Abstract
Lignin has great potential for utilization as a green raw material or as an additive in various industrial applications, such as energy, valuable chemicals, or cost-effective materials. In this study, we assessed a commercial form of lignin isolated using LignoBoost technology (LB lignin) as well as three other types of lignin (two samples of non-wood lignins and one hardwood kraft lignin) isolated from the waste liquors produced during the pulping process. Measurements were taken for elemental analysis, methoxyl and ash content, higher heating values, thermogravimetric analysis, and molecular weight determination. We found that the elemental composition of the isolated lignins affected their thermal stability, activation energies, and higher heating values. The lignin samples examined showed varying amounts of functional groups, inorganic component compositions, and molecular weight distributions. Mean activation energies ranged from 93 to 281 kJ/mol. Lignins with bimodal molecular weight distribution were thermally decomposed in two stages, whereas the LB lignin showing a unimodal molecular weight distribution was decomposed in a single thermal stage. Based on its thermal properties, the LB lignin may find direct applications in biocomposites where a higher thermal resistance is required.
1. Introduction
Lignins have been recently identified as a major potential source of aromatic renewable resources. They represent an excellent alternative feedstock in the production of chemicals and polymers. Reasons for lignins being seen as an important form of future raw materials include the following factors: they exist or are readily available in large quantities; they have accumulated high levels of energy, and therefore represent a potential energy source; they possess interesting properties in terms of colloidal, rheological, as well as adsorption and adhesive properties for new composite materials, dispersants, emulsifiers, additives, adhesives, and binders; they are a potential source for many of the underlying building blocks of chemicals and also a direct source of various kinds of phenolic and aromatic compounds, in addition to being a direct source for a wide range of substitution, oxidation, and electrocatalytic addition reactions. Lignins in the spent pulping liquors of delignification processes have so far been used primarily as an energy source, a use in which they do not always live up to their full economic potential. Recently, lignin valorization has become commercially viable as an important and advanced sustainable process for lignocellulosic biomass-based industries, primarily through depolymerization. Biorefinery is a necessary approach towards the practical considerations of depolymerization techniques [1,2,3,4]. Lignin can be isolated and recovered, for example, after ionic liquid pretreatment; previous results have indicated that the lignins obtained following ionic liquid pretreatment contained the highest molecular mass molecules, did not contain new condensed structures, and were depolymerized[5]. Thus, it may be more amenable to be used as a renewable chemical feedstock for other applications, resulting in decreased biorefinery costs [4,5]. In spite of extensive research, there are several potential difficulties associated with expanding the use of lignins for a wider number of industrial materials and products. The difficulties in lignin valorization are mainly attributed to the complexity and recalcitrance structure of lignins, and the high reactivity of the degraded fractions of lignins, which are prone to condensation reactions [6]. However, these condensation reactions may be useful in the preparation of various polymeric materials, composites, hydrogels, etc. Condensation reactions and the cross-linking of desulfurized kraft lignin with triethyl phosphate have been used for preparing insoluble, hardened resins [3,7,8].
Thermogravimetry, also referred to as a thermoanalytical technique, is a frequently employed technique that can be used to study the thermal properties of biopolymers and to detect changes in the properties of materials. Several studies have reported a mathematical description and quantitative description of lignin decomposition. The approaches used often represent isoconversional models that presume that kinetic parameters, such as the pre-exponential factor and the activation energy, are inconstant during the process of decomposition but are dependent on conversion [9,10]. The disadvantage of integral dependence is a lower sensitivity to small changes (or deviations) in comparison with the differential decay curve [11].
Measurement of a sample’s mass loss as a function of temperature and time is a technique for characterizing material thermal degradation behavior. Depending on whether single or multiple heating rates are applied, the technique used to obtain kinetic parameters is referred to either as a differential or an integral method. From an industrial point of view, there are various types of lignins, whose properties depend on their method of isolation. Each of the methods has its own advantages and disadvantages. Since the modes of lignin production are different, further separation, purification, and processing of lignins can provide various derivatives and other chemicals. Dissolution and fractionation of lignocellulosic material is a critical step for biomass valorization. Table 1 presents the data for kinetic parameters derived for various types of lignin in previously published works. The results show different thermal behaviors for isolated lignins. The activation energy values vary over a wide range, from 8.5 to 361 kJ/mol. One possible explanation for this phenomenon could be the method of isolation and different properties of the various types of lignin. The lignins contain many aromatic structures with various branches and functional groups. In addition, the functional groups in lignins possess an extremely wide range of chemical bonds with different activities.

Table 1.
Kinetic parameters derived for various types of lignin in previously published works.
Depending upon their origin and isolation methods, the complex composition and structure of lignins strongly affect their thermal stability and degradation behavior, and thereby have an impact on the kinetic process. In the present study, the thermal stability of isolated lignins was investigated and the activation energies for thermal degradation were calculated. This work constitutes a significant step towards understanding the complete kinetic scheme of lignin degradation.
2. Materials and Methods
2.1. Black Liquor Characterization and Lignin Sample Denomination
The selected lignins in this work were based on the availability and wide application of their properties. The annual plants such as industrial hemp (Cannabis sativa) and common flax (Linum usitatissimum), used to obtain the black liquor #1, were kindly supplied by OP Papírna Ltd. (Olšany, Czech Republic). The cooking conditions were as follows: active alkali sodium hydroxide and the presence of anthraquinone (AQ). The hardwood (European beech), used to obtain the black liquor #2, was kindly supplied by Bukóza Holding Inc. (Hencovce, Slovak Republic). The cooking conditions were as follows: active alkali sodium hydroxide and sodium sulfide.
The black liquor pH values were determined using a digital 3510 pH-meter (Jenway, Stone, UK) and density was measured by a pycnometer method. The black liquor was dried at 25 °C under a pressure of 0.5 mbar using lyophilization equipment Lyovac GT2 (Leybold Heraeus, Germany) until a constant weight was achieved. The elemental analysis (C, H, N, S, O) was performed using a vario MACRO cube (Elementar, Germany). A detailed chemical characterization of the black liquors is presented in Table 2. The isolated lignins, used in this study, were denoted as follows:

Table 2.
Chemical characterization of black liquors (% of oven-dry weight). Data represent means ± standard deviations.
LB: Kraft lignin powder prepared by the LignoBoost technology (Innventia AB, Stockholm, Sweden, CAS 8068-05-1) (prepared by kraft process from softwood)
NWL-SA: Non-wood lignin obtained from the liquor #1 by precipitation with sulfuric acid at pH = 3 (prepared by soda-AQ pulping of annual plants),
NWL-AA: Non-wood lignin obtained from the liquor #1 by precipitation with acetic acid at pH = 4.3 followed by ultrafiltration (prepared by soda-AQ pulping of annual plants),
HDL: Hardwood kraft lignin obtained from the liquor #2 by precipitation with sulfuric acid at pH = 3 (prepared by kraft pulping of European beech).
Different methods for preparing lignins were chosen to provide different sample properties (purity, content of sulfur, thermal stability, etc.).
2.2. Lignin Recovery Using a Precipitation with Sulfuric Acid
The precipitation of lignin from the black liquors was initially studied as a single step process (lignin samples NWL-SA and HDL) in which a dilute solution of 5% sulfuric acid was added to the black liquor with the pH adjusted to the desired value at a temperature of 50 °C. Then, 100 mL of the black liquor was treated with different amounts of diluted acid to obtain a final pH value of 3 while keeping the temperature constant at 50 °C. After precipitation, the content of each flask was filtered through a pre-weighed oven-dried filter paper using a vacuum filtration unit. The precipitated lignin was washed twice with hot water (total volume 400 mL, pH 6.8) to remove impurities. The lignin was then dried at 25 °C under a pressure of 0.5 mbar using lyophilization equipment (Lyovac GT2) until a constant weight was achieved.
2.3. Lignin Recovery Using Ultrafiltration and Precipitation with Acetic Acid
NWL-AA lignin was isolated from the black liquor #1 by precipitation with acetic acid followed by ultrafiltration. The starting material was diluted with water at a ratio of 1:5. The prepared raw material was heated to 60 °C, stirred with a magnetic stirrer, and titrated with acetic acid to pH 4.3. For ultrafiltration, the membrane of polyethersulfone (PES) with a cut-off 1 kDa was used at the pressure in the range from 3.0 to 4.5 bar.
2.4. Determination of Lignin Higher Heating Values and the Content of Methoxyl Groups
The raw materials obtained were homogenized and adjusted to a powder form. Furthermore, higher heating values (HHVs) of the lignin samples were predicted based on the carbon content using Equation (1) [30]:
where C is the carbon content (wt %) of the sample.
HHV = C × 0.40659
Methoxyl groups (wt %) of the lignin samples were predicted based on the hydrogen and oxygen contents using Equation (2) [31]:
where OCH3 is the content of methoxyl groups in lignin (wt %), H is the hydrogen content (wt %), and O is the oxygen content (wt %) determined by the elemental analysis.
OCH3 = −18.57690 + 4.06580 × H + 0.34543 × O
Carbon content was determined directly by elemental analysis and oxygen content was determined by calculation from elemental analysis. Ash content was determined according to NREL/TP-510-42622 procedure [32]. This test method covers the determination of ash, expressed as the percentage of residue remaining after dry oxidation at 550 to 600 °C.
2.5. Thermogravimetric Analysis
Thermogravimetric analysis (TGA) of lignins was carried out using the thermogravimetric analyzer TGA/DSC 1 (Mettler Toledo AG, Schwerzenbach, Switzerland). The analysis was performed in a reduction atmosphere. The atmosphere was ensured by nitrogen with a purity of 3.0, and the flow rate was 50 mL/min. Measurements were performed in a temperature range from 30 to 800 °C. At the beginning, the sample was conditioned at 30 °C for 3 min. Subsequently the heating rates for each measurement were 1, 3, 5, 7, 10, and 15 °C/min. The measurements were finished at 800 °C for 3 min.
2.6. Kinetic Analyses
Kinetic analyses of non-isothermal processes at a constant heating rate were based on the single step kinetic equation (Equation (3)):
where β is the heating rate, expressed as follows:
where T is the absolute temperature, t is the reaction time, and α is the degree of conversion, which is defined as follows:
where m0 is the initial weight, mi is the weight of the sample at a given time t during the decomposition process, and m∞ is the final weight of the sample at the end of the decomposition. k(T) is a temperature function depending solely on the temperature and f(α) is a conversion function depending solely on the degree of conversion of the process. The temperature function k(T) is usually considered the reaction rate constant, which is generally expressed by the Arrhenius equation (Equation (6)), as follows:
where A is the pre-exponential factor, Eα is the activation energy, and R is the gas constant. Combinations of Equation (3) and Equation (6), after separation of variables and integration, lead to the result:
where Tm is the temperature at which the maximum conversion rate (dα/dT) is achieved. The Arrhenius kinetic parameters A′ and B can be obtained from the measurements with linear heating from the treatment of experimental data by applying the Equation (5), where the relationship between the Arrhenius kinetic parameters and the fitted parameters are as follows:
Here, the presented method based on Equation (7) is an integral isoconversional method. This method is very similar to popular Kissinger–Akahira–Sunose and Flynn–Wall–Ozawa integral methods, but contrary to these methods, our approach does not use any mathematical approximation of the temperature integral of Equation (7). The temperature integral was calculated numerically during the non-linear regression analysis. Therefore, the obtained kinetic parameters were not burdened by the integral approximation´s errors.
2.7. Molecular Weight Distribution of Lignin
Molecular weight and molecular weight distribution (MWD) are important macromolecular traits of lignin that influence both its reactivity and physicochemical properties [33]. The original lignin sample was acetylated before size exclusion chromatography (SEC) analysis using tetrahydrofuran (THF) as the eluent (mobile phase). Lignin was acetylated according to the following procedure. A quantity of 300 mg of lignin was mixed with 3 mL of acetic anhydride and 3 mL of pyridine as catalyst. The reaction was carried out at 25 °C for 18 hours under a nitrogen atmosphere. Acetylated lignin was isolated in a 150 mL mixture of dichloromethane and methane (9:1) while stirring for 30 min. The organic phase was washed with 150 mL HCl (2 M), then 150 mL aqueous NaHCO3. The final washing was carried out with 150 mL of distilled water to remove NaHCO3. The organic phase was removed in a vacuum rotary evaporator under reduced pressure. As calibration standards, polystyrenes were used in the range of 376–2570·103 molar mass.
The MWD of the prepared lignin samples was measured by SEC using an Agilent 1100 instrument (Agilent Technologies, Waldbronn, Germany) with the following installed parts: Isocratic Pump G1310A, UV detector VWDG1314B, column PolarGel, Thermostatted Column Compartment G1316A. The acetylated lignins were dissolved in THF (c = 5 mg/mL), analyzed at a flow rate of 1 mL/min, and monitored at 212 nm.
3. Results and Discussions
3.1. Elemental Composition of Lignins
The impact of various lignin resources and different separation processes resulted in different elemental compositions in the isolated lignin samples, including different elemental ratios of O/C and H/C (Table 3). The elemental composition is relevant for the biomass energy utilization because the energy generated by thermal degradation is associated with the enthalpy of C, H, and S. Therefore, low O/C and H/C ratios are advantageous in the use of lignocellulosic materials for energy and affect HHV [34].

Table 3.
Elemental composition of lignin samples with the ash content and higher heating values.
3.2. Thermal Decomposition of Lignins
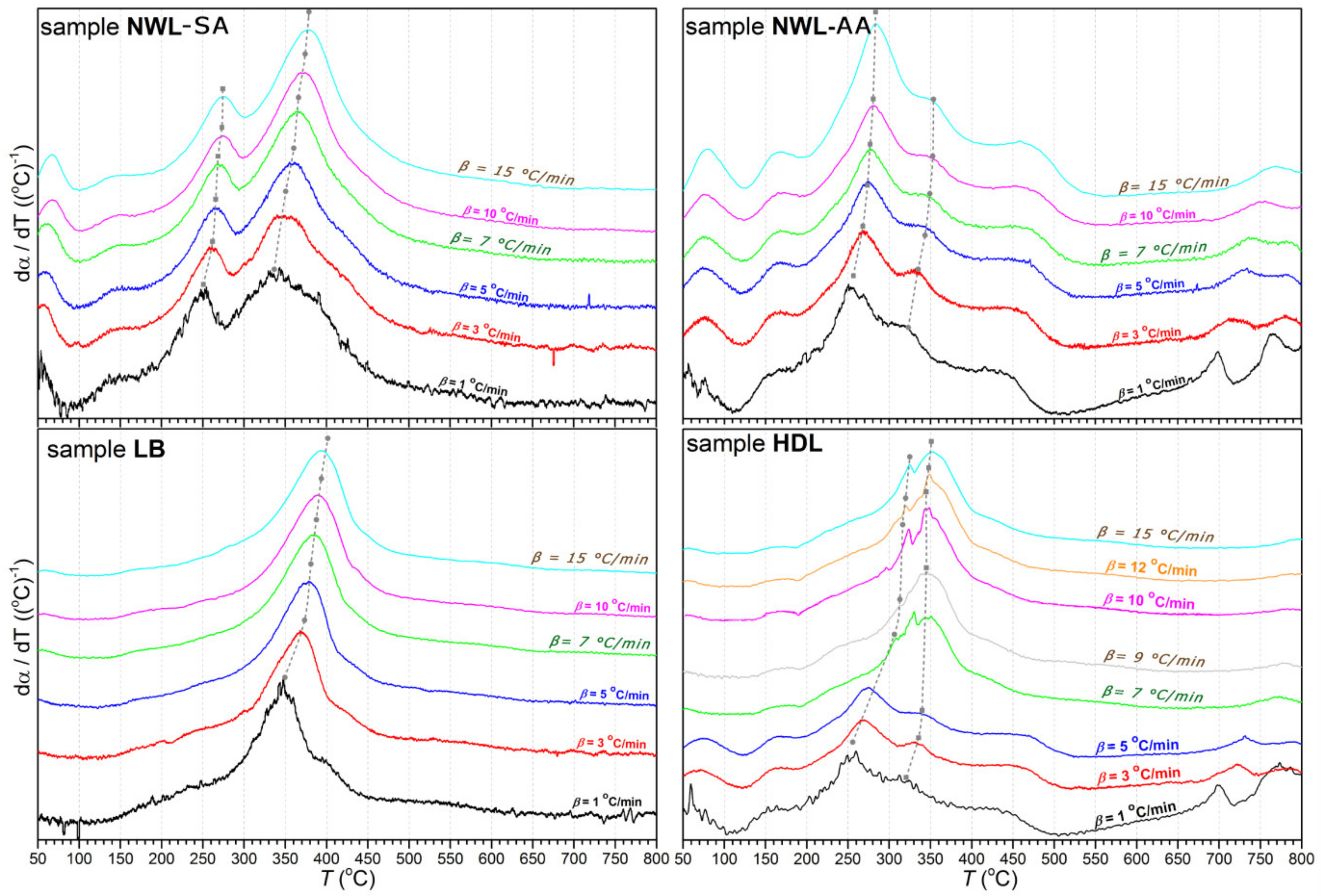
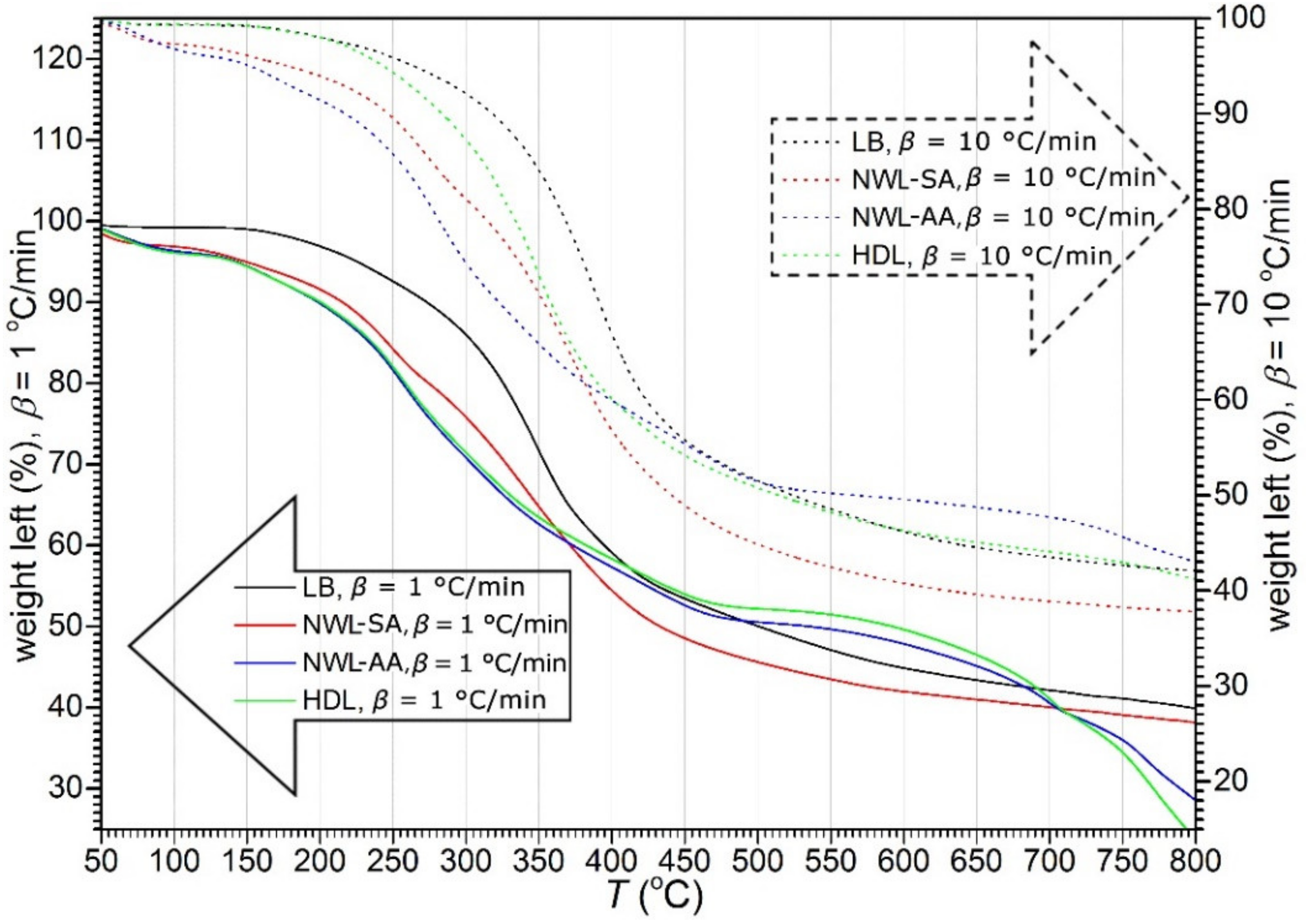
The thermogravimetric (TG) and first derivative of the TG (DTG) curves of lignin samples are plotted in Figure 1; Figure 2, respectively. The curves showed that thermal decomposition occurred over a wide temperature range beginning at approximately 150 °C. A major loss of weight was accompanied by devolatilization with charring. A highly exothermal reaction occurred between 200 and 500 °C, while the char residue at 800 °C varied between 34% and 42%. In addition, an increase in the heating rate resulted in a greater mass loss (Figure 2). This phenomenon can be explained by the restrictions in mass and heat transfer in the particles [35,36,37].
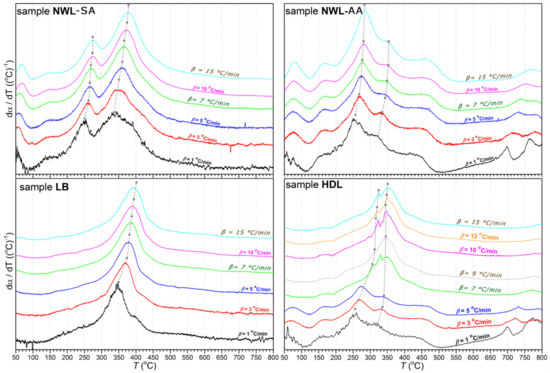
Figure 1.
dα/dT curves of lignin samples at all heating rates (from β = 1 °C/min to 15 °C/min).
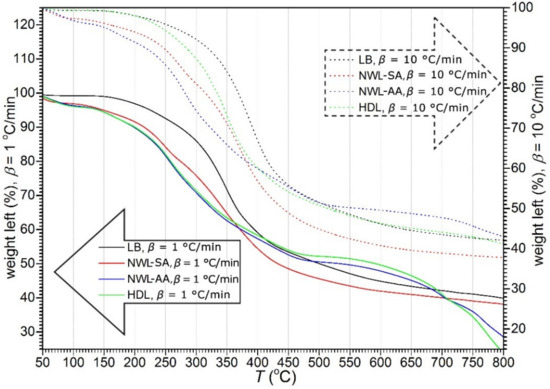
Figure 2.
The derivative thermogravimetry (DTG) curves of lignin samples at two heating rates. Solid lines (belonging to the left axis) represent weight loss during the heating rate β = 1 °C/min and dashed lines (belonging to the right axis) represent weight loss during the heating rate β = 10 °C/min.
Kinetic studies of the thermal degradation processes have been used to obtain a fundamental understanding of the structural changes in isolated lignin fractions. The thermal characteristics of the isolated lignin samples are given in Table 4. Maximum decomposition temperature (Tm) increased for all lignin samples at the heating rate from 1 to 15 °C/min. For the parameter Tdehyd, the trend in changes at the different heating rates was ambiguous. The lowest values for the parameter αdehyd were found for the LB lignin.

Table 4.
Values of characteristic temperatures and conversions observed for the first and second peaks (numbered 1 and 2) at various heating rates.
Kinetic parameters, such as the pre-exponential factors and activation energies, are presented in Table 5. The activation energy values of the lignin samples ranged from 93 to 281 kJ/mol. For the LB lignin, the only value determined was 174.9 kJ/mol. On the contrary, the thermal decomposition of the HDL, NWL-SA, and NWL-AA lignins occurred in two stages with different activation energies. Values of the coefficient of determination (R2) found for the four lignin samples indicated a suitable fit between kinetic parameters (Table 5).

Table 5.
Kinetic parameters obtained by a nonlinear least squares method.
The activation energy values, reported by various authors and given in Table 1, depended on the properties of the isolated lignins and ranged from 8.5 to 197.3 kJ/mol. Lignin properties are mostly affected by the method of isolation from lignocellulosic materials. A similar event occurs during lignin precipitation from liquors, whereby the pH value, temperature, applied precipitating agent and its concentration, stirring mode, etc., had a significant influence on the thermal properties of lignins. In general, thermal stabilities are influenced not only by the inherent structure and various functional groups of lignin polymers, but also by some other structural features of the lignin macromolecules, such as the degree of branching and condensation [38,39]. Lignin monomer units are built into the macromolecule mainly by ether bonds, and the ether bonds between syringyl units are easier to split than those between guaiacyl units. Lignin with a higher proportion of guaiacyl units has a higher activation energy and a higher thermal stability. However, noncovalent interactions can also affect the thermal properties of lignins (e.g., hydrogen bonding) [28]. For the sake of application purposes, these results show the potential of the LB lignin for direct applications in biocomposites where a higher thermal resistance is required. The HDL lignin can be used as an antioxidant, filler, or additive in rubber production.
3.3. Molecular Weight Distribution of Lignins
High average molecular weights were found for the NWL-SA (1933 g/mol) and HDL (1711 g/mol) lignins, which corresponded with the values reported for similar lignin types [40,41]. The other two samples showed substantially lower average molecular weights (i.e., 621 g/mol for the NWL-AA lignin and 622 g/mol for the LB lignin, respectively) (Table 6). In the case of the NWL-AA lignin prepared by ultrafiltration, the conditions for the passage of the membrane fraction were directly created. A pronounced bimodal character of the MWD curve was observed for the NWL-SA lignin, which may indicate the presence of a carbohydrate fraction. On the other hand, the higher molecular weight of the NWL-SA lignin suggests the presence of condensation reactions in lignin during sulfuric acid precipitation. In the case of the LB lignin, the effect of a specific method of preparation on the lignin structure was clear. This sample was characterized by a higher content of low molecular weight fractions. Although all four lignin samples showed varying quantities of methoxyl groups, the relationship between the methoxyl group content and the lignin molecular weight was not significant.

Table 6.
Macromolecular traits of lignin samples determined by size exclusion chromatography.
In addition, all lignin samples showed a similar polydispersity, but their MWD was completely different (distributional curves are not shown). Lignins with bimodal MWDs were thermally decomposed in two stages, whereas the LB lignin with a unimodal MWD was decomposed in a single thermal stage (Table 5). These conditions point out a greater proportion of guaiacyl and syringyl units that tend to disable the stabilization of C-C bonds at C5 position on the aromatic ring. These results have also been confirmed in other studies [40,42].
4. Conclusions
The elemental composition of the four types of isolated lignin affected their thermal stabilities, activation energies, and HHVs. The lignin samples showed varying amounts of functional groups, inorganic component compositions, and molecular weight distributions. An increase in the heating rate resulted in both a greater mass loss of the lignin and an enhanced maximum thermal decomposition temperature. The activation energy values ranged from 93 to 281 kJ/mol. Even though all of the lignin samples showed a similar polydispersity, the distribution of their molecular weight was different. Lignins with bimodal MWDs were thermally decomposed in two stages, whereas the LB lignin showing a unimodal MWD was decomposed in a single thermal stage. Based on its thermal properties, the LB lignin may find direct applications in biocomposites where a higher thermal resistance is required. The HDL lignin can be used as an antioxidant, filler, or additive in rubber production. Although the HDL lignin contains substantially more sulfur, this drawback may be a desirable property during the production of rubber, when the content of sulfur can have a positive effect as a vulcanization agent.
Author Contributions
A.H. and M.J. contributed equally to the conceptualization and design of the work; writing—original draft preparation: A.H., M.J., F.K., and T.B.; writing—review and editing: F.K, T.B., and J.Ď.; software and visualization: A.H.; methodology and formal analysis: A.H., M.J., and I.Š.; investigation: A.H. and F.K.; supervision and critical revision of the manuscript: F.K and J.Ď.; project administration: F.K. and I.Š.; funding acquisition: F.K. All of the authors approved the version of the manuscript submitted for publication.
Funding
This work was supported by the Slovak Research and Development Agency under the contracts Nos. APVV-15-0052, APVV-14-0393, APVV-16-0088, APVV-16-0326, VEGA 1/0403/19, and VEGA 1/0387/18. This article was realized also thanks to the support for infrastructure equipment provided by the Operation Program Research and Development for the project “National Center for Research and Application of renewable energy sources” (ITMS 26240120016, ITMS 26240120028), for the project “Competence center for new materials, advanced technologies and energy” (ITMS 26240220073), and for the project “University science park STU Bratislava” (ITMS 26240220084), co-financed by the European Regional Development Fund.
Acknowledgments
The authors would like to acknowledge the financial support of the Slovak Research and Development Agency and support for infrastructure equipment provided by the Operation Program Research and Development.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef]
- Mattinen, M.-L.; Riviere, G.; Henn, A.; Nugroho, R.W.N.; Leskinen, T.; Nivala, O.; Valle-Delgado, J.J.; Kostiainen, M.A.; Österberg, M. Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials 2018, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Sathitsuksanoh, N.; Holtman, K.M.; Yelle, D.J.; Morgan, T.; Stavila, V.; Pelton, J.; Blanch, H.; Simmons, B.A.; George, A. Lignin fate and characterization during ionic liquid biomass pretreatment for renewable chemicals and fuels production. Green Chem. 2014, 16, 1236–1247. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products—Strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Delmotte, L.; Abdalla, S. Analysis of the Cross-Linking Reaction of Lignin with Triethyl Phosphate by MALDI-TOF and 13C NMR. Polymers 2017, 9, 206. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Gorgens, J.F. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Šimon, P. Isoconversional methods—Fundamentals, meaning and application. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Gašparovič, L.; Labovský, J.; Markoš, J.; Jelemenský, L. Calculation of Kinetic Parameters of the Thermal Decomposition of Wood by Distributed Activation Energy Model (DAEM). Chem. Biochem. Eng. Q. 2012, 26, 45–53. [Google Scholar]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. A systematic study of the kinetic of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Ramiah, M.V. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Chan, R.W.; Krieger, B.B. Kinetics of dielectric-loss microwave degradation of polymers—lignin. J. Appl. Polym. Sci. 1981, 26, 1533–1553. [Google Scholar] [CrossRef]
- Nunn, T.R.; Howard, J.B.; Longwell, J.P.; Peters, W.A. Product compositions and kinetics in the rapid pyrolysis of milled wood lignin. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 844–852. [Google Scholar] [CrossRef]
- Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W. Pyrolysis of lignins: Experimental and kinetics studies. Energy Fuels 2002, 16, 1405–1412. [Google Scholar] [CrossRef]
- Avni, E.; Coughlin, R.W. Kinetic analysis of lignin pyrolysis using non-isothermal TGA data. Thermochim. Acta 1985, 90, 157–167. [Google Scholar] [CrossRef]
- Pasquali, L.C.E.; Herrera, H. Pyrolysis of lignin and IR analysis of residues. Thermochim. Acta 1997, 293, 39–46. [Google Scholar] [CrossRef]
- Rao, T.R.; Sharma, A. Pyrolysis rates of biomass materials. Energy 1998, 23, 973–978. [Google Scholar] [CrossRef]
- Dominguez, J.C.; Oliet, M.; Alonso, M.V.; Gilarranz, M.A.; Rodriguez, F. Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crops Prod. 2008, 27, 150–156. [Google Scholar] [CrossRef]
- Svenson, J.; Pettersson, J.B.C.; Davidsson, K.O. Fast pyrolysis of the main components of birch wood. Combust. Sci. Technol. 2004, 176, 977–990. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, S.; Wang, S.; Guo, X. Thermogravimetric analysis of pyrolysis characteristics of alkali lignin. Trans. China Pulp Paper 2007, 22, 31–34. [Google Scholar]
- Murugan, P.; Mahinpey, N.; Johnson, K.E.; Wilson, M. Kinetics of the pyrolysis of lignin using thermogravimetric and differential scanning calorimetry methods. Energy Fuels 2008, 22, 2720–2724. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Li, B.; Chen, H. TG study on pyrolysis of biomass and its three components under syngas. Fuel 2008, 87, 552–558. [Google Scholar] [CrossRef]
- Mani, T.; Murugan, P.; Mahinpey, N. Determination of distributed activation energy model kinetic parameters using simulated annealing optimization method for nonisothermal pyrolysis of lignin. Ind. Eng. Chem. Res. 2009, 48, 1464–1467. [Google Scholar] [CrossRef]
- Beis, S.H.; Mukkamala, S.; Hill, N.; Joseph, J.; Baker, C.; Jensen, B.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G.; van Heiningen, A.; et al. Fast pyrolysis of lignins. BioResources 2010, 5, 1408–1428. [Google Scholar]
- Yang, Q.; Wu, S.B. Thermogravimetric characteristics of wheat straw lignin. Cellulose Chem. Technol. 2009, 43, 4–6. [Google Scholar]
- Poletto, M.; Zattera, A.J. Materials produced from plant biomass: Part III: Degradation kinetics and hydrogen bonding in lignin. Mat. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef]
- Domburg, G.E.; Sergeeva, V.N. Thermal degradation of sulphuric acid lignins of hard wood. J. Therm. Anal. 1969, 1, 53–62. [Google Scholar] [CrossRef]
- Jablonský, M.; Ház, A.; Orságová, A.; Botková, M.; Šmatko, L.; Kočiš, J. Relationships between elemental carbon contents and heating values of lignins. In Proceedings of the 4th International Conference Renewable Energy Sources 2013, Tatranské Matliare, High Tatras, Slovakia, 21–23 May 2013; pp. 67–72. [Google Scholar]
- Jablonsky, M.; Botkova, M.; Adamovska, J. Prediction of methoxyl groups content in lignin based on ultimate analysis. Cellul. Chem. Technol. 2015, 49, 165–168. [Google Scholar]
- NREL. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Kačík, F.; Ďurkovič, J.; Kačíková, D. Structural characterization of lignin by syringyl to guaiacyl ratio and molecular mass determination. In Lignin: Structural Analysis, Applications in Biomaterials and Ecological Significance; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 67–89. ISBN 978-1-63117-452-0. [Google Scholar]
- Pereira, B.L.C.; de Carneiro, A.C.O.; Carvalho, A.M.M.L.; Colodette, J.L.; Oliveira, A.C.; Fontes, M.P.F. Influence of chemical composition of eucalyptus wood on gravimetric yield and charcoal properties. Bioresources 2013, 8, 4574–4592. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energy 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Ridout, A.J.; Carrier, M.; Görgens, J. Fast pyrolysis of low and high ash paper waste sludge: Influence of reactor temperature and pellet size. J. Anal. Appl. Pyrolysis 2015, 111, 64–75. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, A.; Knoetze, J.H. Using apparent activation energy as a reactivity criterion for biomass pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Quantitative structures and thermal properties of birch lignins after ionic liquid pretreatment. J. Agric. Food Chem. 2013, 61, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sheng, Y.; Sun, S.L.; Yuan, T.Q.; Sun, R.C. Structural characterization and thermal properties of enzymatic hydrolysis lignins. In Lignin: Structural Analysis, Applications in Biomaterials & Ecological Significance; Nova Science Publisher: New York, NY, USA, 2014; p. 109. ISBN 978-1-63117-452-0. [Google Scholar]
- Serrano, L.; Toledano, A.; García, A.; Labidi, J. Obtain lignins for specific applications. In Lignin: Properties and Applications in Biotechnology and Bioenergy; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 139–182. ISBN 978-1-61122-907-3. [Google Scholar]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Paterson, J.R. Lignin: Properties and Applications in Biotechnology and Bioenergy; Nova Science Publisher: New York, NY, USA, 2012; p. 558. ISBN 978-1-61122-907-3. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).