon, E., Martínez-Garza, C., Eds.; CONABIO-UNAM-UAEM: Mexico City, Mexico, 2016; pp. 385–406. [Google Scholar]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of Degraded Tropical Forest Landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef]
- Whitmore, T.C. Canopy Gaps and the Two Major Groups of Forest Trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Swaine, M.D.; Whitmore, T.C. On the definition of ecological species groups in tropical rain forests. Vegetatio 1998, 75, 81–86. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Howe, H.F.; Martínez-Garza, C. Restoring tropical diversity: beating the time tax on species loss. J. Appl. Ecol. 2003, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Holl, K.D.; Zahawi, R.A.; Cole, R.J.; Ostertag, R.; Cordell, S. Planting Seedlings in Tree Islands Versus Plantations as a Large-Scale Tropical Forest Restoration Strategy. Rest. Ecol. 2011, 19, 470–479. [Google Scholar] [CrossRef]
- Huante, P.; Rincon, E.; Acosta, I. Nutrient Availability and growth Rate of 34 Woody Species from a Tropical Deciduous Forest in Mexico. Funct. Ecol. 1995, 9, 849. [Google Scholar] [CrossRef]
- Granados-Victorino, R.L.; Sánchez-González, A.; Martínez-Cabrera, D.; Octavio-Aguilar, P. Estructura y composición arbórea de tres estadios sucesionales de selva mediana subperennifolia del municipio de Huautla, Hidalgo, México. Rev. Mex. Biodivers. 2017, 88, 122–135. [Google Scholar] [CrossRef]
- Alba-García, L. Reclutamiento de leñosas en la selva seca de la localidad de El Limón de Cuauchichinola, Sierra de Huautla, Morelos, México. Ph.D. Thesis, Universidad Autónoma del Estado de Morelos, Cuernavaca, Morelos, Mexico, August 2011. [Google Scholar]
- Casas, A.; Vázquez, M.D.C.; Viveros, J.L.; Caballero, J. Plant management among the Nahua and the Mixtec in the Balsas River Basin, Mexico: An ethnobotanical approach to the study of plant domestication. Hum. Ecol. 1996, 24, 455–478. [Google Scholar] [CrossRef]
- Esquivel, M.J.; Harvey, C.A.; Finegan, B.; Casanoves, F.; Skarpe, C.; Nieuwenhuyse, A. Regeneración Natural de Árboles y Arbustos en Potreros Activos de Nicaragua. Available online: http://repositorio.bibliotecaorton.catie.ac.cr/handle/11554/6769?show=full (accessed on 1 April 2016).
- Bonfil, C.; Trejo, I. Plant Propagation and the Ecological Restoration of Mexican Tropical Deciduous Forests. Ecol. Restor. 2010, 28, 369–376. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, J.S. Experiments on Ecological Restoration of Coal Mine Spoil using Native Trees in a Dry Tropical Environment, India: A Synthesis. New For. 2006, 31, 25–39. [Google Scholar] [CrossRef]
- Mariano, N.A.; Martínez-Garza, C.; Alcalá, R.E. Differential herbivory and successional status in five tropical tree species. Rev. Mex. Biodivers. 2018, 89, 1107–1114. [Google Scholar] [CrossRef]
- González-Tokman, D.M.; Barradas, V.L.; Boege, K.; Domínguez, C.A.; del-Val, E.; Saucedo, E.; Martínez-Garza, C. Performance of 11 tree species under different management treatments in restoration plantings in a tropical dry forest. Rest. Ecol. 2018, 26, 642–649. [Google Scholar] [CrossRef]
- Cheesman, A.W.; Preece, N.D.; Van Oosterzee, P.; Erskine, P.D.; Cernusak, L.A.; Oosterzee, P. The role of topography and plant functional traits in determining tropical reforestation success. J. Appl. Ecol. 2017, 55, 1029–1039. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Campo, J.; Ricker, M.; Tobón, W. Effect of initial soil properties on six-year growth of 15 tree species in tropical restoration plantings. Ecol. Evol. 2016, 6, 8686–8694. [Google Scholar] [CrossRef]
- Huante, P. Growth responses of tropical deciduous tree seedlings to contrasting light conditions. Trees 1993, 7, 202–207. [Google Scholar]
- Huante, P. Influence of mineral nutrient availability on growth of tree seedlings from the tropical deciduous forest. Trees 1994, 9, 93–97. [Google Scholar]
- Markesteijn, L.; Poorter, L.; Bongers, F.; Paz, H.; Sack, L. Hydraulics and life history of tropical dry forest tree species: coordination of species’ drought and shade tolerance. New Phytol. 2011, 191, 480–495. [Google Scholar] [CrossRef] [Green Version]
- Dorado, O.; Maldonado, B.; Arias, D.M.; Sorani, V.; Ramírez, R.; Leyva, E.; Valenzuela, D. Programa de Conservación y Manejo Reserva de la Biosfera Sierra de Huautla, México; CONANP: Mexico City, Mexico, 2005; pp. 26–36.
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen (Para Adaptarlo a las Condiciones de la República Mexicana), 5th ed.; Instituto de Geología, UNAM: Mexico City, Mexico, 2004; pp. 19–20. [Google Scholar]
- CONAGUA. Servicio Metereólogico Nacional de la Comisión del Agua. Available online: https://smn.cna.gob.mx/es/ (accessed on 10 February 2016).
- CONAGUA. Comisión Nacional del Agua. Gerencia Regional Balsas. Available online: http://smn.cna.gob.mx/climatologia/normales/estacion/mor/NORMAL17057.TXT (accessed on 10 February 2016).
- García-Soriano, O.; Poblador de Quilamula, Morelos, Mexico; García-Soriano, E.; Poblador de Quilamula, Morelos, Mexico; Sánchez-García, H.; Poblador de Quilamula, Morelos, Mexico. Personal Communication, 2013.
- Siebe, C.; Jahn, R.; Stahr, K. Manual Para la Descripción y Evaluación Ecológica de Suelos en Campo, 2nd ed.; Instituto de Geología-UNAM: Mexico City, Mexico, 2006. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification Systems for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Rev. Mex. de Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef] [Green Version]
- Tropicos. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 26 November 2018).
- Ceccon, E.; Sanchez, S.; Campo, J. Tree seedling dynamics in two abandoned tropical dry forests of differing successional status in Yucatán, Mexico: a field experiment with N and P fertilization. Plant Ecol. 2004, 170, 277–285. [Google Scholar] [CrossRef]
- Anderson, J.; Ingram, J. Tropical Soil Biology and Fertility: A Handbook of Methods; C.A.B. International: Wallingford, UK, 1993. [Google Scholar]
- StatSoft, I. STATISTICA for Windows [computer program manual]. 7.0. Available online: http://www.statsoft.com (accessed on 10 May 2019).
- Zar, H.J. Biostatistical Analysis, 3rd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Ricker, M.; Ramírez, V.M.P.; Von Rosen, D. A New Method to Compare Statistical Tree Growth Curves: The PL-GMANOVA Model and Its Application with Dendrochronological Data. PLOS ONE 2014, 9, 112396. [Google Scholar] [CrossRef]
- Jara, P.; Martinez, E.; Campo, J. N and P dynamics in the litter layer and soil of Mexican semi-arid forests, state of Morelos. Agric. Ecosyst. 2009, 130, 164–170. [Google Scholar] [CrossRef]
- Paul, K.; Polglase, P.; Nyakuengama, J.; Khanna, P. Change in soil carbon following afforestation. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Parrotta, J.A. Productivity, nutrient cycling, and succession in single- and mixed-species plantations of Casuarina equisetifolia, Eucalyptus robusta, and Leucaena leucocephala in Puerto Rico. Ecol. Manag. 1999, 124, 45–77. [Google Scholar] [CrossRef]
- Carrasco-Carballido, V. Early recruitment dynamics in tropical dry forest restoration settings. Manuscript in preparation.
- Tripathi, N.; Singh, R.S. Influence of different land uses on soil nitrogen transformations after conversion from an Indian dry tropical forest. CATENA 2009, 77, 216–223. [Google Scholar] [CrossRef]
- Yates, C.J.; Norton, D.A.; Hobbs, R.J. Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: implications for restoration. Austral. Ecol. 2000, 25, 36–47. [Google Scholar] [CrossRef]
- Raghubanshi, A.S.; Srivastava, S.C.; Singh, R.S.; Singh, J.S. Nutrient release in leaf litter. Nat. Cell Boil. 1990, 346, 227. [Google Scholar] [CrossRef]
- Yang, M.; Yang, D.; Yu, X. Soil microbial communities and enzyme activities in sea-buckthorn (Hippophae rhamnoides) plantation at different ages. PLOS ONE 2018, 13, e0190959. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Singh, S.D.; Muralikrishna, K.S.; Pathak, H.; Saha, N.D. Soil microbial responses as influenced by Jatropha plantation under rainfed condition in north-west India. Agrofor. Syst. 2018, 92, 47–58. [Google Scholar] [CrossRef]
- Ahirwal, J.; Maiti, S.K. Development of Technosol properties and recovery of carbon stock after 16 years of revegetation on coal mine degraded lands, India. CATENA 2018, 166, 114–123. [Google Scholar] [CrossRef]
- Stern, M.; Quesada, M.; Stoner, K. Changes in Composition and Structure of a Tropical Dry Forest Following Intermittent Cattle Grazing. Rev. Biol. Trop. 2002, 50, 1021–1034. [Google Scholar]
- Griscom, H.P.; Griscom, B.W.; Ashton, M.S. Forest Regeneration from Pasture in the Dry Tropics of Panama: Effects of Cattle, Exotic Grass, and Forested Riparia. Restor. Ecol. 2009, 17, 117–126. [Google Scholar] [CrossRef]
- Gerhardt, K. Effects of root competition and canopy openness on survival and growth of tree seedlings in a tropical seasonal dry forest. Ecol. Manag. 1996, 82, 33–48. [Google Scholar] [CrossRef]
- Barajas-Guzmán, M.G.; Campo, J.; Barradas, V.L. Soil water, nutrient availability and sapling survival under organic and polyethylene mulch in a seasonally dry tropical forest. Plant Soil 2006, 287, 347–357. [Google Scholar] [CrossRef]
- Sampaio, A.B.; Holl, K.D.; Scariot, A. Does Restoration Enhance Regeneration of Seasonal Deciduous Forests in Pastures in Central Brazil? Restor. Ecol. 2007, 15, 462–471. [Google Scholar] [CrossRef]
- Thomas, S.C. Asymptotic height as a predictor of growth and allometric characteristics in malaysian rain forest trees. Am. J. Bot. 1996, 83, 556–566. [Google Scholar] [CrossRef]
- Huante, P.; Rincón, E.; Chapin, F.S., III. Foraging for nutrients, responses to changes in light, and competition in tropical deciduous tree seedlings. Oecologia 1998, 117, 209–216. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Tobon, W.; Campo, J.; Howe, H.F. Drought mortality of tree seedlings in an eroded tropical pasture. Land Degrad. Develop. 2013, 24, 287–295. [Google Scholar] [CrossRef]
- Núñez-Cruz, A.; Bonfil, C. Establecimiento inicial de tres especies del Bosque Tropical Seco en un pastizal degradado: efectos del uso de acolchado y composta. Agrociencias 2013, 47, 609–620. [Google Scholar]
- Bonfil, C.; Barrales-Alcalá, B.; Mendoza-Hernández, P.E.; Alavez, M.; García-Barrios, R. Los límites sociales del manejo y la restauración de ecosistemas: una historia en Morelos. In Experiencias Mexicanas en la Restauración de Los Ecosistemas; Ceccon, E., Martínez-Garza, C., Eds.; CONABIO-UNAM-, UAEM: Mexico City-Cuernavaca, Morelos, Mexico, 2016; pp. 323–345. [Google Scholar]
- Carrasco-Carballido, V. Evaluación de la sucesión natural y la recuperación de la función del ecosistema en la Selva Baja Caducifolia. Manuscript in preparation.
- Reich, P.B.; Hobbie, S.E.; Lee, T.; Ellsworth, D.S.; West, J.B.; Tilman, D.; Knops, J.M.H.; Naeem, S.; Trost, J. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 2005, 440, 922–925. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Peba, L.; Parra-Tabla, V.; Campo, J.; Munguía-Rosas, M.A. Survival and growth of dominant tree seedlings in seasonally tropical dry forests of Yucatan: site and fertilization effects. J. Plant Ecol. 2013, 7, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Román-Dañobeytia, F.J.; Levy-Tacher, S.I.; Aronson, J.; Rodrigues, R.R.; Castellanos-Albores, J. Testing the Performance of Fourteen Native Tropical Tree Species in Two Abandoned Pastures of the Lacandon Rainforest Region of Chiapas, Mexico. Restor. Ecol. 2012, 20, 378–386. [Google Scholar] [CrossRef]
- Paul, M.; Catterall, C.P.; Pollard, P.C.; Kanowski, J. Recovery of soil properties and functions in different rainforest restoration pathways. Ecol. Manag. 2010, 259, 2083–2092. [Google Scholar] [CrossRef]
- Singh, J.S.; Raghubanshi, A.S.; Singh, R.S.; Srivastava, S.C. Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nat. Cell Boil. 1989, 338, 499–500. [Google Scholar] [CrossRef]
- Roy, S.; Singh, J. Seasonal and spatial dynamics of plant-available N and P pools and N-mineralization in relation to fine roots in a dry tropical forest habitat. Soil Boil. Biochem. 1995, 27, 33–40. [Google Scholar] [CrossRef]
- Chapin, F.S.; Vitousek, P.M.; Van Cleve, K. The Nature of Nutrient Limitation in Plant Communities. Am. Nat. 1986, 127, 48–58. [Google Scholar] [CrossRef]
- Rivero-Villar, A.; Templer, P.H.; Parra-Tabla, V.; Campo, J. Differences in nitrogen cycling between tropical dry forests with contrasting precipitation revealed by stable isotopes of nitrogen in plants and soils. Biotropica 2018, 50, 859–867. [Google Scholar] [CrossRef]




| Species | Family | Reference |
|---|---|---|
| Slow-growing species | ||
| Erythrina americana Mill. | Fabaceae | [23] |
| Crescentia alata Kunth | Bignoniaceae | [45] |
| Lysiloma divaricatum (Jacq.) J.F. Macbr. | Fabaceae | [24] |
| Fast-growing species | ||
| Spondias mombin L. | Anacardiaceae | [26] |
| Acacia coulteri Benth. 1 | Fabaceae | [23] |
| Leucaena esculenta (DC) Benth. | Fabaceae | [25] |
| Soil Properties | Year | Restoration Intervention | ||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | F (1, 92) | Minimal | Maximal | F (1,92) | |
| pH | 6.61 ± 0.10 | 6.63 ± 0.11 | 0.020 ns | 6.57 ± 0.10 | 6.66 ± 0.11 | 0.39 ns |
| SOC (g m−2) | 19.16 ± 0.54 | 24.16 ± 0.97 | 19.71 ** | 21.8 ± 0.79 | 21.4 ± 0.93 | 0.150 ns |
| total N (g m−2) | 1.55 ± 0.04 | 1.54 ± 0.05 | 0.2 ns | 1.56 ± 0.04 | 1.53 ± 0.05 | 0.18 ns |
| C:N ratio | 12.48 ± 0.3 | 15.94 ± 0.6 | 25.97 ** | 14.1 ± 0.47 | 14.2 ± 0.05 | 0.23 ns |
| NH4+ (g m−2) | 76.61 ± 3.85 | 20.34 ± 0.66 | 206.27 *** | 46.7 ± 4.7 | 50.1 ± 5.08 | 0.73 ns |
| NO3− (g m−2) | 53.12 ± 4.3 | 11.42 ± 0.95 | 133.4 *** | 32.9 ± 4.45 | 31.6 ± 4.25 | 0.14 ns |
| NO3−:NH4+ | 0.74 ± 0.6 | 0.54 ± 0.35 | 6.62 * | 0.68 ± 0.06 | 0.60 ± 0.04 | 0.08 ns |
| total P (mg m−2) | 107.13 ± 2.56 | 106.84 ± 3.69 | 0.004 ns | 107.9 ± 2.9 | 106.7 ± 3.42 | 0.18 ns |
| available P (mg m−2) | 11.45 ± 0.30 | 10.54 ± 0.44 | 2.85 ns | 10.5 ± 0.38 | 11.2 ± 0.38 | 0.85 ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Carballido, V.; Martínez-Garza, C.; Jiménez-Hernández, H.; Márquez-Torres, F.; Campo, J. Effects of Initial Soil Properties on Three-Year Performance of Six Tree Species in Tropical Dry Forest Restoration Plantings. Forests 2019, 10, 428. https://doi.org/10.3390/f10050428
Carrasco-Carballido V, Martínez-Garza C, Jiménez-Hernández H, Márquez-Torres F, Campo J. Effects of Initial Soil Properties on Three-Year Performance of Six Tree Species in Tropical Dry Forest Restoration Plantings. Forests. 2019; 10(5):428. https://doi.org/10.3390/f10050428
Chicago/Turabian StyleCarrasco-Carballido, Valentina, Cristina Martínez-Garza, Héctor Jiménez-Hernández, Flavio Márquez-Torres, and Julio Campo. 2019. "Effects of Initial Soil Properties on Three-Year Performance of Six Tree Species in Tropical Dry Forest Restoration Plantings" Forests 10, no. 5: 428. https://doi.org/10.3390/f10050428





