Impacts of Change in Atmospheric CO2 Concentration on Larix gmelinii Forest Growth in Northeast China from 1950 to 2010
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tree-Ring Data
2.2. Tree-Ring Approach
2.2.1. Chronology Development
2.2.2. Dendroclimatic Analysis
2.3. CO2 Fertilization Test
2.4. Description of the InTEC Model
2.4.1. Model Inputs
2.4.2. Stemwood Biomass Growth
2.4.3. Baseline and Residual Stemwood Biomass Growth
2.4.4. The Partitioning Approach to Attribute Growth Variation to Various Factors
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2007: Synthesis Report. 2007. Available online: http://www.ipcc.ch/ (accessed on 5 January 2019).
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Piao, S.; Sitch, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Wang, X.; Ahlström, A.; Anav, A.; Canadell, J.G.; Cong, N.; et al. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Chang. Boil. 2013, 19, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.K.; Reed, S.C.; Cleveland, C.C.; Ballantyne, A.P.; Anderegg, W.R.; Wieder, W.R.; Liu, Y.Y.; Running, S.W. Large divergence of satellite and Earth system model estimates of global terrestrial CO2 fertilization. Nat. Clim. Chang. 2015, 6, 306–310. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhuang, Q.L.; Chen, M.; Gu, L.H. Quantifying spatially and temporally explicit CO2 fertilization effects on global terrestrial ecosystem carbon dynamics. Ecosphere 2016, 7, e01391. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Michalak, A.M.; Schwalm, C.; Ciais, P.; King, A.W.; Fang, Y.; Schaefer, K.; Wei, Y.; Cook, R.B.; Fisher, J.B.; et al. Uncertainty in the response of terrestrial carbon sink to environmental drivers undermines carbon-climate feedback predictions. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; DeLucia, E.H.; Gielen, B.; Calfapietra, C.; Giardina, C.P.; King, J.S.; Ledford, J.; McCarthy, H.R.; Moore, D.J.; Ceulemans, R.; et al. Forest response to elevated CO2 is conserved across a broad range of productivity. Proc. Natl. Acad. Sci. USA 2005, 102, 18052–18056. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.H.; Morgan, J.; Norby, R. CO2 fertilization: When, where, howmuch? In Terrestrial Ecosystems in a Changing World Series: Global Change—The IGBP Series; Canadell, J.G., Pataki, D., Pitelka, L., Eds.; Springer: Berlin, Germany, 2007; pp. 9–21. [Google Scholar]
- Hickler, T.; Smith, B.; Prentice, I.C.; Mjöfors, K.; Miller, P.; Arneth, A.; Sykes, M.T. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob. Chang. Biol. 2008, 14, 1531–1542. [Google Scholar] [CrossRef]
- Girardin, M.P.; Bernier, P.Y.; Raulier, F.; Tardif, J.C.; Conciatori, F.; Guo, X.J. Testing for a CO2, fertilization effect on growth of canadian boreal forests. J. Geophys. Res. Biogeosci. 2015, 116, G01012. [Google Scholar]
- Wang, G.G.; Chhin, S.; Bauerle, W. Effect of natural atmospheric CO2 fertilization suggested by open-grown white spruce in a dry environment. Glob. Chang. Boil. 2006, 11, 1–10. [Google Scholar]
- Chen, Z.; Zhang, X.; He, X.; Davi, N.K.; Li, L.; Bai, X. Response of radial growth to warming and CO2, enrichment in southern Northeast China: A case of Pinus tabulaeformis. Clim. Chang. 2015, 130, 559–571. [Google Scholar] [CrossRef]
- Huang, J.G.; Zhang, Q.B. Tree-rings and climate for the last 680 years in Wulan area of northeastern Qinghai-Tibetan Plateau. Clim. Chang. 2007, 80, 369–377. [Google Scholar] [CrossRef]
- Gedalof, Z.; Berg, A.A. Tree ring evidence for limited direct CO2 fertilization of forests over the 20th century. Glob. Biogeochem. Cycles 2010, 24, GB3027. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Briffa, K.R.; Jones, P.D. A tree-ring densitometric transect from Alaska to Labrador. Comparisons of ring-width and maximum-latewood-density chronologies in the conifer belt of northern North America. Int. J. Biometeorol. 1993, 37, 151–169. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Mielikäinen, K. Climatic signals in annual growth variation of Norway spruce (Picea abies) along a transect from central Finland to the Arctic timberline. Can. J. Res. 2000, 30, 769–777. [Google Scholar] [CrossRef]
- Girardin, M.P.; Bouriaud, O.; Hogg, E.H.; Kurz, W.; Zimmermann, N.E.; Metsaranta, J.M.; de Jong, R.; Frank, D.C.; Esper, J.; Büntgen, U.; et al. No growth stimulation of Canada’s boreal forest under half-century of combined warming and CO2 fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, E8406–E8414. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, F.A. The response of natural systems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Field, C.B.; Jackson, R.B.; Mooney, H.A. Stomatal responses to increased CO2: Implications from the plant to the global scale. Plant Cell Environ. 1995, 18, 1214–1225. [Google Scholar] [CrossRef]
- Huang, J.G.; Bergeron, Y.; Denneler, B.; Berninger, F.; Tardif, J. Response of Forest Trees to Increased Atmospheric CO2. Crit. Rev. Plant Sci. 2007, 26, 265–283. [Google Scholar] [CrossRef]
- Jacoby, G.C.; D’Arrigo, R.D. Tree-rings, carbon dioxide and climatic change. Proc. Natl. Acad. Sci. USA 1997, 94, 8350–8353. [Google Scholar] [CrossRef]
- Chen, W.J.; Chen, J.M.; Cihlar, J. An integrated terrestrial carbon-budget model based on changes indisturbance, climate, and atmospheric chemistry. Ecol. Model. 2000, 135, 55–79. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R. A Time Series Approach to Tree-Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Ahmed, M.; Wahab, M.; Khan, N.; Zafar, M.U.; Palmer, J. Tree-ring chronologies from upper indus basin of Karakorum Range, Pakistan. Pak. J. Bot. 2010, 42, 295–307. [Google Scholar]
- Cook, E.R.; Buckley, B.M.; D’Arrigo, R.D.; Peterson, M.J. Warm-season temperature since 1600 AD. Reconstruction from Tasmanian tree-rings and their relationship to large scale sea surface temperature anomalies. Clim. Dyn. 2000, 16, 79–91. [Google Scholar] [CrossRef]
- Wigley, T.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Fritts, H.C.; Vaganov, E.A.; Sviderskaya, I.V.; Shashkin, A.V. Climatic variation and tree-ring structure in conifers: Empirical and mechanistic models of tree-ring width, number of cells, cellsize, cell-wall thickness and wood density. Clim. Res. 1991, 1, 97–116. [Google Scholar] [CrossRef]
- Sen, P.K. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Gilbert, R.O. Statistical Methods for Environmental Pollution Monitoring; Van Nostrand Reinhold: New York, NY, USA, 1987. [Google Scholar]
- Farquhar, G.D.; Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilationin leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Ju, W.; Cihlar, J.; Price, D.; Liu, J.; Chen, W.; Pan, J.; Black, A.; Barr, A. Spatial distribution of carbon sources and sinks in Canada’s forests. Tellus Ser. B 2003, 55, 622–641. [Google Scholar]
- Ju, W.; Chen, J.M. Distribution of soil carbon stocks in Canada’sforests and wetland simulated based on drainage class, topography and remote sensing. Hydrol. Process. 2005, 19, 77–94. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matterlevels in the Great Plains grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Townsend, A.R.; Braswell, B.H.; Holland, E.A.; Penner, J.E. Spatial and temporal patterns in potential terrestrial carbon storage resulting from deposition of fossil fuel derived nitrogen. Ecol. Appl. 1996, 6, 806–814. [Google Scholar] [CrossRef]
- Climatic Research Unit. Available online: http://www.cru.uea.ac.uk/cru/data/ (accessed on 9 January 2019).
- Earth System Research Laboratory. Available online: https://www.esrl.noaa.gov/ (accessed on 9 January 2019).
- FRSOC. Forest Resources Statistics of China (2009–2014); Department of Forest Resources Management State Forestry Administration: Beijing, China, 2016.
- Zhang, X.; Sun, S.; Yong, S.; Zhou, Z.; Wang, R. Vegetation Map of the People’s Republic of China; Geography Press: Beijing, China, 2007. [Google Scholar]
- Liu, J.; Chen, J.M.; Cihlar, J.; Park, W.M. A Process-Based Boreal Ecosystem Productivity Simulator Using Remote Sensing Inputs. Remote Sens. Environ. 1997, 62, 158–175. [Google Scholar] [CrossRef]
- Carbon Dioxide Information Analysis Center (CDIAC). Available online: http://cdiac.esd.ornl.gov/ftp/trends/CO2/maunaloa.CO2 (accessed on 12 January 2019).
- Deng, F.; Chen, J.M.; Plummer, S.; Chen, M.; Pisek, J. Algorithm for global leaf area index retrieval using satellite imagery. IEEE Trans. Geosci. Remote Sens. 2006, 44, 2219–2229. [Google Scholar] [CrossRef] [Green Version]
- IGBP-DIS. Available online: http://daac.ornl.gov/cgi-bin/dsviewer.pl?ds_id=569 (accessed on 12 January 2019).
- ORNL. Available online: https://www.ornl.gov/ (accessed on 12 January 2019).
- IIASA. Available online: http://daac.ornl.gov/SOILS/guides/HWSD.html (accessed on 12 January 2019).
- Wang, B.; Li, M.; Fan, W.; Zhang, F.M. Quantitative simulation of C budgets in a forest in Heilongjiang province, China. iForest 2017, 10, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Li, M.; Fan, W.; Yu, Y.; Chen, J. Relationship between net primary productivity and forest stand age under different site conditions and its implications for regional carbon cycle study. Forests 2018, 5, 1. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, J.M.; Yang, X.; Fan, W.; Li, M.; He, L. Influence of site index on the relationship between forest net primary productivity and stand age. PLoS ONE 2017, 12, e0177084. [Google Scholar] [CrossRef]
- Dong, L.H.; Zhang, L.J.; Li, F.R. A compatible system of biomass equations for three conifer species in Northeast, China. Ecol. Manag. 2014, 329, 306–317. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, W.Y.; Li, M.Z. Forest carbon rates at different scales in Northeast China forest area. Chin. J. Appl. Ecol. 2012, 23, 341–346. [Google Scholar]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. Rev. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhang, Y.D.; Mcrae, D.J. Spatial and age-dependent tree-ring growth responses of Larix gmelinii to climate in northeastern China. Trees 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Rolland, C. Long term changes in the radial growth of the mountain pine (Pinus uncinata) in the French Alps as influenced by climate. Dendrochronologia 1996, 14, 255–262. [Google Scholar]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Hendrickson, L.; Ball, M.C.; Wood, J.T.; Chow, W.S.; Furbank, R.T. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2010, 27, 795–809. [Google Scholar] [CrossRef]
- Lavigne, M.B. Stem growth and respiration of young balsam fir trees in thinned and unthinned stands. Can. J. For. Res. 1988, 18, 483–489. [Google Scholar] [CrossRef]
- Wu, C.; Hember, R.A.; Chen, J.M.; Kurz, W.A.; Price, D.T.; Boisvenue, C.; Gonsamo, A.; Ju, W. Accelerating Forest Growth Enhancement due to Climate and Atmospheric Changes in British Colombia, Canada over 1956–2001. Sci. Rep. 2014, 4, 4461. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Kirschbaum, M.U.; Kurz, W.A.; Sanz, M.J.; Schlamadinger, B.; Yamagata, Y. Factoring out natural and indirect human effects on terrestrial carbon sources and sinks. Environ. Sci. Policy 2007, 10, 370–384. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.; Hom, J.; McCullough, K. Separating effects of changes in atmospheric composition, climate, and land-use on carbon sequestration of U.S. mid-Atlantic temperate forests. Ecol. Manag. 2009, 259, 151–164. [Google Scholar] [CrossRef]
- Kӧrner, C.; Farquhar, G.D.; Wong, S.C. Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 1991, 99, 343–351. [Google Scholar] [CrossRef]
- Handa, I.T.; Kӧrner, C.; Hättenschwiler, S. A test of the tree-line carbon limitation hypothesis by in situ CO2 enrichment and defoliation. Ecology 2005, 86, 1288–1300. [Google Scholar] [CrossRef]
- Morgan, J.A.; Pataki, D.E.; Körner, C.; Clark, H.; Del Grosso, S.J.; Grünzweig, J.M.; Knapp, A.K.; Mosier, A.R.; Newton, P.C.D.; Niklaus, P.A.; et al. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 2004, 140, 11–25. [Google Scholar] [CrossRef]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional responses of plants to elevated atmospheric CO2—Do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 2004, 162, 253–280. [Google Scholar] [CrossRef]
- Brienen, R.J.; Gloor, W.E.; Zuidema, P.A. Detecting evidence for CO2 fertilization from tree ring studies: The potential role of sampling biases. Glob. Biogeochem. Cycles 2012, 26, GB1025. [Google Scholar] [CrossRef]
- Miller, P.M.; Eddleman, L.E.; Miller, J.M. The response of juvenile and small adult western juniper (Juniperus occidentalis) to nitrate and ammonium fertilization. Can. J. Bot. 1991, 69, 2344–2352. [Google Scholar] [CrossRef]
- Thomas, R.; Canham, C.; Weathers, K.; Goodale, C. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Solberg, S.; Dobbertin, M.; Reinds, G.J.; Lange, H.; Andreassen, K.; Fernandez, P.G.; Hildingsson, A.; de Vries, W. Analyses of the impact of changes in atmospheric deposition and climate on forest growth in European monitoring plots: A stand growth approach. For. Ecol. Manag. 2009, 258, 1735–1750. [Google Scholar] [CrossRef]







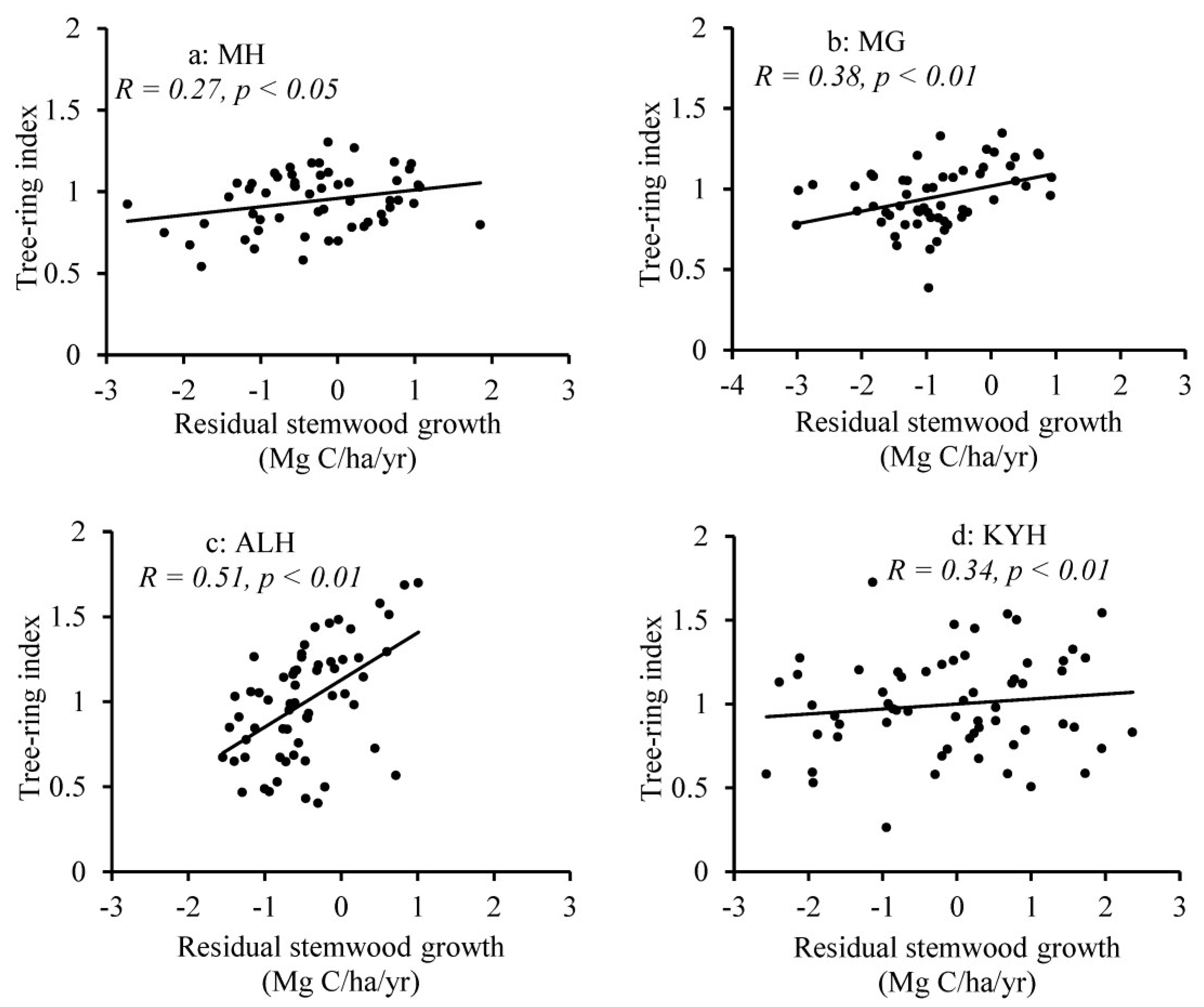

| Site | Longitude (E) | Latitude (N) | Elev. (m) | T (°C) | P (mm) | Length of Chronology | N |
|---|---|---|---|---|---|---|---|
| MH | 122.13 | 53.30 | 617 | −5.1 | 437 | 1802–2005 | 61 |
| MG | 121.80 | 52.23 | 695 | −6.0 | 465 | 1760–2005 | 61 |
| ALH | 123.48 | 50.84 | 135 | −3.9 | 471 | 1782–2010 | 27 |
| KYH | 122.38 | 50.64 | 779 | −3.7 | 461 | 1715–2010 | 22 |
| Sites | MH | MG | ALH | KYH |
|---|---|---|---|---|
| Mean index | 0.92 | 0.94 | 1.01 | 0.98 |
| Std. | 0.34 | 0.23 | 0.40 | 0.23 |
| Skewness | 0.89 | 0.28 | 1.02 | 0.03 |
| Kurtosis | 4.66 | 2.55 | 5.37 | 2.74 |
| Mean Sensitivity | 0.24 | 0.17 | 0.31 | 0.21 |
| Serial correlation | 0.65 | 0.68 | 0.47 | 0.39 |
| Rbar | 0.26 | 0.30 | 0.26 | 0.31 |
| EPS | 0.97 | 0.98 | 0.93 | 0.94 |
| Parameters | a | b | a1 | a2 | a3 | a4 | R2 | RMSE | Samples |
|---|---|---|---|---|---|---|---|---|---|
| Values | −0.726 | 1.038 | 0.019 | 91.639 | 710236 | 0.073 | 0.997 | 0.051 | 29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Li, M.; Fan, W.; Yu, Y.; Jia, W. Impacts of Change in Atmospheric CO2 Concentration on Larix gmelinii Forest Growth in Northeast China from 1950 to 2010. Forests 2019, 10, 454. https://doi.org/10.3390/f10050454
Wang B, Li M, Fan W, Yu Y, Jia W. Impacts of Change in Atmospheric CO2 Concentration on Larix gmelinii Forest Growth in Northeast China from 1950 to 2010. Forests. 2019; 10(5):454. https://doi.org/10.3390/f10050454
Chicago/Turabian StyleWang, Bin, Mingze Li, Wenyi Fan, Ying Yu, and Weiwei Jia. 2019. "Impacts of Change in Atmospheric CO2 Concentration on Larix gmelinii Forest Growth in Northeast China from 1950 to 2010" Forests 10, no. 5: 454. https://doi.org/10.3390/f10050454
APA StyleWang, B., Li, M., Fan, W., Yu, Y., & Jia, W. (2019). Impacts of Change in Atmospheric CO2 Concentration on Larix gmelinii Forest Growth in Northeast China from 1950 to 2010. Forests, 10(5), 454. https://doi.org/10.3390/f10050454






