Phytophthora ramorum and Phytophthora gonapodyides Differently Colonize and Contribute to the Decomposition of Green and Senesced Umbellularia californica Leaves in a Simulated Stream Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Overview
2.2. Experiment Preparation
2.2.1. Leaves
2.2.2. Microcosms and Water
2.2.3. Phytophthora Inoculum
2.2.4. Experiment Conditions and Sampling
2.3. Data Collection
2.4. Analysis
2.4.1. Phytophthora Colonization
2.4.2. Leaf Decomposition
3. Results
3.1. Phytophthora Leaf Colonization
3.2. Sporulation
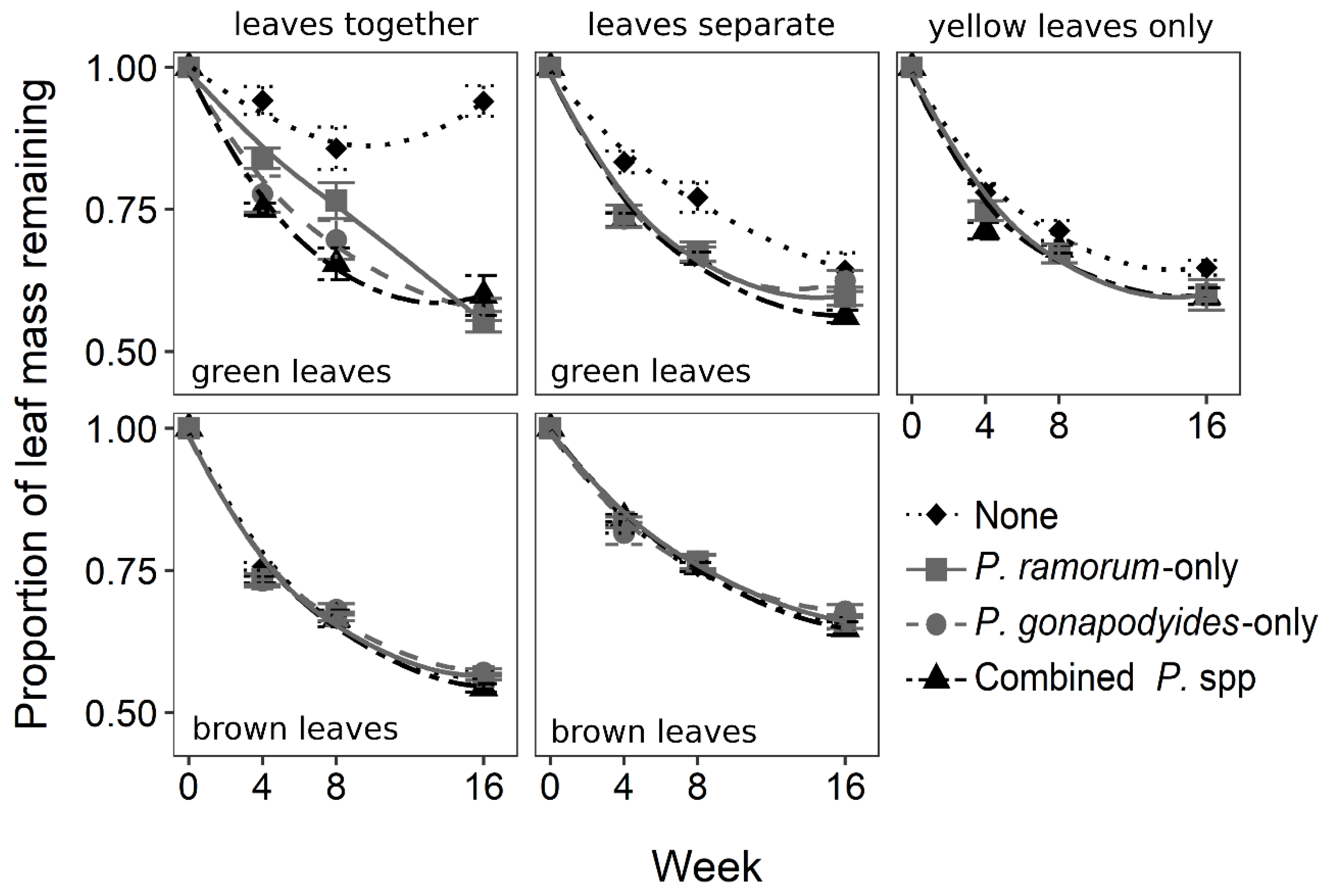
3.3. Leaf Decomposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erwin, D.C.; Ribeiro, O.K. Phytophthora diseases worldwide.; APS Press: St. Paul, Minnesota, USA, 1996; ISBN 978-0-89054-212-. [Google Scholar]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Phytophthora beyond agriculture. Annu. Rev. Phytopathol. 2012, 50, 359–378. [Google Scholar] [CrossRef]
- Hansen, E.M. Alien forest pathogens: Phytophthora species are changing world forests. Boreal Environ. Res. 2008, 13, 33–44. [Google Scholar]
- Kroon, L.P.; Brouwer, H.; de Cock, A.W.; Govers, F. The genus Phytophthora anno 2012. Phytopathology 2012, 102, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Cooke, D.E.L.; Duncan, J.M.; Hansen, E.M. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides-P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycol. Res. 2003, 107, 277–290. [Google Scholar] [CrossRef]
- Brazee, N.J.; Wick, R.L.; Hulvey, J.P. Phytophthora species recovered from the Connecticut River Valley in Massachusetts, USA. Mycologia 2016, 108, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Chastagner, G.; Oak, S.; Omdal, D.; Ramsey-Kroll, A.; Coats, K.; Valachovic, Y.; Lee, C.; Hwang, J.; Jeffers, S.; Elliott, M.; et al. Spread of Phytophthora ramorum from nurseries into waterways—implications for pathogen establishment in new areas. In Proceedings of the Sudden Oak Death Fourth Science Symposium; Gen. Tech. Rep. PSW-GTR-229; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2010; pp. 22–28. [Google Scholar]
- Dunstan, W.A.; Howard, K.; Hardy, G.E.S.; Burgess, T.I. An overview of Australia’s Phytophthora species assemblage in natural ecosystems recovered from a survey in Victoria. IMA Fungus 2016, 7, 47–58. [Google Scholar] [CrossRef]
- Greslebin, A.G.; Hansen, E.M.; Winton, L.M.; Rajchenberg, M. Phytophthora species from declining Austrocedrus chilensis forests in Patagonia, Argentina. Mycologia 2005, 97, 218–228. [Google Scholar] [CrossRef]
- Hansen, E.M.; Reeser, P.W.; Sutton, W. Phytophthora borealis and Phytophthora riparia, new species in Phytophthora ITS Clade 6. Mycologia 2012, 11–349. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Gallegly, M.E.; Richardson, P.A.; Kong, P.; Moorman, G.W. Phytophthora irrigata, a new species isolated from irrigation reservoirs and rivers in Eastern United States of America. FEMS Microbiol. Lett. 2008, 285, 203–211. [Google Scholar] [CrossRef]
- Huai, W.-X.; Tian, G.; Hansen, E.M.; Zhao, W.-X.; Goheen, E.M.; Gruenwald, N.J.; Cheng, C. Identification of Phytophthora species baited and isolated from forest soil and streams in northwestern Yunnan province, China. For. Pathol. 2013, 43, 87–103. [Google Scholar]
- Hwang, J.; Oak, S.W.; Jeffers, S.N. Recovery of Phytophthora species from drainage points and tributaries within two forest stream networks: a preliminary report. N. Z. J. For. Sci. 2011, 41S, S83–S87. [Google Scholar]
- Jung, T.; Stukely, M.J.C.; Hardy, G.E.S.T.J.; White, D.; Paap, T.; Dunstan, W.A.; Burgess, T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: evolutionary and ecological implications. Persoonia - Mol. Phylogeny Evol. Fungi 2011, 26, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Nagel, J.H.; Slippers, B.; Wingfield, M.J.; Gryzenhout, M. Multiple Phytophthora species associated with a single riparian ecosystem in South Africa. Mycologia 2015, 107, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Gryzenhout, M.; Wingfield, B.D.; Wingfield, M.J.; Burgess, T.I. Surveys of soil and water reveal a goldmine of Phytophthora diversity in South African natural ecosystems. IMA Fungus 2013, 4, 123–131. [Google Scholar] [CrossRef]
- Reeser, P.W.; Sutton, W.; Hansen, E.M.; Remigi, P.; Adams, G.C. Phytophthora species in forest streams in Oregon and Alaska. Mycologia 2011, 103, 22–35. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Zhou, Y.; Lamour, K. Oomycetes baited from streams in Tennessee 2010–2012. Mycologia 2013, 105, 1516–1523. [Google Scholar] [CrossRef]
- Sims, L.L.; Sutton, W.; Reeser, P.; Hansen, E.M. The Phytophthora species assemblage and diversity in riparian alder ecosystems of western Oregon, USA. Mycologia 2015, 107, 889–902. [Google Scholar] [CrossRef]
- Stamler, R.A.; Sanogo, S.; Goldberg, N.P.; Randall, J.J. Phytophthora Species in Rivers and Streams of the Southwestern United States. Appl. Environ. Microbiol. 2016, 82, 4696–4704. [Google Scholar] [CrossRef]
- Bily, D.S.; Diehl, S.V.; Cook, M.; Wallace, L.E.; Sims, L.L.; Watson, C.; Baird, R.E. Temporal and Locational Variations of a Phytophthora spp. Community in an Urban Forested Water Drainage and Stream-runoff System. Southeast. Nat. 2018, 17, 176–201. [Google Scholar] [CrossRef]
- Jung, T.; Durán, A.; von Stowasser, E.S.; Schena, L.; Mosca, S.; Fajardo, S.; González, M.; Ortega, A.D.N.; Bakonyi, J.; Seress, D.; et al. Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. For. Pathol. 2018, 48, e12443. [Google Scholar] [CrossRef]
- Hansen, E.M.; Wilcox, W.F.; Reeser, P.W.; Sutton, W. Phytophthora rosacearum and P. sansomeana, new species segregated from the Phytophthora megasperma “complex”. Mycologia 2009, 101, 129–135. [Google Scholar] [CrossRef]
- Hüberli, D.; Hardy, G.S.J.; White, D.; Williams, N.; Burgess, T.I. Fishing for Phytophthora from Western Australia’s waterways: a distribution and diversity survey. Australas. Plant Pathol. 2013, 42, 251–260. [Google Scholar] [CrossRef]
- Hulvey, J.; Gobena, D.; Finley, L.; Lamour, K. Co-occurrence and genotypic distribution of Phytophthora species recovered from watersheds and plant nurseries of eastern Tennessee. Mycologia 2010, 102, 1127–1133. [Google Scholar] [CrossRef]
- Hwang, J.; Jeffers, S.N.; Oak, S.W. Aquatic habitats-a reservoir for population diversity in the genus Phytophthora. Phytopathology 2010, 100, S150–S151. [Google Scholar]
- Marano, A.V.; Jesus, A.L.; de Souza, J.I.; Jerônimo, G.H.; Gonçalves, D.R.; Boro, M.C.; Rocha, S.C.O.; Pires-Zottarelli, C.L.A. Ecological roles of saprotrophic Peronosporales (Oomycetes, Straminipila) in natural environments. Fungal Ecol. 2016, 19, 77–88. [Google Scholar] [CrossRef]
- Hardham, A.R.; Blackman, L.M. Molecular cytology of Phytophthora-plant interactions. Australas. Plant Pathol. 2010, 39, 29–35. [Google Scholar] [CrossRef]
- Gessner, M.O.; Chauvet, E.; Dobson, M. A Perspective on Leaf Litter Breakdown in Streams. Oikos 1999, 85, 377–384. [Google Scholar] [CrossRef]
- Gessner, M.O.; Schwoerbel, J. Leaching kinetics of fresh leaf-litter with implications for the current concept of leaf-processing in streams. Arch. Für Hydrobiol. 1989, 115, 81–90. [Google Scholar]
- Bärlocher, F.; Boddy, L. Aquatic fungal ecology–How does it differ from terrestrial? Fungal Ecol. 2016, 19, 5–13. [Google Scholar] [CrossRef]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Effects of Resource Limitation on a Detrital-Based Ecosystem. Ecol. Monogr. 1999, 69, 409–442. [Google Scholar] [CrossRef]
- Cooper, W.S. The Broad-Sclerophyll Vegetation of California. In World Vegetation Types; Eyre, S.R., Ed.; The Geographical Readings series; Palgrave Macmillan: Basingstoke, UK, 1971; pp. 149–156. ISBN 978-0-333-11031-7. [Google Scholar]
- Cobb, R.C.; Eviner, V.T.; Rizzo, D.M. Mortality and community changes drive sudden oak death impacts on litterfall and soil nitrogen cycling. New Phytol. 2013, 200, 422–431. [Google Scholar] [CrossRef]
- Shreve, F. The Vegetation of a Coastal Mountain Range. Ecology 1927, 8, 27–44. [Google Scholar] [CrossRef]
- Rundel, P.W.; Sturmer, S.B. Native plant diversity in riparian communities of the Santa Monica mountains, California. Madroño 1998, 45, 93–100. [Google Scholar]
- Davidson, J.M.; Patterson, H.A.; Wickland, A.C.; Fichtner, E.J.; Rizzo, D.M. Forest type influences transmission of Phytophthora ramorum in California oak woodlands. Phytopathology 2011, 101, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M.; Garbelotto, M.; Hansen, E.M. Phytophthora ramorum: Integrative Research and Management of an Emerging Pathogen in California and Oregon Forests. Annu. Rev. Phytopathol. 2005, 43, 309–335. [Google Scholar] [CrossRef]
- Davidson, J.M.; Wickland, A.C.; Patterson, H.A.; Falk, K.R.; Rizzo, D.M. Transmission of Phytophthora ramorum in Mixed-Evergreen Forest in California. Phytopathology 2005, 95, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.M.; Patterson, H.A.; Rizzo, D.M. Sources of Inoculum for Phytophthora ramorum in a Redwood Forest. Phytopathology 2008, 98, 860–866. [Google Scholar] [CrossRef]
- DiLeo, M.V.; Bostock, R.M.; Rizzo, D.M. Phytophthora ramorum does not cause physiologically significant systemic injury to California bay laurel, its primary reservoir host. Phytopathology 2009, 99, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Elosegi, A.; Pozo, J. Litter Input. In Methods to Study Litter Decomposition; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, Netherlands, 2005; pp. 3–11. ISBN 978-1-4020-3348-3. [Google Scholar]
- Avila-Ospina, L.; Moison, M.; Yoshimoto, K.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Gil Nam, H. Leaf Senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Aram, K.; Rizzo, D.M. Distinct Trophic Specializations Affect How Phytophthora ramorum and Clade 6 Phytophthora spp. Colonize and Persist on Umbellularia californica Leaves in Streams. Phytopathology 2018, 108, 858–869. [Google Scholar] [CrossRef]
- Murphy, S.K.; Lee, C.; Valachovic, Y.; Bienapfl, J.; Mark, W.; Jirka, A.; Owen, D.R.; Smith, T.F.; Rizzo, D.M. Monitoring Phytophthora ramorum distribution in streams within California watersheds. In Proceedings of the Sudden Oak Death Third Symposium; US Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2005; Vol. PSW-GTR-214, pp. 409–411. [Google Scholar]
- Werres, S.; Marwitz, R.; De Cock, A.W.; Bonants, P.J.; De Weerdt, M.; Themann, K.; Ilieva, E.; Baayen, R.P. Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycol. Res. 2001, 105, 1155–1165. [Google Scholar] [CrossRef]
- Hansen, E.M.; Parke, J.L.; Sutton, W. Susceptibility of Oregon Forest Trees and Shrubs to Phytophthora ramorum : A Comparison of Artificial Inoculation and Natural Infection. Plant Dis. 2005, 89, 63–70. [Google Scholar] [CrossRef]
- DiLeo, M.V.; Bostock, R.M.; Rizzo, D.M. Microclimate Impacts Survival and Prevalence of Phytophthora ramorum in Umbellularia californica, a Key Reservoir Host of Sudden Oak Death in Northern California Forests. PLoS ONE 2014, 9, e98195. [Google Scholar] [CrossRef]
- Maloney, P.E.; Lynch, S.C.; Kane, S.F.; Jensen, C.E.; Rizzo, D.M. Establishment of an emerging generalist pathogen in redwood forest communities. J. Ecol. 2005, 93, 899–905. [Google Scholar] [CrossRef]
- Wood, S.E.; Gaskin, J.F.; Langenheim, J.H. Loss of monoterpenes from Umbellularia californica leaf litter. Biochem. Syst. Ecol. 1995, 23, 581–591. [Google Scholar] [CrossRef]
- Medeiros, A.O.; Pascoal, C.; Graça, M.A.S. Diversity and activity of aquatic fungi under low oxygen conditions. Freshw. Biol. 2009, 54, 142–149. [Google Scholar] [CrossRef]
- Bärlocher, F. Leaf Mass Loss Estimated by Litter Bag Technique. In Methods to Study Litter Decomposition; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Dordrecht, Netherlands, 2005; pp. 37–42. ISBN 978-1-4020-3348-3. [Google Scholar]
- Adair, E.C.; Hobbie, S.E.; Hobbie, R.K. Single-pool exponential decomposition models: potential pitfalls in their use in ecological studies. Ecology 2010, 91, 1225–1236. [Google Scholar] [CrossRef]
- Newell, S.Y.; Fell, J.W. Do halophytophthoras (marine Pythiaceae) rapidly occupy fallen leaves by intraleaf mycelial growth. Can. J. Bot. 1995, 73, 761–765. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K. The arcsine is asinine: the analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-128. 2016. Available online: http://CRAN.R-project.org/package=nlme (accessed on 28 March 2019).
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2015. Available online: https://www.R-project.org/ (accessed on 28 March 2019).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69(1), 1–33. [Google Scholar] [CrossRef]
- Johnston, S.F.; Cohen, M.F.; Torok, T.; Meentemeyer, R.K.; Rank, N.E. Host Phenology and Leaf Effects on Susceptibility of California Bay Laurel to Phytophthora ramorum. Phytopathology 2015, 106, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Meshriy, M.; Hüberli, D.; Harnik, T.Y.; Miles, L.; Reuther, K.D.; Garbelotto, M. Variation in susceptibility of Umbellularia californica (Bay Laurel) to Phytophthora ramorum. In Proceedings of the Sudden oak death second science symposium; Gen. Tech. Rep. PSW-GTR-196; Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture: Pacific Southwest Research Station: Albany, CA, USA, 2006; pp. 87–89. [Google Scholar]
- Goralka, R.J.L.; Schumaker, M.A.; Langenheim, J.H. Variation in chemical and physical properties during leaf development in California bay tree (Umbellularia californica): Predictions regarding palatability for deer. Biochem. Syst. Ecol. 1996, 24, 93–103. [Google Scholar] [CrossRef]
- Wong, M.K.; Goh, T.-K.; Hodgkiss, I.J.; Hyde, K.D.; Ranghoo, V.M.; Tsui, C.K.; Ho, W.-H.; Wong, W.S.; Yuen, T.-K. Role of fungi in freshwater ecosystems. Biodivers. Conserv. 1998, 7, 1187–1206. [Google Scholar] [CrossRef]
- Richards, T.A.; Soanes, D.M.; Jones, M.D.M.; Vasieva, O.; Leonard, G.; Paszkiewicz, K.; Foster, P.G.; Hall, N.; Talbot, N.J. Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proc. Natl. Acad. Sci. 2011, 108, 15258–15263. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Yan, H.-Z.; Liu, L.-F.; Liou, R.-F. Functional characterization of a gene family encoding polygalacturonases in Phytophthora parasitica. Mol. Plant. Microbe Interact. 2008, 21, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Blackman, L.M.; Cullerne, D.P.; Torreña, P.; Taylor, J.; Hardham, A.R. RNA-Seq Analysis of the Expression of Genes Encoding Cell Wall Degrading Enzymes during Infection of Lupin (Lupinus angustifolius) by Phytophthora parasitica. PloS ONE 2015, 10, e0136899. [Google Scholar] [CrossRef]
- Beakes, G.W.; Glockling, S.L.; Sekimoto, S. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 2011, 249, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.C.; Whipps, J.M. The Evolution of Modes of Nutrition in Fungi Parasitic on Terrestrial Plants. Biol. Rev. 1980, 55, 341–362. [Google Scholar] [CrossRef]
- Navarro, S.; Sims, L.; Hansen, E. Pathogenicity to alder of Phytophthora species from riparian ecosystems in western Oregon. For. Pathol. 2015, 45, 358–366. [Google Scholar] [CrossRef]
- Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C.; Grünwald, N.J. Phytophthora Community Structure Analyses in Oregon Nurseries Inform Systems Approaches to Disease Management. Phytopathology 2014, 104, 1052–1062. [Google Scholar] [CrossRef]
- Yakabe, L.E.; Blomquist, C.L.; Thomas, S.L.; MacDonald, J.D. Identification and frequency of Phytophthora species associated with foliar diseases in California ornamental nurseries. Plant Dis. 2009, 93, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Andrews, J.; Douhan, L.; Douhan, G.; Rizzo, D.M. Fungi associated with leaves of California bay laurel. Phytopathology 2005, 95, S4. [Google Scholar]
- Aram, K. The Ecology of Phytophthora ramorum and Resident Phytophthora in California Streams. Ph.D. Dissertation, University of California, Davis, Davis, California, 2017. [Google Scholar]
- Graça, M.A.S.; Bärlocher, F.; Gessner, M.O. Methods to study litter decomposition a practical guide; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2005; ISBN 978-1-4020-3466-4. [Google Scholar]
- Themann, K.; Werres, S.; Lüttmann, R.; Diener, H.A. Observations of Phytophthora spp. in Water Recirculation Systems in Commercial Hardy Ornamental Nursery Stock. Eur. J. Plant Pathol. 2002, 108, 337–343. [Google Scholar] [CrossRef]

| wk | day | wk | day | wk | day | wk | day | wk | day | wk | day | wk | day | Total | |||
| 7 | 52 | 10 | 71 | 16 | 111 | 18 | 123 | 19 | 133 | 20 | 141 | 22 | 151 | ||||
| Water | Inoculum | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg |
| nst | Pr | 3 | - | 3 | - | 4 | - | 3 | - | 0 | - | 1 | - | 1 | - | 15 | - |
| st | 5 | - | 5 | - | 5 | - | 3 | - | 3 | - | 2 | - | 0 | - | 23 | - | |
| nst | Pg | - | 5 | - | 5 | - | 5 | - | 4 | - | 0 | - | 0 | - | 1 | - | 20 |
| st | - | 3 | - | 5 | - | 5 | - | 4 | - | 3 | - | 2 | - | 2 | - | 24 | |
| nst | Pr + Pg | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 23 |
| st | 0 | 5 | 0 | 5 | 0 | 5 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 21 | |
| wk | day | wk | Day | wk | day | wk | day | wk | day | wk | day | wk | day | Total | ||||
| 0 | 2 | 10 | 69 | 14 | 100 | 17 | 117 | 19 | 134 | 22 | 153 | 28 | 193 | |||||
| Water | Leaf | Inoculum | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg |
| nst | Green | Pr | 2 | - | 2 | - | 5 | - | 1 | - | 0 | - | 0 | - | 0 | - | 10a | - |
| st | 5 | - | 0 | - | 5 | - | 3 | - | 5 | - | 2 | - | 0 | - | 20 | - | ||
| nst | Brown | 5 | - | 0 | 1 | 2 | - | 0 | - | 1 | - | 0 | - | 0 | - | 8 | 1 | |
| st | 5 | - | 0 | - | 1 | - | 0 | - | 0 | - | 0 | - | 0 | - | 6 | - | ||
| nst | Green | Pg | - | 5 | - | 5 | - | 5 | - | 4 | - | 4 | - | 5 | - | 2 | - | 30 a |
| st | - | 5 | - | 5 | - | 5 | - | 5 | - | 4 | - | 5 | - | 1 | - | 30 | ||
| nst | Brown | - | 5 | - | 5 | - | 5 | - | 3 | - | 2 | - | 4 | - | 2 | - | 26 | |
| st | - | 5 | - | 5 | - | 5 | - | 4 | - | 3 | - | 4 | - | 1 | - | 27 | ||
| nst | Green | Pr + Pg | 0 | 4 | 0 | 3 | 0 | 5 | 0 | 3 | 0 | 4 | 0 | 5 | 0 | 2 | 0 | 26 a |
| st | 0 | 5 | 0 | 4 | 0 | 5 | 0 | 1 | 0 | 3 | 0 | 2 | 0 | 3 | 0 | 23 | ||
| nst | Brown | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 4 | 0 | 1 | 0 | 4 | 0 | 1 | 0 | 25 | |
| st | 1 | 5 | 0 | 5 | 0 | 5 | 0 | 4 | 0 | 1 | 0 | 4 | 0 | 1 | 1 | 25 | ||
| wk | Day | wk | day | wk | day | wk | day | wk | day | wk | day | wk | day | Total | ||
| 8 | 54 | 12 | 85 | 14 | 97 | 15 | 102 | 17 | 119 | 20 | 138 | 25 | 178 | |||
| Inoculum | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg | Pr | Pg |
| Pr | 3 | - | 4 | - | 4 | - | 4 | - | 4 | - | 1 | - | 0 | - | 20 | - |
| Pr + Pg | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aram, K.; Rizzo, D.M. Phytophthora ramorum and Phytophthora gonapodyides Differently Colonize and Contribute to the Decomposition of Green and Senesced Umbellularia californica Leaves in a Simulated Stream Environment. Forests 2019, 10, 434. https://doi.org/10.3390/f10050434
Aram K, Rizzo DM. Phytophthora ramorum and Phytophthora gonapodyides Differently Colonize and Contribute to the Decomposition of Green and Senesced Umbellularia californica Leaves in a Simulated Stream Environment. Forests. 2019; 10(5):434. https://doi.org/10.3390/f10050434
Chicago/Turabian StyleAram, Kamyar, and David M. Rizzo. 2019. "Phytophthora ramorum and Phytophthora gonapodyides Differently Colonize and Contribute to the Decomposition of Green and Senesced Umbellularia californica Leaves in a Simulated Stream Environment" Forests 10, no. 5: 434. https://doi.org/10.3390/f10050434
APA StyleAram, K., & Rizzo, D. M. (2019). Phytophthora ramorum and Phytophthora gonapodyides Differently Colonize and Contribute to the Decomposition of Green and Senesced Umbellularia californica Leaves in a Simulated Stream Environment. Forests, 10(5), 434. https://doi.org/10.3390/f10050434





