Degradation of Ecosystem Services and Deforestation in Landscapes With and Without Incentive-Based Forest Conservation in the Ecuadorian Amazon
Abstract
:1. Introduction
2. Materials and Methods
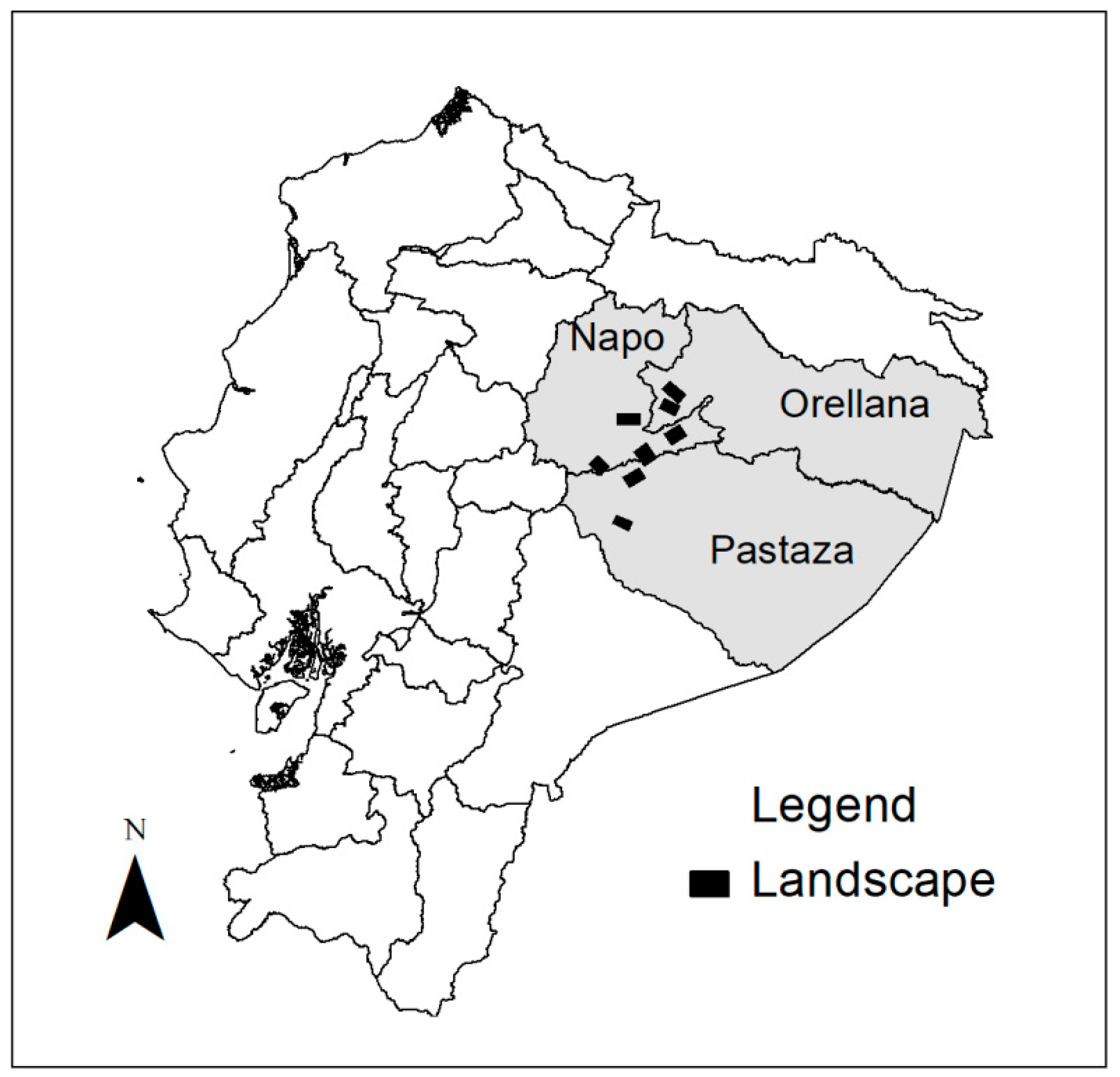
2.1. Study Area
2.2. Data Collection
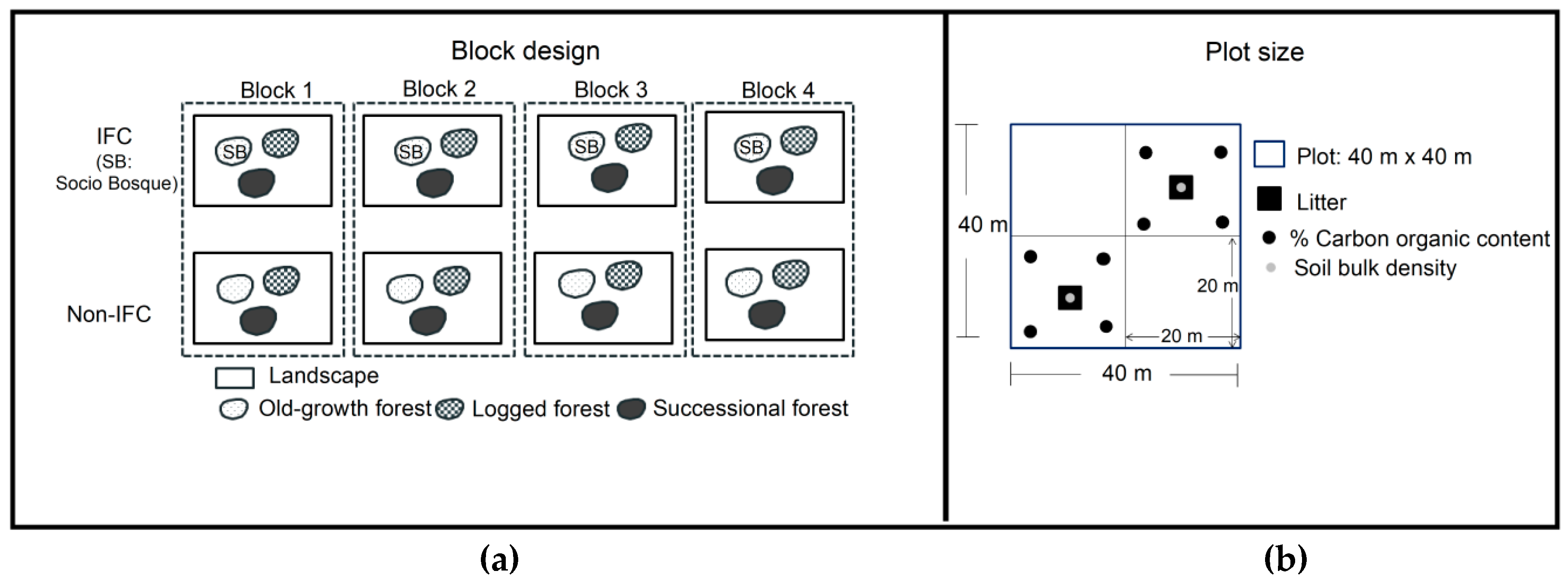
2.2.1. Plot Design
- Ht = Total tree height;
- DBH = Diameter at breast height.
- IVI = Importance index value;
- DoR = Relative dominance;
- DR = Relative density;
- FR = Relative frequency.
- A1 = Forest area at the beginning of the period;
- A2 = Forest area at the end of the period.
2.2.2. Statistical Analysis
3. Results
3.1. Degradation
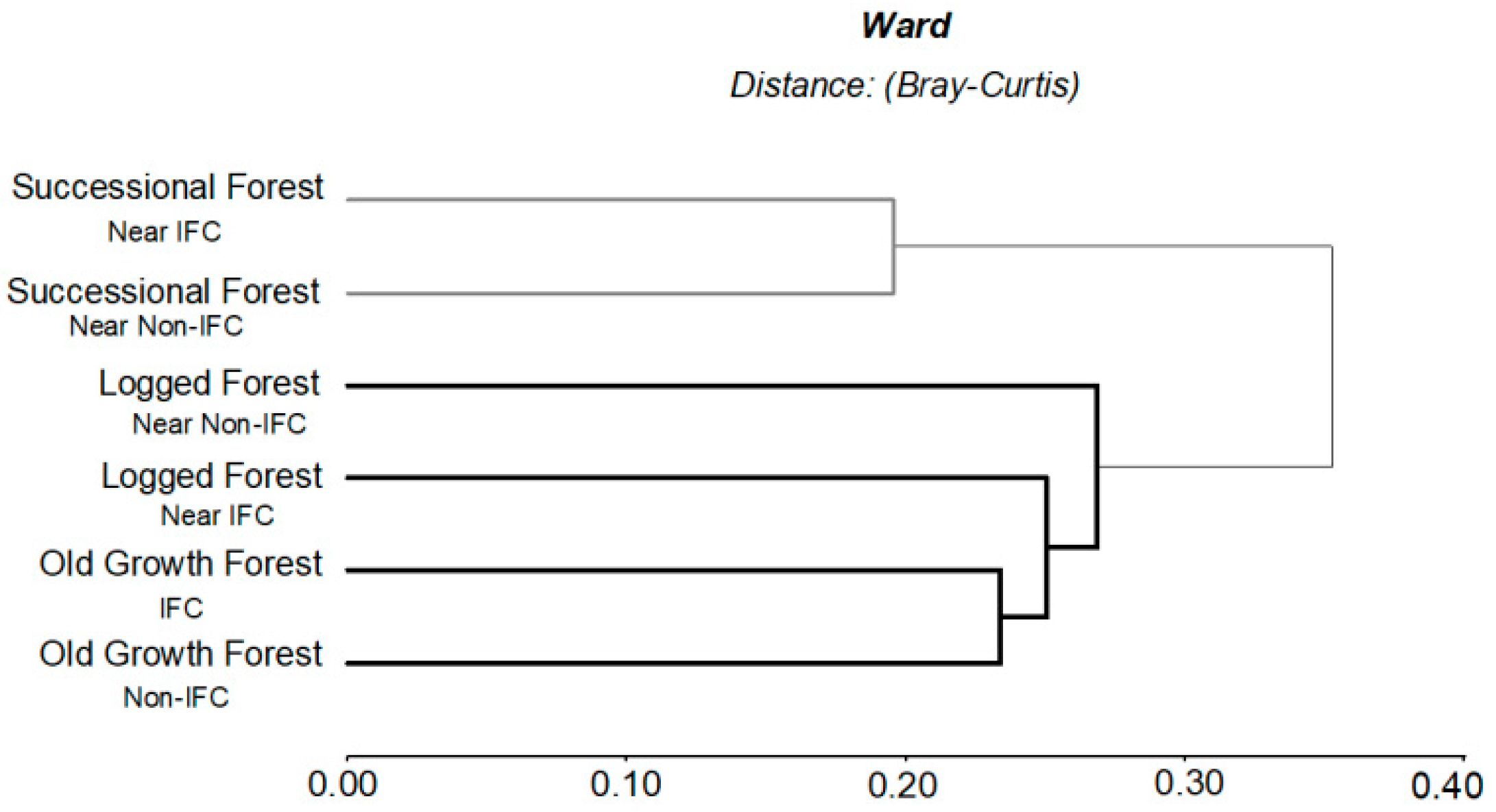
3.1.1. Influence of Forest Types on Ecosystem Services Degradation
3.1.2. Influence of IFC, Non-IFC and Forest Types on Ecosystem Services Degradation
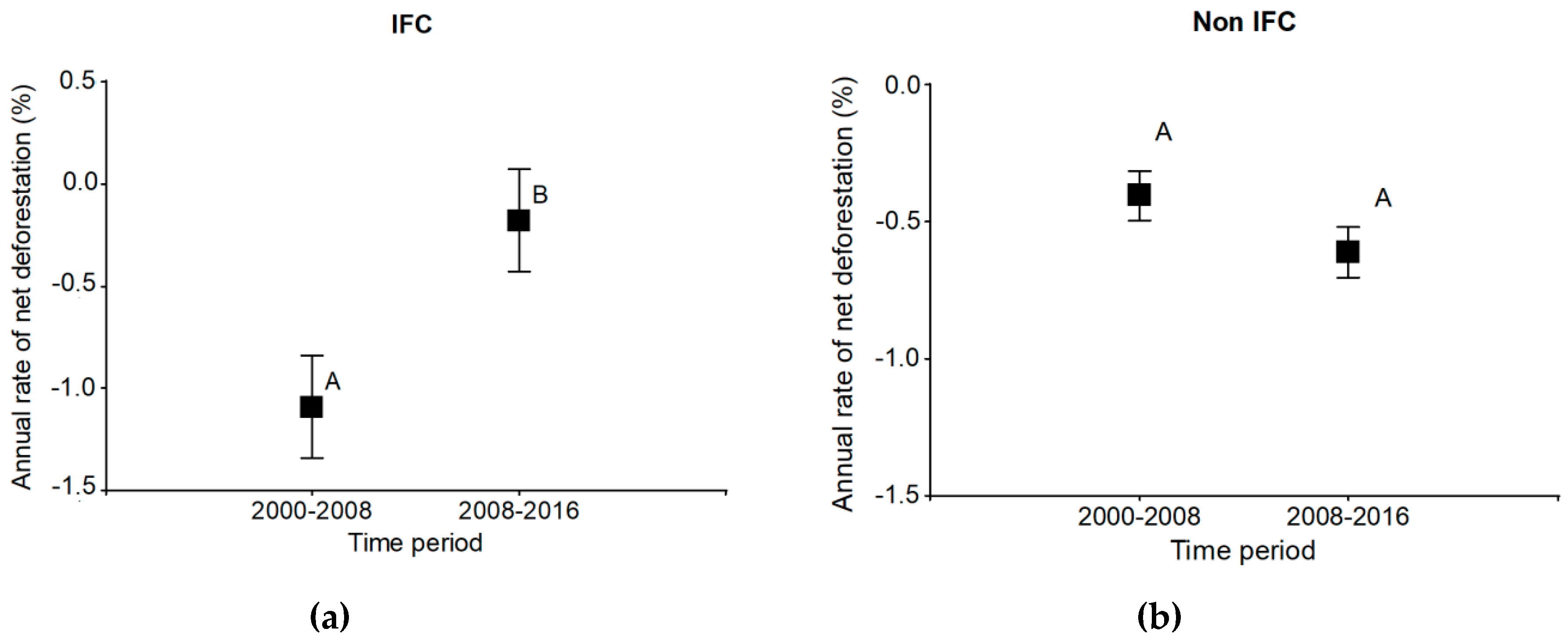
3.2. Deforestation
4. Discussion
4.1. Degradation
4.1.1. Influence of Forest Types on Ecosystem Services Degradation
4.1.2. Influence of IFC, Non-IFC and Forest Types on Ecosystem Services Degradation
4.2. Deforestation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Environmental Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Landscape | Forest Cover 1 (%) | Agricultural Land 1 (%) | Ecosystem 2 | Soil Type 3 | Soil Texture 4 | Mean Distance from Plots to Roads (m) | Altitude m a.s.l. | ||
| (mean) | Min. | Max | |||||||
| IFC-1 (block 1) | 71.52 | 27.31 | tropical lowland forest | Inceptisol/Andisol | medium–moderately coarse | 3328.86 | 887.9 | 560.0 | 1180.0 |
| Non-IFC-1 (block 1) | 57.6 | 39.32 | tropical lowland forest | Inceptisol/Andisol | medium–moderately coarse | 1087.61 | 667.0 | 479.0 | 1049.0 |
| IFC-2 (block 2) | 72.62 | 25.77 | tropical lowland forest | Inceptisol/Andisol | fine | 1312.41 | 525.6 | 403.0 | 758.0 |
| Non-IFC-2 (block 2) | 81.84 | 16.77 | tropical lowland forest | Inceptisol/Andisol | fine–medium | 1302.99 | 568.6 | 403.0 | 1028.0 |
| IFC-3 (block 3) | 65.03 | 28.59 | tropical lowland forest | Inceptisol | fine–medium | 1487.63 | 429.4 | 348.0 | 688.0 |
| Non-IFC-3 (block 3) | 50.17 | 43.13 | tropical lowland forest | Inceptisol | fine–medium | 826.91 | 372.2 | 299.0 | 600.0 |
| IFC-4 (block 4) | 61.83 | 34.21 | tropical lowland forest | Inceptisol/Andisol | fine–medium | 988.60 | 552.7 | 331.0 | 819.0 |
| Non-IFC-4 (block 4) | 49.37 | 48.16 | tropical lowland forest | Inceptisol/Andisol | fine–medium | 1181.18 | 451.3 | 315.0 | 654.0 |
| Demographic Variables (Socio-Economic) | |||
|---|---|---|---|
| Landscape | Population Density (hab. per km2) 1 | Mean Distance from Households within Landscapes to Forest (km) 2 | Distance from Landscape to Large Cities (km) 2 |
| IFC-1 (block 1) | 12.68 | 2.82 | 24.00 |
| Non-IFC-1 (block 1) | 9.44 | 2.65 | 30.42 |
| IFC-2 (block 2) | 9.00 | 2.38 | 46.83 |
| Non-IFC-2 (block 2) | 9.33 | 2.03 | 60.50 |
| IFC-3 (block 3) | 18.09 | 2.05 | 35.84 |
| Non-IFC-3 (block 3) | 21.24 | 1.43 | 58.24 |
| IFC-4 (block 4) | 24.22 | 0.83 | 10.20 |
| Non-IFC-4 (block 4) | 29.77 | 1.12 | 16.80 |
| Factor | IFC vs. non-IFC | ||
|---|---|---|---|
| IFC Mean (std.err) | Non-IFC Mean (std.err) | p-Value | |
| Forest (%) | 67.75 (5.67) | 59.75 (5.67) | 0.3569 |
| Agricultural land (%) | 28.97 (5.07) | 36.85 (5.07) | 0.3143 |
| Mean Altitude m a.s.l. | 598.90 (84.21) | 514.78 (84.21) | 0.5064 |
| Mean Distance from plots to roads (m) | 1779.38 (379.25) | 1099.67 (379.25) | 0.2520 |
| Population Density (hab. per km2) | 16.00 (4.22) | 17.45 (4.22) | 0.8166 |
| Distance from households within the landscapes to forest (km) | 2.02 (0.39) | 1.81 (0.39) | 0.7099 |
| Distance from landscape to large cities (km) | 29.22 (9.39) | 41.49 (9.39) | 0.3911 |
| Variable | IFC (mean/Std. err.) | Non-IFC (mean/Std. err.) | p-Value |
|---|---|---|---|
| Successional forest age | 20 (1.26) | 19 (1.26) | 0.6439 |
| Variable | IFC | S.E. | Non-IFC | S.E. |
|---|---|---|---|---|
| AGC (Mg C·ha−1) | 126.62 | 4.81 | 121.23 | 4.93 |
| DOM (Mg C·ha−1) | 11.37 | 0.54 | 10.94 | 0.52 |
| SOC (Mg C·ha−1) | 51.44 | 5.31 | 48.70 | 5.31 |
| Total carbon (Mg C·ha−1) | 191.77 | 10.43 | 185.97 | 10.43 |
| Volume (m3 ha−1) | 303.39 | 15.65 | 283.23 | 15.94 |
| Richness (#species/plot) | 46.11 | 2.56 | 46.64 | 2.56 |
| Variable | Old Growth Forest | S.E. | Logged Forest | S.E. | Successional Forest | S.E. |
|---|---|---|---|---|---|---|
| AGC (Mg C·ha−1) | 165.63 | 5.87 | 112.97 | 5.87 | 93.17 | 5.75 |
| DOM (Mg C·ha−1) | 11.60 | 0.67 | 11.86 | 0,64 | 10.01 | 0.64 |
| SOC (Mg C·ha−1) | 52.57 | 5.39 | 48.19 | 5.39 | 49.45 | 5.39 |
| Total carbon (Mg C·ha−1) | 236.85 | 10.40 | 176.75 | 10.40 | 153.01 | 10.40 |
| Volume (m3 ha−1) | 387.29 | 16.09 | 264.78 | 16.09 | 227.85 | 16.42 |
| Richness (#species/plot) | 55.17 | 2.26 | 49.92 | 2.26 | 34.04 | 2.26 |
| Variable | Old-Growth Forest with IFC | S.E. | Old-Growth Forest in Non-IFC Landscapes | S.E. | Logged Forest Near IFC | S.E. | Logged Forest in Non-IFC Landscapes | S.E. | Successional Forest Near IFC | S.E. | Successional Forest in Non-IFC landscapes | S.E. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGC (Mg C·ha−1) | 159.36 | 7.94 | 171.90 | 8.28 | 125.37 | 7.94 | 100.57 | 8.28 | 95.13 | 7.94 | 91.20 | 7.94 |
| DOM (Mg C·ha−1) | 12.02 | 0.97 | 11.18 | 0.93 | 12.85 | 0.93 | 10.88 | 0.89 | 9.26 | 0.93 | 10.76 | 0.89 |
| SOC (Mg C·ha−1) | 50.91 | 5.78 | 54.24 | 5.78 | 51.26 | 5.78 | 45.12 | 5.78 | 52.16 | 5.78 | 46.75 | 5.78 |
| Total carbon (Mg C·ha−1) | 227.60 | 12.78 | 246.10 | 12.78 | 190.39 | 12.78 | 163.10 | 12.78 | 157.32 | 12.78 | 148.71 | 12.78 |
| Volume (m3 ha−1) | 380.28 | 22.35 | 394.30 | 23.16 | 283.62 | 23.16 | 245.95 | 22.3 | 246.28 | 22.35 | 209.42 | 24.07 |
| Richness (#species/plot) | 55.17 | 3.19 | 55.17 | 3.19 | 46.67 | 3.19 | 53.17 | 3.19 | 36.50 | 3.19 | 31.58 | 3.19 |
| IFC–Non-IFC | Annual net Deforestation Rate (%) 2000–2008 | Annual net Deforestation Rate (%) 2008–2016 | Tendency ↓↑ |
|---|---|---|---|
| IFC 1 | −0.77 | 0.78 | ↓ |
| Non-IFC 1 | −0.36 | −0.49 | ↑ |
| IFC 2 | −1.23 | −0.33 | ↓ |
| Non-IFC 2 | −0.55 | −0.68 | ↑ |
| IFC 3 | −1.31 | −0.41 | ↓ |
| Non-IFC 3 | −0.53 | −0.43 | ↓ |
| IFC 4 | −1.06 | −0.76 | ↓ |
| Non-IFC 4 | −0.18 | −0.85 | ↑ |
References
- MEA. Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis. Millennium Ecosystem Assessment; Island Press: Washington, DC, USA, 2005; pp. 1–155. [Google Scholar]
- The State of Forests in the Amazon Basin, Congo Basin, and Southeast Asia. A Report Prepared for the Summit of the Three Rainforest Basins; FAO: Rome, Italy, 2011; pp. 1–80. [Google Scholar]
- IPCC. Ecosystems and Their Goods and Services; IPCC: Cambridge, UK, 2001; pp. 237–315. [Google Scholar]
- Saatchi, S.; Harris, N.; Brown, S.; Lefsky, M.; Mitchard, E.; Salas, W.; Zutta, B.; Buermann, W.; Lewis, S.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, K.; Ashton, M.; Craven, D.; Griscom, H. Carbon Dynamics of Tropical Forests; Ashton, M., Tyrrell, M., Spalding, D., Gentry, B., Eds.; Springer: New York, NY, USA, 2012; pp. 51–75. [Google Scholar]
- Tovo, A.; Suweis, S.; Formentin, M.; Favretti, M.; Volkov, I.; Banavar, J.R.; Azaele, S.; Maritan, A. Upscaling species richness and abundances in tropical forests. Sci. Adv. 2017, 3, e1701438. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Riitters, K.H.; Wickham, J.D.; Jones, K.B.; Smith, E.R.; Coulston, J.W.; Wade, T.G.; Smith, J.H.; O’Neill, R.V. Fragmentation of Continental United States Forests. Ecosystems 2002, 5, 815–822. [Google Scholar] [CrossRef]
- Asner, G.P.; Powell, G.V.N.; Mascaro, J.; Knapp, D.E.; Clark, J.K.; Jacobson, J.; Kennedy-Bowdoin, T.; Balaji, A.; Paez-Acosta, G.; Victoria, E.; et al. High-resolution forest carbon stocks and emissions in the Amazon. Proc. Acad. Sci. 2010, 107, 16738–16742. [Google Scholar] [CrossRef] [Green Version]
- Keenan, R.J.; Reams, G.A.; Achard, F.; De Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015. How the World´s Forest Change? 2nd ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; pp. 1–45. [Google Scholar]
- FAO. Assessment of Forest and Carbon Stocks, 1990–2015. Reduce Overall Emissions, but Increase Degration; Food and Agriculture Organization of the United Nations: Roma, Italy, 2015; pp. 1–4. [Google Scholar]
- MAE. Deforestación del Ecuador Continental Periodo 2014–2016; Ministry of Environment: Quito, Ecuador, 2017; pp. 1–37.
- FAO. Global Forest Resources Assessment 2015; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; pp. 1–244. [Google Scholar]
- Lewis, S.L.; Malhi, Y.; Phillips, O. Fingerprinting the impacts of global change on tropical forests. Philos. Trans. R. Soc. Lond. 2004, 359, 437–462. [Google Scholar] [CrossRef]
- Mena, C.; Bilsborrow, R.; McClain, M. Socioeconomic Drivers of Deforestation in the Northern Ecuadorian Amazon. Environ. Manag. 2006, 37, 802–815. [Google Scholar] [CrossRef]
- Sierra, R. Patrones y Factores de Deforestación en el Ecuador Continental, 1990–2010. Y un Acercamiento a los Próximos 10 Años; Conservación Internacional Ecuador y Forest Trends: Quito, Ecuador, 2013; pp. 1–57. [Google Scholar]
- Wasserstrom, R.; Southgate, D. Deforestation, Agrarian Reform and Oil Development in Ecuador, 1964–1994. Nat. Resour. 2013, 4, 31–44. [Google Scholar] [CrossRef]
- Asner, G.; Knapp, D.; Broadbent, E.; Oliveira, P.; Keller, M.; Silva, J. Selective Logging in the Brazilian Amazon. Science 2005, 310, 480–482. [Google Scholar] [CrossRef]
- Blanc, L.; Echard, M.; Herault, B.; Bonal, D.; Marcon, E.; Chave, J.; Baraloto, C. Dynamics of aboveground carbon stocks in a selectively logged tropical forest. Ecol. Appl. 2009, 19, 1397–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.; Tobias, J.; Sheil, D.; Meijaard, E.; Laurance, W. Maintaining ecosystem function and services in logged tropical forests. Trends Ecol. Evol. 2014, 29, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, J.; Asner, G.; Heil, M.; Coe, M.; DeFries, R.; Gibbs, H.; Howard, E.; Olson, S.; Patz, J.; Ramankutty, N.; et al. Amazonia revealed: Forest degradation and loss of ecosystem good and services in the Amazon basin. Ecol. Soc. Am. 2007, 5, 25–32. [Google Scholar] [CrossRef]
- Cardinale, B.; Duffy, E.; Gonzalez, A.; Hooper, D.; Perrings, C.; Venail, P.; Mace, G.; Tilman, D.; Wardle, D.; Kinzig, A.; et al. Biodiversityloss anditsimpactonhumanity. Nature 2012, 486, 59. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; DeFries, R.; Asner, G.; Barford, C.; Bonan, G.; Carpenter, S.; Chapin, S.; Coe, M.; Daily, G.; Gibbs, H.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Berry, P.; Simpson, G.; Haslett, J.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.; Hector, A.; Hooper, D.; Huston, M.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [Green Version]
- Naeem, S.; Thompson, L.; Lawler, S.; Lawton, J.; Woodfin, R. Declining biodiversity can alter the performance of ecosystems. Nature 1997, 368, 734–737. [Google Scholar] [CrossRef]
- Ricketts, T.; Watson, K.; Koh, I.; Ellis, A.; Nicholson, C.; Posner, S.; Sonter, L. Disaggregating the evidence linking biodiversity and ecosystem services. Nat. Commun. 2016, 7, 13106. [Google Scholar] [CrossRef] [Green Version]
- Leadley, P.; Pereira, H.; Alkemade, R.; Fernandez-Manjarrés, J.; Proença, V.; Scharlemann, J.; Walpole, M. Biodiversity Scenarios: Projections of 21st Century Change in Biodiversity and Associated Ecosystem Services; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010; p. 132. [Google Scholar]
- Hososuma, N.; Herold, M.; De-Sy, V.; De-Fries, R.; Brockhaus, M.; Verchot, L.; Angelsen, A.; Romijn, E. An assessment of deforestation and forest degradation drivers in developing countries. Environ. Res. Lett. 2012, 7, 044009. [Google Scholar] [CrossRef]
- Kissinger, G.; Herold, M.; De-Sy, V. Drivers of Deforestation and Forest Degradation: A Synthesis Report for REDD+ Policymakers; Lexeme Consulting: Vancouver, BC, Canada, 2012; pp. 1–47. [Google Scholar]
- Bunker, D.; DeClerck, F.; Bradford, J.; Colwell, R.; Perfecto, I.; Phillips, O.; Sankaran, M.; Naeem, S. Species Loss and Aboveground Carbon Storage in a Tropical Forest. Science 2005, 310, 1029–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwing, J. Degradation of forest through logging and fire in the eastern Brazilian Amazon. For. Ecol. Manag. 2002, 157, 131–141. [Google Scholar] [CrossRef]
- Sist, P.; Nascimiento, F. Sustainability of reduced-impact logging in the Eastern Amazon. For. Ecol. Manag. 2007, 243, 199–209. [Google Scholar] [CrossRef]
- West, T.; Vidal, E.; Putz, F. Forest biomass recovery after conventional and reduced-impact logging in Amazonian Brazil. For. Ecol. Manag. 2014, 314, 59–63. [Google Scholar] [CrossRef]
- Rutishauser, E.; Hérault, B.; Baraloto, C.; Blanc, L.; Descroix, L.; Sotta, E.D.; Ferreira, J.; Kanashiro, M.; Mazzei, L.; d’Oliveira, M. Rapid tree carbon stock recovery in managed Amazonian forests. Curr. Biol. 2015, 25, R787–R788. [Google Scholar] [CrossRef] [Green Version]
- Andam, K.; Ferrano, P.; Pfaff, A.; Sánchez-Azofeifa, A.; Robalino, J. Measuring the effectiveness of protected area networks in reducing deforestation. Proc. Natl. Acad. Sci. USA 2008, 105, 16089–16094. [Google Scholar] [CrossRef] [Green Version]
- Bruner, A.; Gullison, R.; Rice, R.; Da-Fonseca, G. Effectiveness of Parks in Protecting Tropical Biodiversity. Science 2000, 291, 125–127. [Google Scholar] [CrossRef]
- Nagendra, H. Do Parks Work? Impact of Protected Areas on Land Cover Clearing. AMBIO 2008, 37, 330–337. [Google Scholar] [CrossRef]
- Morales-Hidalgo, D.; Oswalt, S.; Somanathan, E. Status and trends in global primary forest, protected areas, and areas designated for conservation of biodiversity from the Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.; Chomitz, K. Protected Area Effectiveness in Reducing Tropical Deforestation A Global Analysis of the Impact of Protection Status; The World Bank: Washington, DC, USA, 2009; p. 40. [Google Scholar]
- Cuesta, F.; Peralvo, M.; Baquero, F.; Bustamante, M.; Merino, A.; Muriel, P.; Freile, J.; Torres, O. Áreas Prioritarias para la Conservación del Ecuador Continental; Ministerio del Ambiente, CONDESAN, Pontificia Universidad Católica del Ecuador, GIZ: Quito, Ecuador, 2015; p. 109. [Google Scholar]
- Andrade, G.; Rhodes, J. Protected Areas and Local Communities: An Inevitable Partnership toward Successful Conservation Strategies? Ecol. Soc. 2012, 17, 1–14. [Google Scholar] [CrossRef]
- Corbera, E.; Kosoy, N.; Martinez, M. Equity implications of marketing ecosystem services in protected areas and rural communities: Case studies from Meso-America. Glob. Environ. Chang. 2007, 17, 365–380. [Google Scholar] [CrossRef]
- Duchelle, A.; Almeyda, A.; Wunder, S.; Börner, J.; Kainer, K. Smallholder Specialization Strategies along the Forest Transition Curve in Southwestern Amazonia. World Dev. 2014, 64, S149–S158. [Google Scholar] [CrossRef] [Green Version]
- DeKonig, F.; Aguinaga, M.; Bravo, M.; Chiu, M.; Lascano, M.; Tozada, T.; Suarez, L. Bridging the gap between forest conservation and poverty alleviation: The Ecuadorian Socio Bosque program. Environ. Sci. Policy 2011, 14, 531–542. [Google Scholar] [CrossRef]
- Grima, N.; Singh, S.; Smetschka, B.; Ringhofer, L. Payment for Ecosystem Services (PES) in Latin America: Analysing the performance of 40 case studies. Ecosyst. Serv. 2016, 17, 24–32. [Google Scholar] [CrossRef]
- Wunder, S. Payments for environmental services and the poor: Concepts and preliminary evidence. Environ. Dev. Econ. 2008, 13, 279–297. [Google Scholar] [CrossRef]
- Palacios, W.; Freire, J. Recursos forestales y territorios indigenas en Ecuador. In Proceedings of the Memorias del Primer Encuentro Andino de Derecho Forestal Ambiental con Enfoque Comunitario, Quito, Ecuador, 13–15 October 2004; pp. 116–119. [Google Scholar]
- Añazco, M.; Morales, M.; Palacios, W.; Vega, E.; Cuesta, A. Sector Forestal Ecuatoriano: Propuestas para una Gestión Forestal Sostenible. Serie de Investigación y Sistematización; Programa Regional ECOBONA—INTERCOOPERATION: Quito, Ecuador, 2010; Volume 8, pp. 1–169. [Google Scholar]
- Bray, D.; Duran, E.; Ramos, V.; Mas, J.; Velazquez, A.; McNab, R.; Barry, D.; Radachowsky, J. Tropical Deforestation, Community Forests, and Protected Areas in the Maya Forest. Ecol. Soc. 2008, 13, 1–20. [Google Scholar] [CrossRef]
- Nepstad, D.; Schwartzman, S.; Bamberger, B.; Santilli, M.; Ray, D.; Schlesinger, P.; Lefebvre, P.; Alencar, A.; Prinz, E.; Fiske, G.; et al. Inhibition of Amazon Deforestation and Fire by Parks and Indigenous Lands. Conserv. Biol. 2006, 20, 65–73. [Google Scholar] [CrossRef]
- Porter-Bolland, L.; Ellis, E.; Guariguata, M.; Ruiz-Mallén, I.; Negrete-Yankelevich, S.; Reyes-García, V. Community managed forests and forest protected areas: An assessment of their conservation effectiveness across the tropics. For. Ecol. Manag. 2011, 268, 6–17. [Google Scholar] [CrossRef]
- Jones, K.; Holland, M.; Naughton-Treves, L.; Morales, M.; Suarez, L.; Keenan, K. Forest conservation incentives and deforestation in the Ecuadorian Amazon. Environ. Conserv. 2016, 44, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Mohebalian, P.; Aguilar, F. Beneath the Canopy: Tropical Forests Enrolled in Conservation Payments Reveal Evidence of Less Degradation. Ecol. Econ. 2018, 143, 64–73. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, A.; Pfaff, A.; Robalinno, J.; Boomhower, J. Costa Rica’s Payment for Environmental Services Program: Intention, Implementation, and Impact. Conserv. Biol. 2007, 21, 1165–1173. [Google Scholar] [CrossRef]
- Pfaff, A.; Robalino, J.; Sanchez-Azofeifa, A. Payments for Environmental Services: Empirical analysis for Costa Rica; Terry Sanford Institute of Public Policy, Duke University: Durham, NC, USA, 2008; pp. 404–424. [Google Scholar]
- Armenteras, D.; Rodríguez, N.; Retana, J. Are conservation strategies effective in avoiding the deforestation of the Colombian Guyana Shield? Biol. Conserv. 2009, 142, 1411–1419. [Google Scholar] [CrossRef]
- Defries, R.; Hansen, A.; Newton, A.; Hansen, M. Increasing isolation of protected areas in tropical forests over the past twenty years. Ecol. Appl. 2005, 15, 19–26. [Google Scholar] [CrossRef]
- FAO. FRA 2015. Term and Definitions; Forest Resources Assessment Working Paper 180; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; pp. 1–31. [Google Scholar]
- IPBES. Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES: Bonn, Germany, 2018; pp. 1–44. [Google Scholar]
- Mitchard, E. The Hectares Indicators: A Review of Earth Observation Methods for Detecting and Measuring Forest Change in the Tropics; School of GeoSciences, University of Edinburgh: Edinburgh, UK, 2016; pp. 1–57. [Google Scholar]
- Edwards, D.; Gilroy, J.; Woodcock, P.; Edwards, F.; Larsen, T.; Andrews, D.; Derhé, M.; Docherty, T.; Hsu, W.; Mitchell, S. Land-sharing versus land-sparing logging: Reconciling timber extraction with biodiversity conservation. Glob. Chang. Biol. 2014, 20, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Abson, D.; Butsic, V.; Chappell, M.; Ekroos, J.; Hanspach, J.; Kuemmerle, T.; Smith, H.; von-Wehrden, H. Land sparing versus land sharing: Moving forward. Conserv. Lett. 2014, 7, 149–157. [Google Scholar] [CrossRef]
- Green, R.; Cornell, S.; Scharlemann, J.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef]
- Runting, R.; Griscom, B.; Struebig, M.; Satar, M.; Meijaard, E.; Burivalova, Z.; Cheyne, S.; Deere, N.; Game, E.; Putz, F. Larger gains from improved management over sparing–sharing for tropical forests. Nat. Sustain. 2019, 2, 53. [Google Scholar] [CrossRef]
- Griscom, B.; Goodman, R. Reframing the sharing vs. sparing debate for tropical forestry landscapes. J. Trop. For. Sci. 2015, 27, 145. [Google Scholar]
- MAE. Resultados Socio Bosque 2018; Ministry of Environment: Quito, Ecuador, 2018.
- MAE. Programa Socio Bosque; Ministry of Environment: Quito, Ecuador, 2016; p. 2.
- MAE. Sistematizacion Socio Bosque 2012; Ministry of Environment: Quito, Ecuador, 2012; pp. 1–43.
- MAE. Estadísticas de Patromonio Natural. Datos de Bsoques, Ecosistemas, Especies, Carbono y Deforestación del Ecuador Continental; Ministry of Environment: Quito, Ecuador, 2015; pp. 1–19.
- MAE; EcoCiencia; IUCN. La Biodiversidad del Ecuador. Informe 2000; Ministerio del Ambiente del Ecuador: Quito, Ecuador; EcoCiencia: Quito, Ecuador; Unión Mundial para la Naturaleza: Gland, Switzerland, 2001; p. 368.
- Pitman, N.; Terborgh, J.; Silman, M.; Núñez, P.L.; Neill, D.; Cerón, C.; Palacios, W.; Aulestia, M. Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology 2001, 82, 2101–2117. [Google Scholar] [CrossRef]
- Chazdon, R. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014; pp. 1–429. [Google Scholar]
- Killmann, W. Proceedings: Expert Meeting on Harmonizing Forest-Related Definitions for Use by Various Stakeholders, Rome, Italy, 23–25 January 2002; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Bonilla-Bedoya, S.; Estrella-Bastidas, A.; Ordoñez, M.; Sanchez, A.; Herrera, M. Patterns of timber harvesting and its relationship with sustainable forest management in the western Amazon, Ecuador case. J. Sustain. For. 2017, 36, 433–453. [Google Scholar] [CrossRef]
- MAE. Aprovechamiento de Recursos Forestales en el Ecuador y Procesos de Infracciones y Decomisos; Ministerio del Ambiente del Ecuador: Quito, Ecuador, 2011; pp. 1–28.
- Congo, G. Evolución del Aprovechamiento Forestal de Origen Legal en la Provincia de Napo; Universidad Tecnica del Norte: Ibarra, Ecuador, 2016; p. 115. [Google Scholar]
- MAE. Información de Planes de Aprovechamiento Forestal 2015–2016. Provincias de Napo, Orellana y Pastaza. Data Base: Ministerio del Ambiente del Ecuador; Ministerio del Ambiente del Ecuador: Quito, Ecuador, 2017.
- Brown, S.; Lugo, A. Tropical secondary forest. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.; Chave, J.; Terborgh, J.; Foster, R.; Nunez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-Diversity in Tropical Forest Trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Morlon, H.; Chuyong, G.; Condit, R.; Hubbell, S.; Kenfack, D.; Thomas, D.; Valencia, R.; Green, J. A general framework for the distance-decay of similarity in ecological communities. Ecol. Lett. 2008, 11, 904–917. [Google Scholar] [CrossRef] [Green Version]
- Harper, K.; Macdonald, E.; Burton, P.; Chen, J.; Brosofske, K.; Saunders, S.; Euskirchen, E.; Roberts, D.; Jaiteh, M.; Esseen, P.A. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Sesnie, S.; Bryan, F.; Paul, G.; Zayra, R. Landscape-Scale Environmental and Floristic Variation in Costa Rican Old-Growth Rain Forest Remnants. Biotropica 2009, 41, 16–26. [Google Scholar] [CrossRef]
- MacDicken, K.G. A Guide to Monitoring Carbon Storage in Forestry and Agroforestry Proyects; Winrock International: Arlington, MA, USA, 1997; pp. 1–87. [Google Scholar]
- Pearson, T.; Walker, S.; Brown, S. Sourcebook for Land Use, Land-Use Change and Forest Projects; Winrock International and the BioCarbon Fund of the World Bank: Arlington, MA, USA, 2005; p. 57. [Google Scholar]
- Ravindranath, N.; Ostwald, M. Carbon Inventory Methods Handbook for Greenhouse Gas Inventory, Carbon Mitigation and Roundwood Production Projects; Springer Science & Business Media: Berlin, Germany, 2008; p. 299. [Google Scholar]
- IPCC. IPCC 5th Assessment Report “Climate Change 2013: The Physical Science Basis”; IPCC: Stockholm, Sweden, 2013. [Google Scholar]
- Chave, J.; Rejou-Mechain, M.; Burquez, A.; Chidumayo, E.; Colgan, M.; Delitti, W.; Duque, A.; Eid, T.; Fearnside, P.; Goodman, R.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.A.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Data from: Towards a Worldwide Wood Economics Spectrum; Dryad Digital Repositor: Durham, NC, USA, 2009. [Google Scholar]
- Chave, J.; Coomes, D.A.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- MAE. Propiedades Anatómicas, Físicas y Mecanicas de 93 Especies Forestales; Ministerios del Ambiente del Ecuador: Quito, Ecuador, 2014; p. 169.
- Aguirre, Z.; Loja, A.; Solano, C.; Aguirre, N. Especies Forestales más Aprovechadas en la Región Sur del Ecuador; Universidad Nacional de Loja: Loja, Ecuador, 2015; pp. 1–80. [Google Scholar]
- Goodman, R.; Phillips, O.; Castillo, D.; Freitas, L.; Tapia, S.; Monteagudo, A.; Baker, T. Amazon palm biomass and allometry. For. Ecol. Manag. 2013, 310, 994–1004. [Google Scholar] [CrossRef]
- Chave, J. Medición de Densidad de Madera en Árboles Tropicales; Université Paul Sabatier: Touluse, France, 2006; pp. 1–7. [Google Scholar]
- Williamson, B.; Wiemann, M. Measuring wood specific gravity correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar] [CrossRef]
- FAO. Estimación del Volumen Forestal y Predicción del Redimiento, con Referencia Especial en los Tropicos; FAO: Rome, Italy, 1981; pp. 1–93. [Google Scholar]
- Chamber, J.; Higuchi, N.; Schimel, J.; Ferreira, L.; Melack, J. Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon. Oecologia 2000, 122, 380–388. [Google Scholar] [CrossRef]
- Soil Survey, S. Kellogg Soil Survey Laboratory Methods Manual; Report No. ed.; Natural Resources Conservation Service, U.S. Department of Agriculture: Washington, DC, USA, 2014; pp. 1–1030.
- Mostacedo, B.; Fredericksen, T. Manual de Métodos Básicos de Muestreo y Análisis en Ecología Vegetal; Proyecto de Manejo Forestal Sostenible (BOLFOR): Santa Cruz, Bolivia, 2000; pp. 1–81. [Google Scholar]
- Magurran, A.; McGill, B. Biological Diversity: Frontiers in Measurement and Assessment; OUP Oxford: Oxford, UK, 2011; pp. 1–151. [Google Scholar]
- Curtis, J.; McIntosh, R. The Interrelations of Certain Analytic and Synthetic Phytosociological Characters. Ecol. Soc. Am. 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Chisholm, R.; Muller-Landau, H.; Abdul, R.; Bebber, D.; Bin, Y.; Bohlman, S.; Bourg, N.; Brinks, J.; Bunyavejchewin, S.; Butt, N.; et al. Scale-dependent relationships between tree species richness and ecosystem function in forests. J. Ecol. 2013, 101, 1214–1224. [Google Scholar] [CrossRef]
- Keith, H.; Mackey, B.; Lindenmayer, D. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.; Phillips, O.; Malhi, Y.; Almeida, S.; Arrollo, L.; Di Fiore, A.; Erwin, T.; Higuchi, N.; Killeen, T.; Laurance, S.; et al. Increasing biomass in Amazonian forest plots. Philos. Trans. R. Soc. 2004, 359, 353–365. [Google Scholar] [CrossRef]
- Valencia, R.; Codit, R.; Muller-Landau, H.; Hernandez, C.; Navarrete, H. Dissecting biomass dynamics in a large Amazonian forest plot. J. Trop. Ecol. 2009, 25, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Eguiguren, P. Los Efectos de Intervenciones Forestales y la Variabilidad Climática Sobre a Dinámica a Largo plaz de Bosques Tropicales en el Noreste de Costa Rica. Master’s Thesis, Centro Agronómico Tropical de Investigación y Enseñanza CATIE, Turrialba, Costa Rica, 2013; pp. 1–75. [Google Scholar]
- Mejia, E.; Pacheco, P.; Morocho, J.; Alarcón, S. Actors, Intermediation and Forest Control in the Amazon; Mejia, E., Pacheco, P., Eds.; CIFOR: Bogor, Indonesia, 2014. [Google Scholar]
- MAE; FAO. Resultados de la Evaluación Nacional Forestal; Ministerio del Ambiente del Ecuador: Quito, Ecuador, 2014; pp. 1–316.
- Dammer, L.; Bowyer, C.; Breitmayer, E.; Eder, A.; Nanni, S.; Allen, B.; Carus, M.; Essel, R. Mapping Study on Cascading Use of Wood Products; World Wide Fund for Nature (WWF): Grand, Switzerland, 2016; pp. 1–55. [Google Scholar]
- Chazdon, R.L.; Broadbent, E.N.; Rozendaal, D.M.A.; Bongers, F.; Zambrano, A.M.A.; Aide, T.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; et al. Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2016, 2, e1501639. [Google Scholar] [CrossRef] [PubMed]
- Stanturf, J.; Kant, P.; Lillesø, J.; Mansourian, S.; Kleine, M.; Graudal, L.; Madsen, P. Forest Landscape Restoration as a Key Component of Climate Change Mitigation and Adaptation; IUFRO, Ed.; International Union of Forest Research Organizations (IUFRO): Vienna, Austria, 2015; Volume 34, p. 76. [Google Scholar]
- Rai, N.; Uhl, C. Forest product use, conservation and livelihoods: The case of Uppage fruit harvest in the Western Ghats, India. Conservation and Society. Conserv. Soc. 2004, 2, 289–313. [Google Scholar]
- Shahabuddin, G.; Rao, M. Do community-conserved areas effectively conserve biological diversity? Global insights and the Indian context. Biol. Conserv. 2010, 143, 2926–2936. [Google Scholar] [CrossRef]
- Gautam, A.; Shivakoti, G.; Webb, E. Forest Cover Change, Physiography, Local Economy, and Institutions in a Mountain Watershed in Nepal. Environ. Manag. 2004, 33, 48–61. [Google Scholar] [CrossRef]
- Vuohelainen, A.; Coad, L.; Marthews, T.; Malhi, Y.; Killeen, T. The effectiveness of contrasting protected areas in preventing deforestation in Madre de Dios, Peru. Environ. Manag. 2012, 50, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Naughton-Treves, L.; Holland, M.; Brandon, K. The role of protected areas in conserving Biodiversity and sustaining local livelihoods. Annu. Rev. Environ. Resour. 2005, 30, 219–252. [Google Scholar] [CrossRef]
- INEC. Censo de Población y Vivienda; Instituto Nacional de Estadísticas y Censos: Madrid, Spain, 2010. [Google Scholar]
- Finegan, B.; Delgado, D.; Camacho, M.; Zamora, N. Timber Production and Plant Biodiversity Conservation in a Costa Rican Rain Forest: An Experimental Study and its Lessons for Adaptive Sustainability Assessment; European Forest Institute: Nancy, France, 2001; pp. 123–134. [Google Scholar]
- Magnusson, W.; Lima, O.; Quintiliano, F.; Higuchi, N.; Ferreira, J. Logging activity and tree regeneration in an Amazonian forest. For. Ecol. Manag. 1999, 113, 67–74. [Google Scholar] [CrossRef]
- Verburg, R.; Van-Eijk-Bos, C. Effects of selective logging on tree diversity, composition and plant functional type patterns in a Bornean rain forest. J. Veg. Sci. 2003, 14, 99–110. [Google Scholar] [CrossRef]
- Wright, J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.; Araujo, I.; Ávila-Pires, T.; Bonaldo, A.; Costa, J.; Esposito, M.; Ferreira, L.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, L.; Lee, T.; Koh, L.; Brook, B.; Gardner, T.; Barlow, J.; Peres, C.; Bradshaw, C.; Laurance, W.; Lovejoy, T.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Thanichanon, P.; Schmidt-Vogt, D.; Messerli, P.; Heinimann, A.; Epprecht, M. Secondary Forests and Local Livelihoods along a Gradient of Accessibility: A Case Study in Northern Laos. Soc. Nat. Resour. 2013, 26, 1283–1299. [Google Scholar] [CrossRef]
- Morse, W.C.; Schedlbauer, J.L.; Sesnie, S.E.; Finegan, B.; Harvey, C.A.; Hollenhorst, S.J.; Kavanagh, K.L.; Stoian, D.; Wulfhorst, J.D. Consequences of Environmental Service Payments for Forest Retention and Recruitment in a Costa Rican Biological Corridor. Ecol. Soc. 2009, 14, 1–20. [Google Scholar] [CrossRef]
- Cuenca, P.; Robalino, J.; Arriagada, R.; Echeverria, C. Are government incentives effective for avoided deforestation in the tropical Andean forest? PLoS ONE 2018, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- MAE. Mapa de Cobertura y Uso de la Tierra del Ecuador Continental; Ministerio del Ambiente del Ecuador: Quito, Ecuador, 2016.
- SIGTIERRAS. Mapa Digital, Órdenes de Suelos del Ecuador. Sistema Nacional de Información y Gestión de Tierras Rurales e Infraestructura Tecnológica; MAGAP: Quito, Ecuador, 2017. [Google Scholar]
- MAGAP. Mapa de suelos del Ecuador Ministerio de Agricultura, Ganeria y Pesca del Ecuador; MAGAP: Quito, Ecuador, 2002. [Google Scholar]


| Equation | Reference | |
|---|---|---|
| (1) | Chave et al. [89] | |
| (2) | Goodman et al. [94] | |
| (3) | Pearson et al. [86] Ravindranath and Ostwald [87] | |
| (4) | Pearson et al. [86] Ravindranath and Ostwald [87] | |
| (5) | Pearson et al. [86] | |
| (6) | Pearson et al. [86] Ravindranath and Ostwald [87] | |
| (7) | Pearson et al. [86] Ravindranath and Ostwald [87] | |
| (8) | FAO [97] | |
| (9) | Chamber et al. [98] | |
| (10) | Pearson et al. [86] Ravindranath and Ostwald [87] | |
| Dependent Variable | IFC vs. non-IFC | Forest Type | Interaction: Forest Types Influenced by IFC and Non-IFC | R2 | n |
|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | |||
| AGC (Mg C·ha−1) | 0.4624 | <0.0001 *** | 0.0696 * | 0.61 | 70 |
| DOM (Mg C·ha−1) | 0.6044 | 0.0974 * | 0.1619 | 0.12 | 67 |
| SOC (Mg C·ha−1) | 0.3738 | 0.2755 | 0.1753 | 0.53 | 72 |
| Total carbon (Mg C·ha−1) | 0.6208 | <0.0001 *** | 0.0459 ** | 0.66 | 72 |
| Timber volume (m3 ha−1) | 0.4330 | <0.0001 *** | 0.3498 | 0.57 | 68 |
| Richness (#sp./plot) | 0.8933 | <0.0001 *** | 0.0570 * | 0.65 | 72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguiguren, P.; Fischer, R.; Günter, S. Degradation of Ecosystem Services and Deforestation in Landscapes With and Without Incentive-Based Forest Conservation in the Ecuadorian Amazon. Forests 2019, 10, 442. https://doi.org/10.3390/f10050442
Eguiguren P, Fischer R, Günter S. Degradation of Ecosystem Services and Deforestation in Landscapes With and Without Incentive-Based Forest Conservation in the Ecuadorian Amazon. Forests. 2019; 10(5):442. https://doi.org/10.3390/f10050442
Chicago/Turabian StyleEguiguren, Paúl, Richard Fischer, and Sven Günter. 2019. "Degradation of Ecosystem Services and Deforestation in Landscapes With and Without Incentive-Based Forest Conservation in the Ecuadorian Amazon" Forests 10, no. 5: 442. https://doi.org/10.3390/f10050442
APA StyleEguiguren, P., Fischer, R., & Günter, S. (2019). Degradation of Ecosystem Services and Deforestation in Landscapes With and Without Incentive-Based Forest Conservation in the Ecuadorian Amazon. Forests, 10(5), 442. https://doi.org/10.3390/f10050442