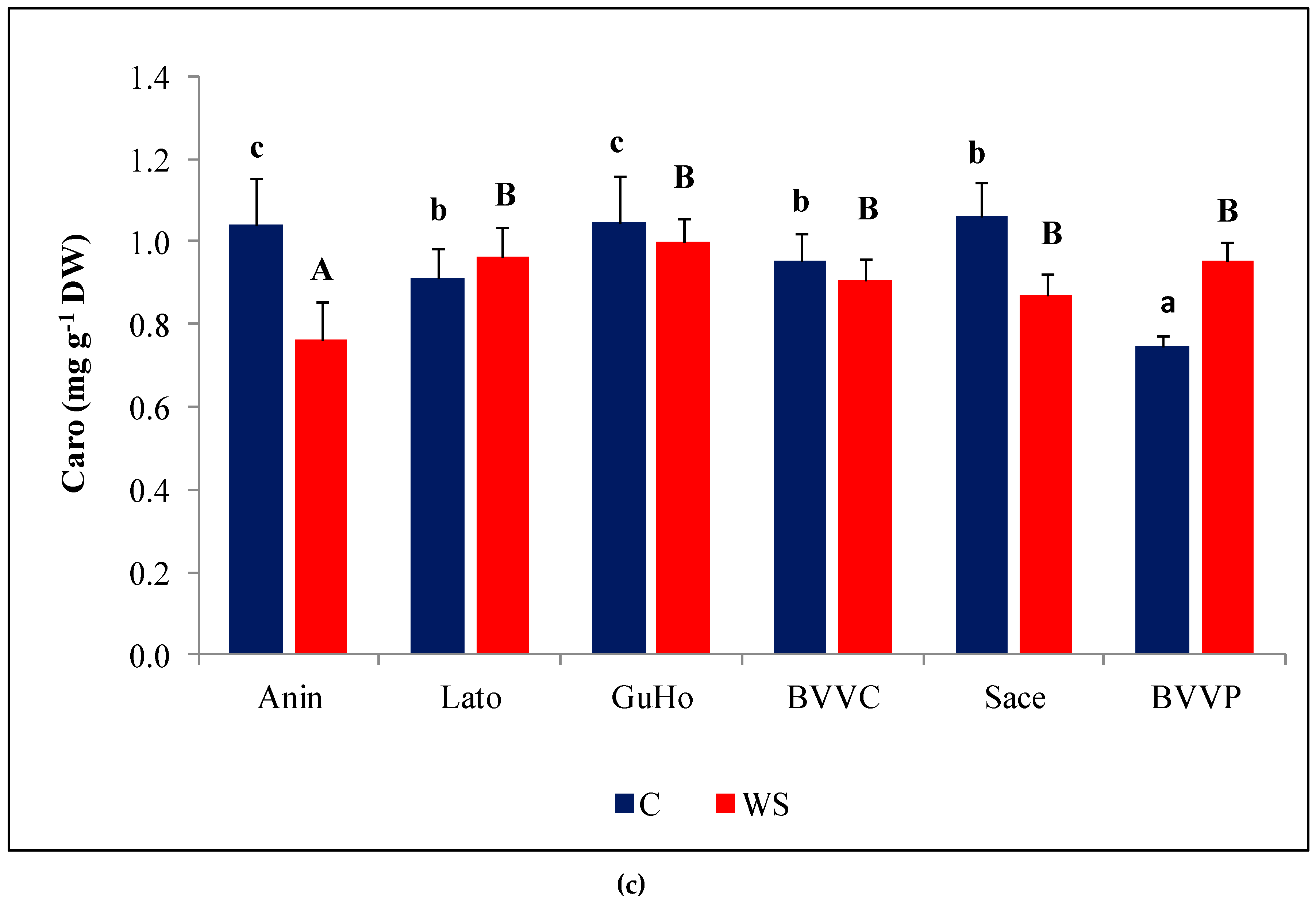Abstract
European larch (Larix decidua Mill.) has been reported either as more tolerant or as more sensitive to drought than conifers with perennial leaves. Previous studies have revealed that Carpathian populations of European larch display a high genetic variability. A comparative study of the responses of these populations to drought stress at the seedling stage might allow the identification of drought tolerant genotypes and reliable drought stress biomarkers, which could be eventually used for the early detection of drought effects in larch, not only under control greenhouse conditions, but also in their natural stands. Growth responses were analyzed in larch seedlings from six Romanian Carpathian populations, submitted to one month of mild drought stress under controlled conditions. Levels of photosynthetic pigments (chlorophylls a and b, and carotenoids), osmolytes (proline and total soluble sugars), monovalent cations (Na+ and K+), and malondialdehyde (MDA) and non-enzymatic antioxidants (total phenolics and flavonoids) were compared with control treatments and between populations. Growth and the pattern of the biochemical responses were very similar in the six populations. Drought stress lead to stem length decrease in all population, whereas reduction of fresh weight of needles was significant only in one population (BVVC), and reduction of water content of needles in two populations (BVVC and GuHo). The optimal biochemical traits for an early detection of drought symptoms in this species is the increase—in most populations—of total soluble sugars, MDA, and total phenolic compounds, whereas K+ reduction was significant in all populations. Photosynthetic pigments remained unchanged, except for the Anin population where they were reduced under stress. Multivariate principal component and hierarchical clustering analyses confirmed the impact of drought in the growth and physiology of European larch, and revealed that the humidity of the substrate was positively correlated with the growth parameters and the levels of K+ in needles, and negatively correlated with the levels of MDA, total soluble sugars, total phenolic compounds, and flavonoids in needles.
1. Introduction
Drought, as a consequence of climate change, is a threat to many forest stands, which will have to cope with new adverse environmental conditions within their current natural habitats [1,2,3,4,5]. European coniferous forests are among those ecosystems forecasted to be susceptible to the effects of global warming; the negative ones such as drought and disturbance risk will probably outweigh the favorable effects of increasing atmospheric CO2 and warmer temperatures [6,7,8]. In addition, deforestation and continuous soil degradation contribute to the acceleration of the climate change effects. The predicted environmental changes will affect natural areas of growth for a large number of tree species, but trees like other plants can respond in different ways. Existing forest stands may withstand these changing climate conditions for some time, but long-term responses might depend on the capability of species to adapt [6]. This capability can be determined by the variation within and between species in their physiological responses to changes such as water deficiency. A high phenotypic plasticity of forest species is essential for surviving stressful conditions [7].
Depending on their intensity, the abiotic stress factors can have significant influences on wood formation. This impact not only is dependent on the tree species but also varies across provenances within a species [9]. Drought can significantly affect germination capacity of seeds, as well as the development of seedlings and also cause decrease of plant biomass of the coniferous species [10]. Prolonged droughts may inhibit the uptake of water and mineral nutrients by plants, induce carbon starvation, but also affect translocation of primary and secondary metabolites, and facilitate biotic attack [1,11].
Summer droughts and heat waves predicted for Central and Southern Europe bring new challenges for the conservation of European forests [12]. New measures should be implemented in order to mitigate the effects of deleterious environmental conditions such as drought. One such strategy would be the replacement of sensitive populations with more tolerant ones of the same species, which could potentially respond better to the forecasted climate changes during their life cycle [13]. In the case of coniferous species with a long life span, assessment of drought tolerance is more effective at the seedling stage, when a large number of genotypes can be screened in a relatively brief period. Moreover, seed germination and seedling development represents the bottleneck of the biological cycle of plants, and in trees, seedling survival is a key factor for tree recruitment, and evolution of forests [14]. There is an urgent need to increase our knowledge on seedling responses of forest species to drought in areas where future extensive drought periods are forecasted. Responses to drought are heritable but variable within populations of one species [5], and are often being conditioned by the environmental conditions in which parental plants develop [15]. Therefore, for better management of forest stands, as well as for reforestation and afforestation programs, not only a genetic, but also a screening of the phenotype plasticity of different populations of the same species are highly recommended [16].
European larch (Larix decidua Mill.) has a disjunctive distribution in Europe, with a center in the subalpine belt of the Central Alps, extending from southeastern France and southwestern Italy to eastern Poland and central Romania, in a large altitudinal range, from 160 m in the Polish lowland to 2900 m altitude in the western Alps [17] The total natural area of European larch in Romania is small, representing only 0.3% of the national forest area [18], whereas in Europe it represents 1% of total forest and 4% of coniferous forest. On the other hand, in Romania as in many other European countries, European larch was introduced in many new locations outside its natural range, but also its native range has been extended by foresters [19,20]. European larch has been traditionally used due to its high-quality wood, resistance to rot, durability, strength, and moderately high density, with excellent toughness and stiffness. Due to its fast growth rate, frugality, and pioneer characteristics, it is highly indicated in reforestation and afforestation programs. Besides its interest as an important timber species in Europe, L. decidua also has a high potential as an ornamental tree, much appreciated as being one of the few coniferous species with deciduous foliage with changing colors, frequently used not only in forest landscaping but also in many urban areas.
The present study analyses responses to mild drought stress in seedlings of different Carpathian provenances of European larch. A previous study [21] in which these provenances were genetically characterized revealed that these populations, despite displaying high levels of heterozygosis at the individual level and variation among individuals within the same population, had a moderate degree of genetic differentiation, indicating that some alleles are more frequent in certain populations than in others. This genetic differentiation, albeit limited, may have important implications for adaptation to specific environments, as natural selection and other microevolutive forces may have favored the fixation or increase of frequency of certain adaptive alleles or genomic regions for adaptation to local conditions in the different populations evaluated. Due to global warming, drought represents an increasing threat for many Southern and Central European forest stands, including many of those located in Romania. The comparative analysis of the responses to drought in seedlings with different provenances, as well as among individuals, will allow the selection of genotypes better adapted to drought to be used in reforestation programs. The study could also provide useful information for management of parks and other green areas. The aim of the study was two-fold: (i) to assess the responses to drought in seedlings from six populations of L. decidua from the Romanian Carpathian Mountains; and, (ii) to identify the underlying biochemical traits related to these responses.
2. Materials and Methods
2.1. Plant Material
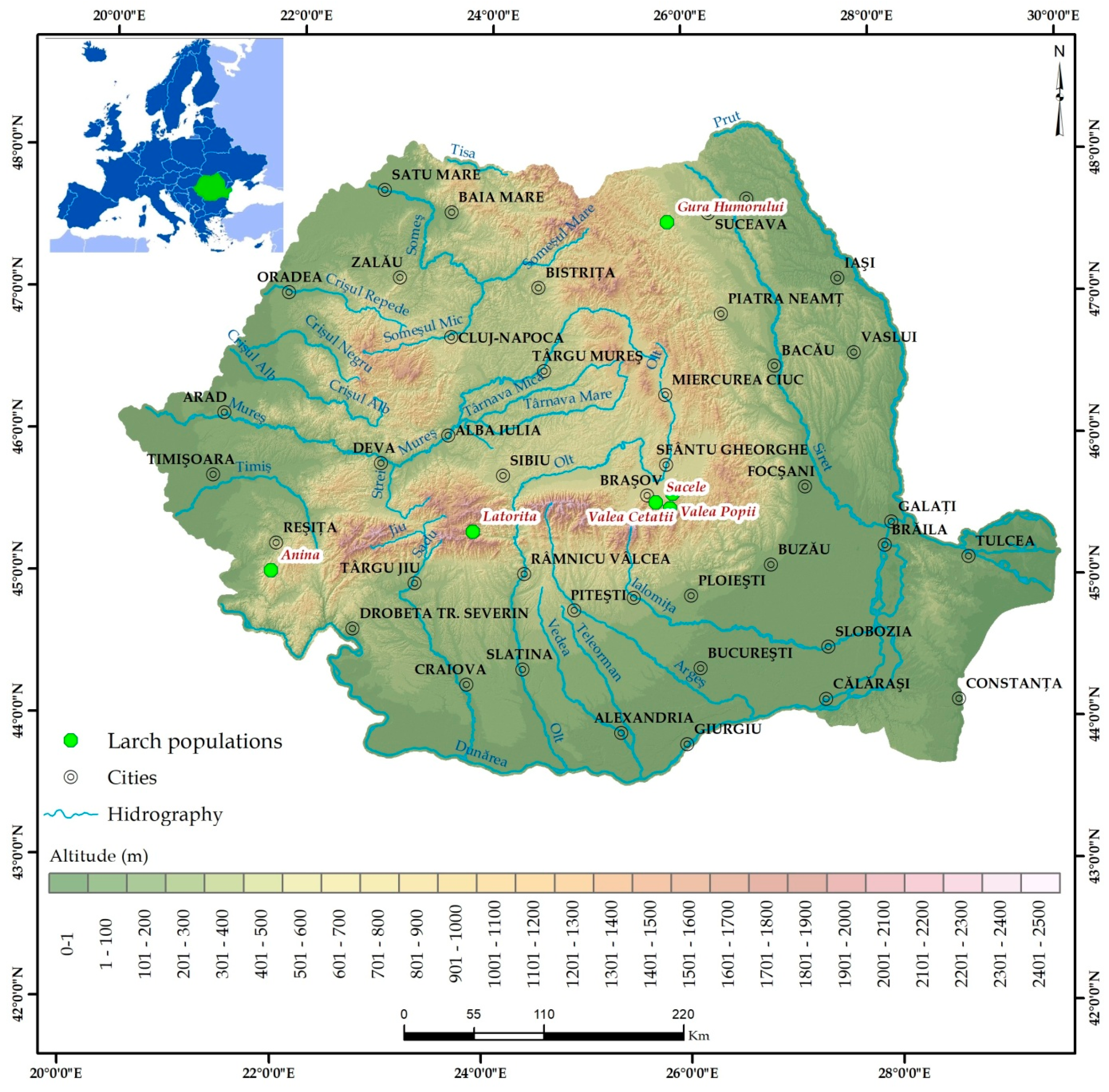
Plants were obtained by germination of seeds sampled from six genetically different populations of European larch [21] from Romania: Anina (Anin), Latorita (Lato), Gura Humorului (GuHo), Brasov Valea Cetatii (BVVC), Sacele (Sace), and Brasov Valea Popii (BVVP) (Figure 1).
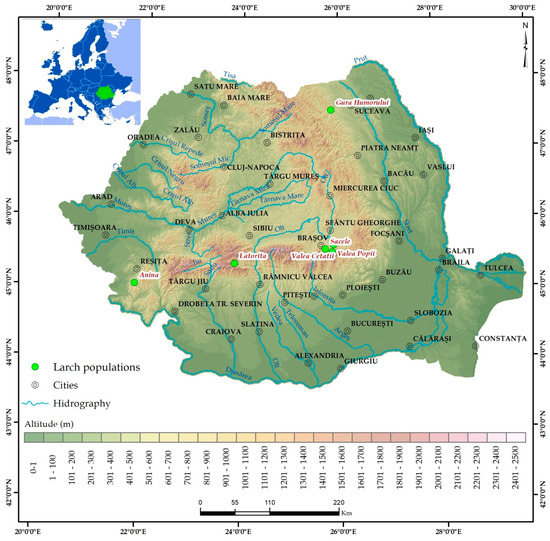
Figure 1.
Origin of the European larch seeds used to obtain the seedlings.
Seeds were sown on a mixture of peat (50%), perlite (25%), and vermiculite (25%) and grown during the whole experiment in standard 1 L pots under the following controlled conditions: long-day photoperiod (16 h of light), temperature of 23 °C during the day and 17 °C at night, and 50–80% relative humidity, in the greenhouses of the Institute for Plant Molecular and Cell Biology (IBMCP), Polytechnic University of Valencia (UPV), Valencia, Spain. The seedlings were watered with Hoagland’s nutrient solution [22] in the two-month growth period before applying stress treatments.
Plantlets with odd or irregular growth were discarded in order to maintain uniformity among the studied provenances. Plants of each provenance were then distributed at random in two treatments (drought vs. control), with five biological replicas per provenance and per treatment and placed in plastic trays (10 pots per tray). Those subjected to drought were not irrigated for the whole duration of the treatment, whereas the substrate of control plants was kept moist by watering twice per week with 1 L Hoagland’s nutrient solution applied to each tray. After 30 days, treatments were stopped, seedlings were sampled, and the length of stems and the total weight of needles were measured. A fraction of the needles of each plant was oven-dried at 65 °C for 72 h and weighed again for the calculation of the percentage of dry weight (DW).
All measurements and biochemical assays were performed using leaf material, which was harvested at the end of the applied treatments. The effect of applied drought on plant growth was studied by measurements of stem length, total fresh weight (hereafter FW) of the needles, and water content percentage (WC) in the needles, calculated according to Al Hassan et al. [23]. Dry weight vapor pressure deficit and evapotranspiration in the above-mentioned greenhouse conditions were estimated according to standard calculations [24,25].
2.2. Substrate Analysis
Substrate humidity was determined by the gravimetric method: a fraction of each substrate sample was weighed (SW), dried in an oven at 105 °C until reaching constant weight, and then weighed again (DSW), and the substrate water content was calculated as
Substrate humidity % = [(SW − DSW)/SW] × 100
2.3. Quantification of Photosynthetic Pigments
Photosynthetic pigments, chlorophyll a (Chl a), chlorophyll b (Chl b), and total carotenoids (Caro), which are renown to be good stress biomarkers, were quantified from 0.05 g fresh leaf tissue by the method of Lichtenthaler and Wellburn [26]. Fresh leaf material was ground in the presence of liquid nitrogen, before being extracted in 80% (v/v) ice-cold acetone, by mixing overnight in an orbital shaker. The absorbance of the supernatant was measured at 663, 646, and 470 nm. Calculation of pigment concentrations was done according to the equations
Chl a (µg mL−1) = 12.21 × (A663) − 2.81 × (A646)
Chl b (µg mL−1) = 20.13 × (A646) − 5.03 × (A663)
Caro (µg mL−1) = (1000 × A470 − 3.27 × [Chl a] − 10 × [Chl b])/229
Chlorophyll and carotenoid contents were finally expressed in mg g−1 DW.
2.4. Measurement of Cationic Needle Contents
Contents of sodium (Na+) and potassium (K+) were determined in the needles of all studied provenances. Measurements were performed according to Weimberg [27]; the extracts were prepared by boiling 0.05 g of dried and ground needle tissue for one hour, after being extracted in water, followed by filtration through a 0.45 μm nylon filter. Na+ and K+ were quantified with a PFP7 flame photometer (Jenway Inc., Staffordshire, UK).
2.5. Osmolyte Quantification
Two types of osmolytes known to play a key role in abiotic stress responses and tolerance were measured in the needles of the studied plants: proline (Pro) and total soluble sugars (TSS). For both osmolytes, the extracts were prepared from fresh leaf tissue. For proline, the ninhydrin/acetic acid method [28] was utilized. Samples of 0.05 g fresh material prepared in 2 mL of a 3% (w/v) sulfosalicylic acid solution, incubated for an hour at 95 °C, cooled on ice, were extracted with toluene. Absorbance was then measured at 520 nm, and Pro was calculated and expressed in µmol g−1 DW. On the other hand, total soluble sugars were measured according to Dubois et al. [29]. Extraction was done with 80% (v/v) methanol on a rocker shaker for 24 h, before having the supernatant mixed with concentrated sulfuric acid and 5% phenol. Absorbance was then measured at 490 nm. A standard curve was obtained with glucose solutions of known concentration, and TSS contents were expressed as ‘mg equivalent of glucose’ per gram of DW.
2.6. Measuring Malondialdehyde (MDA) and Antioxidant Compounds
Fresh leaf material (0.05 g) was extracted in 80% methanol, using a rocker shaker, for 24–48 h for malondialdehyde (MDA), total phenolic compounds (TPC), and total flavonoids (TF). MDA content in the extracts was determined according to the method described by Hodges et al. [30]. The samples were mixed with 0.5% thiobarbituric acid (TBA) prepared in 20% TCA (or with 20% TCA without TBA for the controls), and then incubated at 95°C for 20 min. The absorbance of the supernatants was measured at 532 nm. The non-specific absorbance at 600 and 440 nm was subtracted and MDA concentration was determined using the equations described by Hodges et al. [30]. Total phenolic compounds (TPC) were quantified via the protocol of Blainski et al. [31], based on the reaction with the Folin–Ciocalteu reagent and measurement of absorbance at 765 nm, using gallic acid (GA) as the standard. TPC concentrations were expressed as GA equivalents (mg eq. GA g−1 DW). Total flavonoids (TF) contents were also calculated spectrophotometrically, after reaction of the extracts with NaNO2, followed by AlCl3 in the presence of NaOH, according to the protocol described by Zhishen et al. [32]. The absorbance was read at 510 nm using catechin as the standard. TF concentration was expressed as equivalents of catechin (mg eq. C g−1 DW).
2.7. Statistical Analysis
Data were analyzed using the program SPSS for Windows (SPSS Inc. Chigago, IL, USA). Before the analysis of variance, the Shapiro–Wilk test was used to check for validity of normality assumption and Levene’s test for the homogeneity of variance. If ANOVA requirements were accomplished, the significance of the differences among provenances within each treatment was tested with a one-way ANOVA, followed by post hoc comparisons using the Tukey’s HSD test at a significance level of p = 0.05. The pairwise comparisons between the control and stress treatments was performed using the calculated LSD value (p = 0.05) for the interaction between provenance and treatment in a two-way ANOVA. The mean values for combination of provenance and treatment were used for a principal component analysis (PCA). Hierarchical cluster analysis (HCA) and the corresponding heatmap were performed using the ClustVis tool [33]. Unit variance scaling for the normalized and centred data was used. Distance measures for the HCA were based on Pearson correlations, and the average clustering method with the tightest cluster first for the tree ordering option were used.
3. Results
3.1. Substrate Humidity
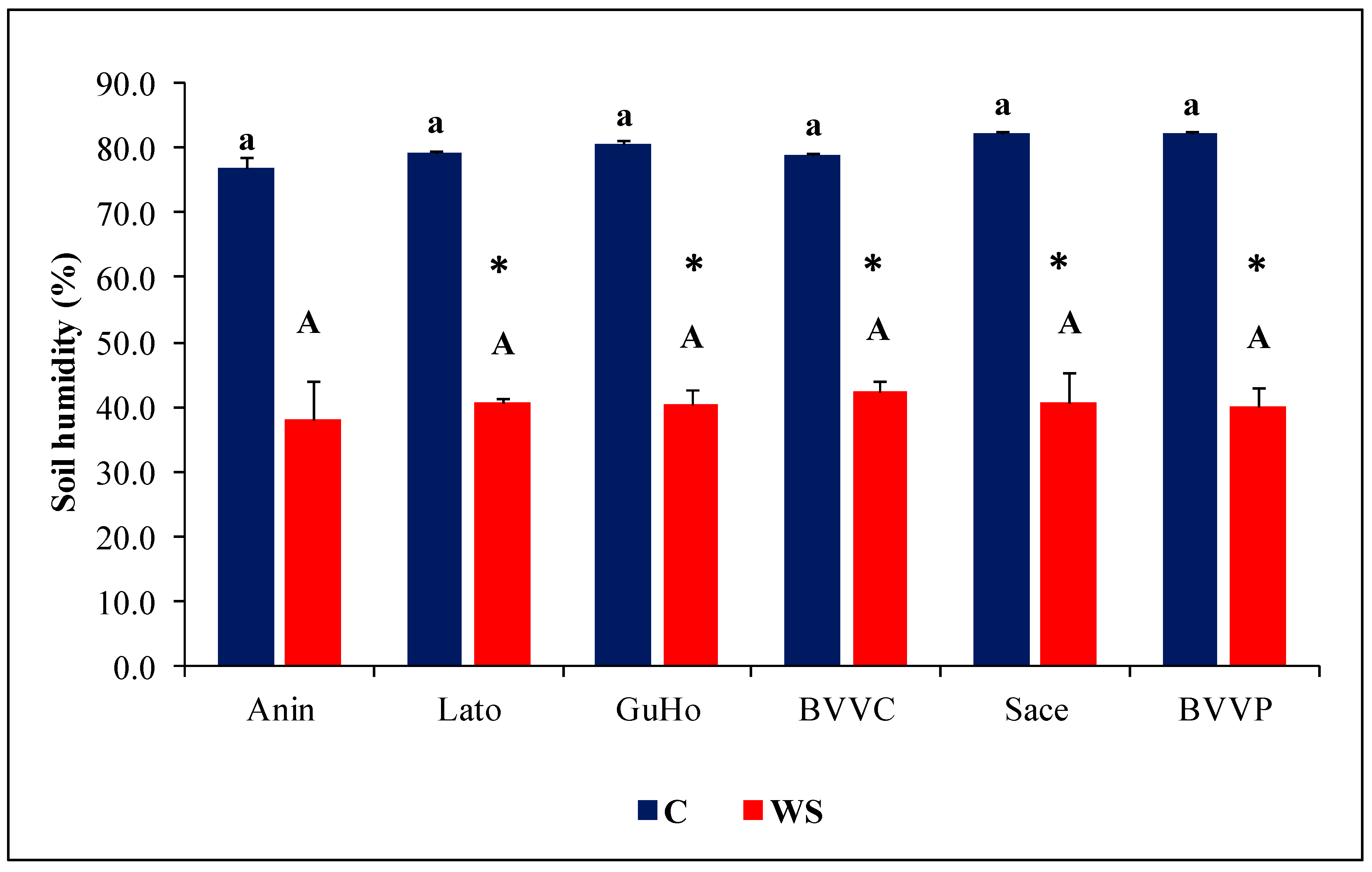
One month of irrigation withholding induced a reduction of more than half of the humidity of the substrate in all analyzed pots with respect to their controls. There were no significant differences between the different provenances either in the mean values of the control or of the drought treatments (Figure 2).
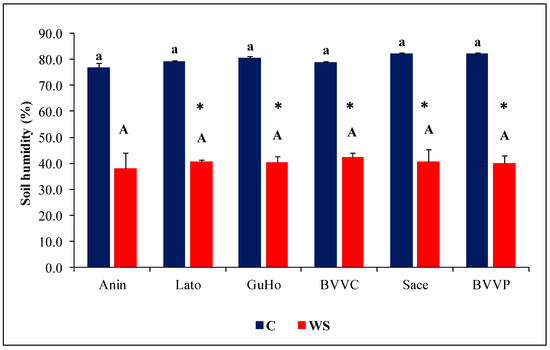
Figure 2.
Pot substrate humidity (%) after one month of treatments of six Romanian provenances of L. decidua (Mean ± SE, n = 5). Comparing provenances, different lowercase letters indicate significant differences between control plants, and different uppercase letters indicate significant differences between drought-affected plants, according to Tukey’s test (α = 0.05). Asterisks indicate a significant difference between treatments within the same provenance, according to the LSD value for the interaction (p = 0.05).
3.2. Growth Parameters
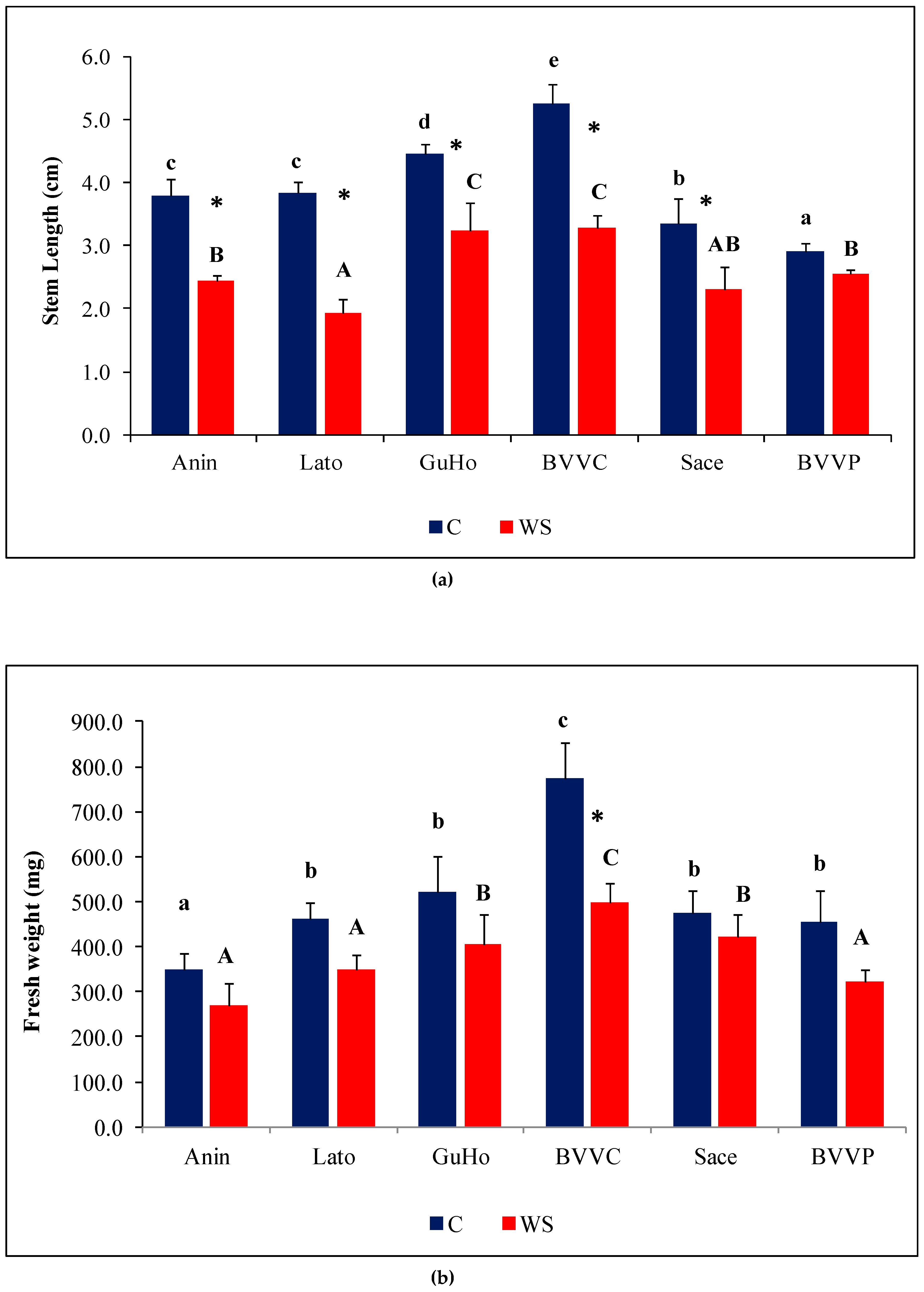
Mean seedling length varied significantly under control conditions, from 2.9 cm (provenance BVVP) to 5.3 cm (provenance BVVC) as can be seen in Figure 3a. One month of drought affected seedlings’ growth, as the stem length of affected plantlets of five provenances showed a significant relative decrease in comparison to their respective controls (Figure 3a). The degree of this decrement, however, was not uniform in the studied provenances, as BVVP seedlings recorded a non-significant decrease of 12% in stem length under drought stress, whereas in the Lato provenance a 50% decrease was observed under similar conditions. In the remaining provenances, the stress-induced reduction of SL was roughly one-third.
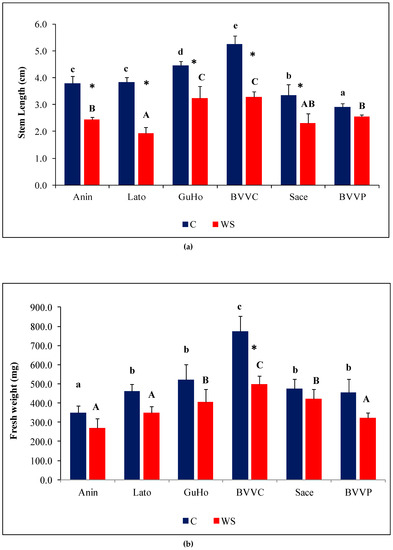
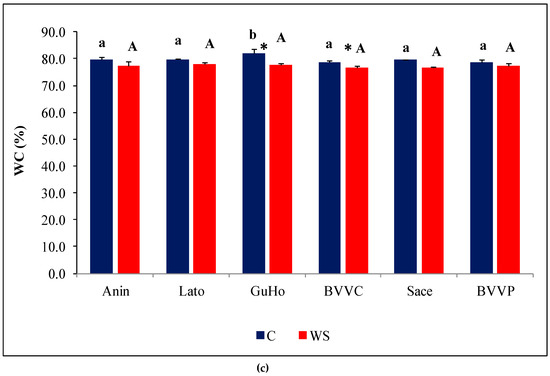
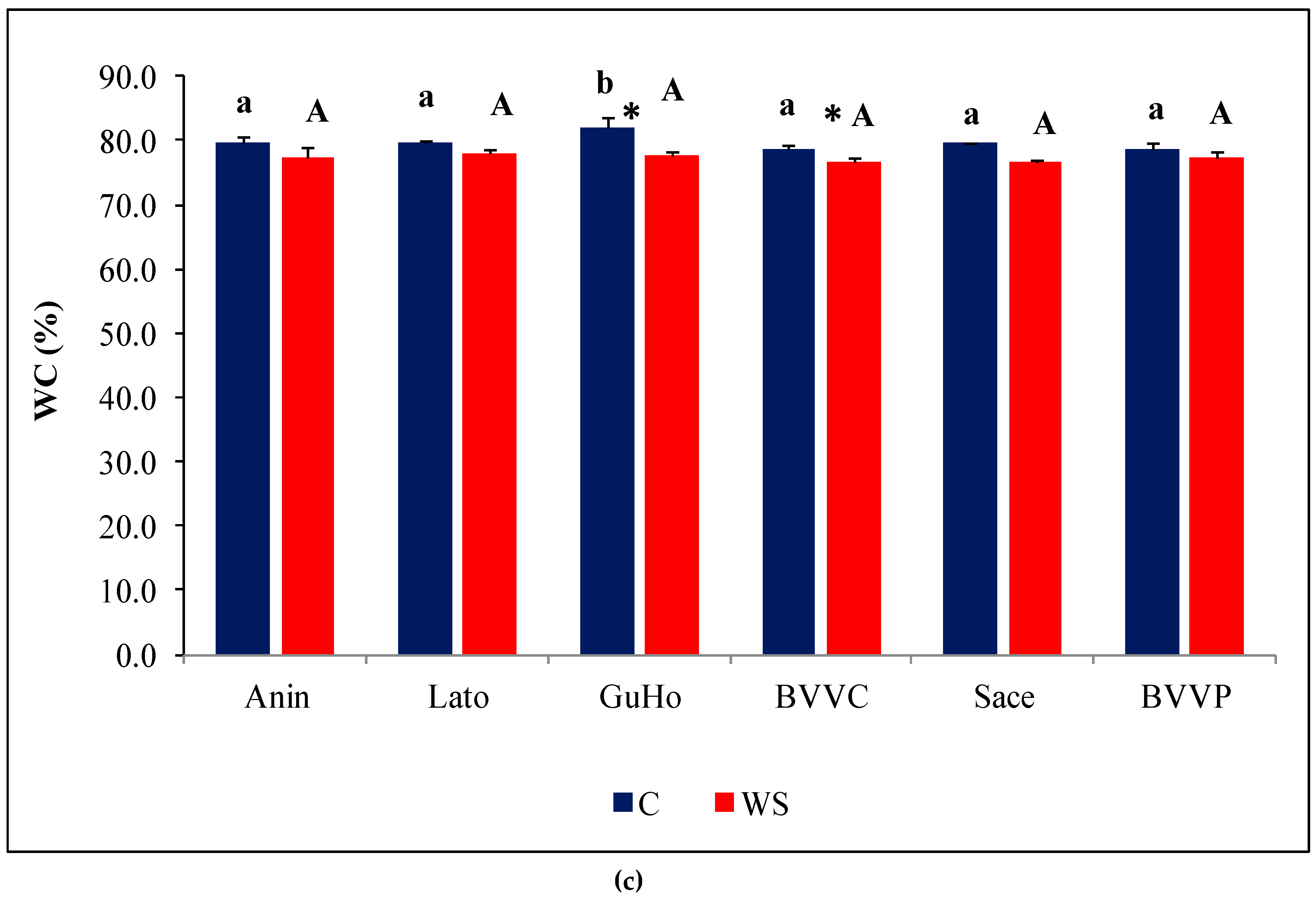
Figure 3.
Growth parameters of six Romanian provenances of L. decidua after one month of applied drought stress (Mean ± SE, n = 5): (a) Stem length elongation (cm); (b) Needles fresh weight (mg); (c) Needles water content percentage (WC). Comparing provenances, different lowercase letters indicate significant differences between control plants, and different uppercase letters indicate significant differences between drought-affected plants, according to Tukey’s test (α = 0.05). Asterisks indicate a significant difference between treatments within the same provenance, according to the LSD value for the interaction (p = 0.05).
Fresh weight of needles within each of the treatments varied among the provenances. Mean values of plants from the control treatment of BVVC doubled those from Anin. The same pattern of variation was found when comparing plants from the drought treatments. Regarding the way one month of absence of irrigation affected the plants, only differences registered in the BVVC provenance were significant (Figure 3b). Expressed in percentages, this reduction ranges from about 35% in BVVC to 11% in Sace. Another investigated growth parameter was needle water content percentage (WC), that revealed near identical values in all water-stressed plantlets across the studied provenances, at around 77% (Figure 3c). However, the reductions in WC compared to the corresponding non-stressed controls were slight, and statistically significant only in two of the analyzed provenances (GuHo and BVVC), suggesting that larch seedlings are relatively resistant to drought-induced needle dehydration or due to the mild level of drought stress. The vapor pressure deficit calculated for the greenhouse conditions during the whole experiment was of 0.87 kP, a value that corresponds to an optimal environment for a healthy transpiration of plants; evapotranspiration in the pots from the drought treatments ranged from 9.4 to 11.6 mL/day and plant/pot.
3.3. Photosynthetic Pigments
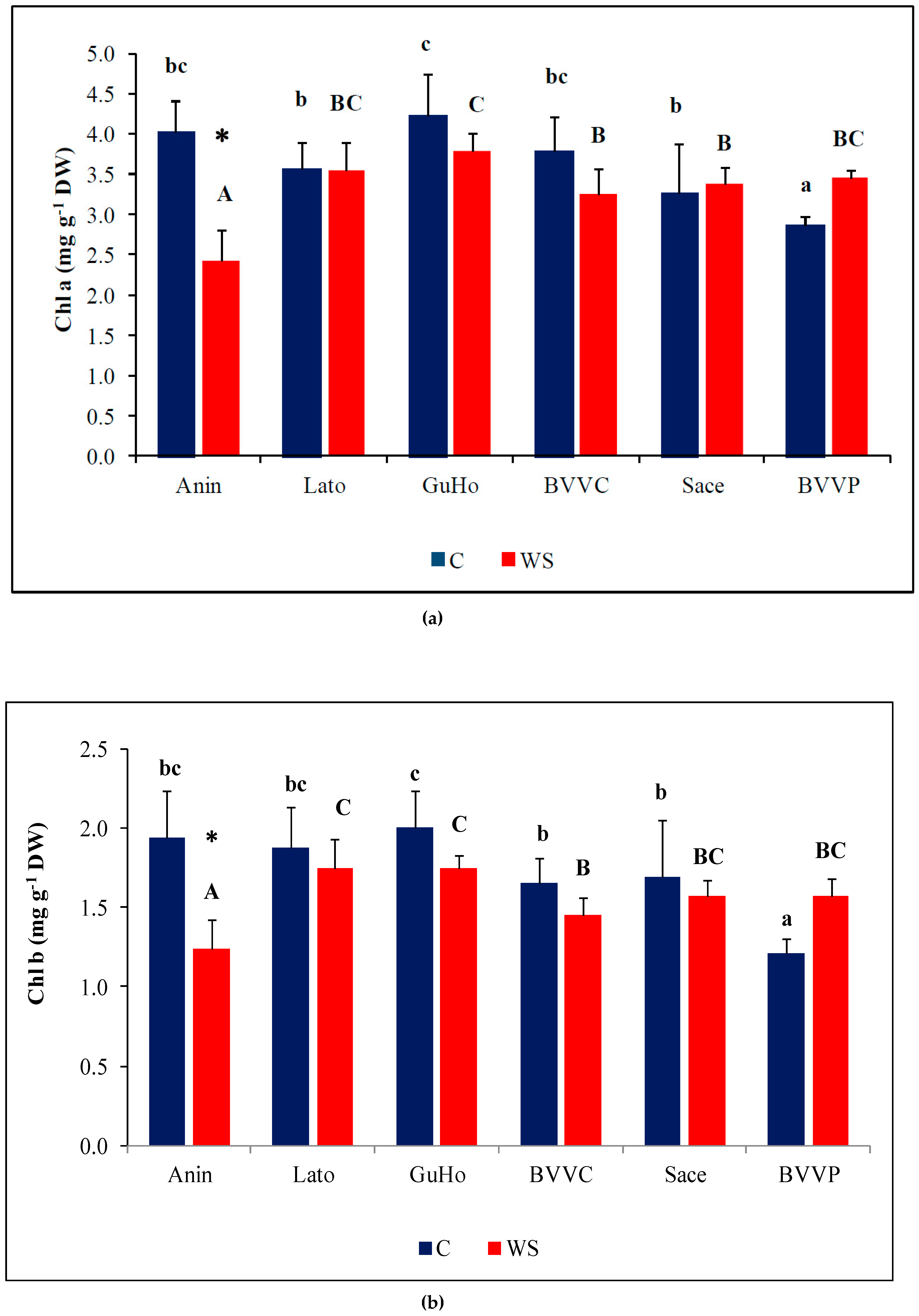
Photosynthetic pigments showed a small but significant variation among provenances, both in control and drought treatments (Figure 4), but drought induced a significant reduction of Chl a and Chl b only in plants from the Anin provenance (Figure 4a,b). Caro showed only small, non-significant variations in all analyzed provenances (Figure 4c).
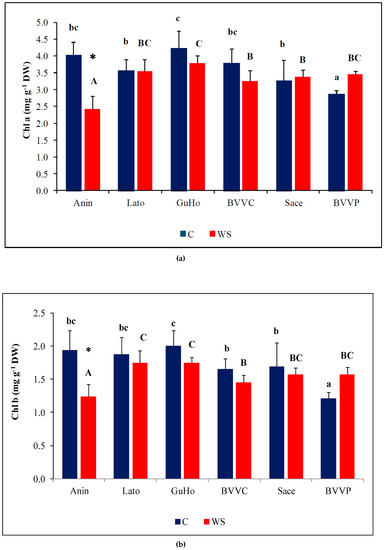
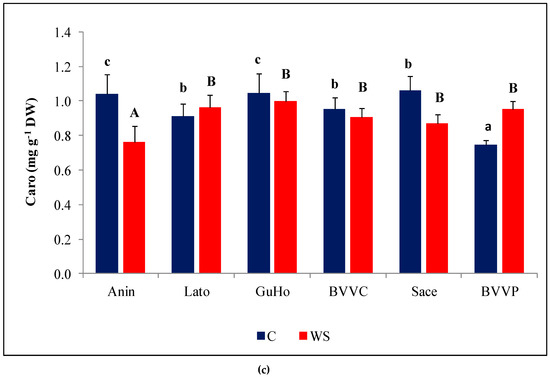
Figure 4.
Photosynthetic pigments in the needles of six Romanian provenances of L. decidua after one month of applied drought stress (Mean ± SE, n = 5): (a) Chlorophyll a (Chl a) expressed in mg g−1 DW; (b) Chlorophyll b (Chl b) expressed in mg g−1 DW; (c) total carotenoids (Caro) expressed in mg g−1 DW. Comparing provenances, different lowercase letters indicate significant differences between control plants, and different uppercase letters indicate significant differences between drought-affected plants, according to Tukey’s test (α = 0.05). Asterisks indicate a significant difference between treatments within the same provenance, according to the LSD value for the interaction (p = 0.05).
3.4. Cation Contents in Needles
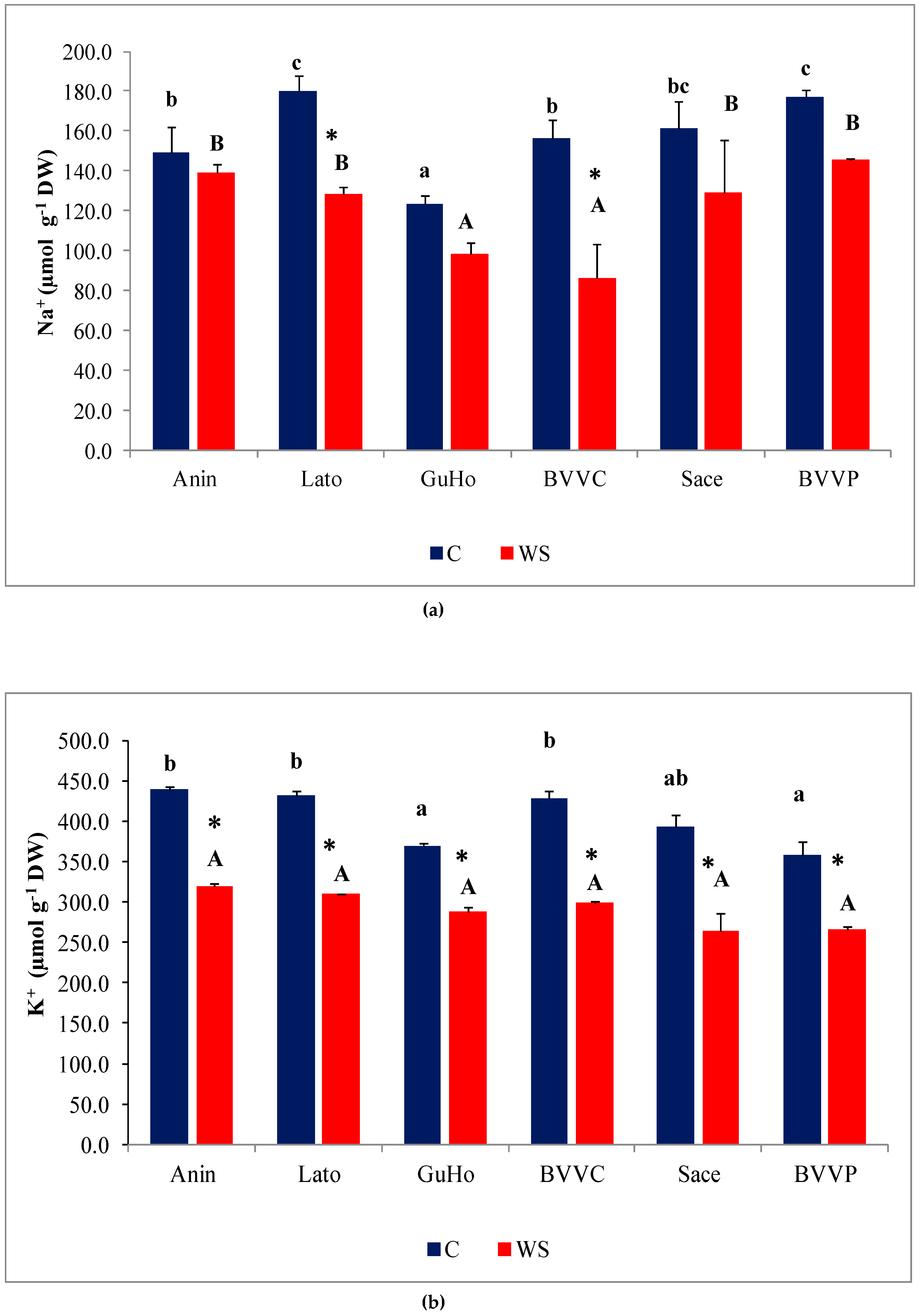
Upon the drought treatment, mean Na+ contents decreased in the needles of plants from all six European larch provenances (Figure 5a), although the decrease with respect to the values measured in their respective controls was only significant in provenances Lato (28%) and BVVC (35%). Needle K+ levels also decreased in response to drought, in plants across all studied provenances (Figure 5b), and this reduction was significant in all provenances. It must be noted, however, that the K+ control levels in needles were approximately double those of Na+. The decrement of K+ contents with respect to control values ranged between 30% and 35% in the selected L. decidua provenances.
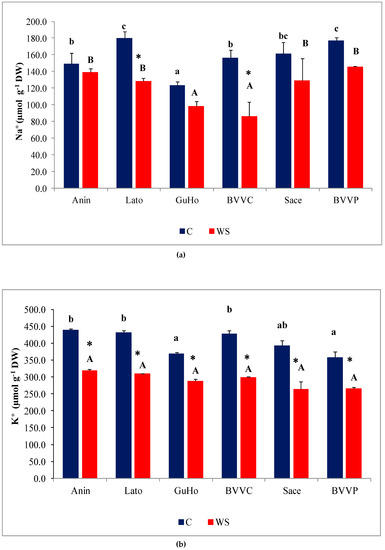
Figure 5.
Needle cationic contents of six Romanian provenances of L. decidua after one month of applied drought stress (Mean ± SE, n = 5): (a) Sodium (Na+) expressed in µmol g−1 DW; (b) potassium (K+) expressed in µmol g−1 DW. Comparing provenances, different lowercase letters indicate significant differences between control plants, and different uppercase letters indicate significant differences between drought-affected plants, according to Tukey’s test (α = 0.05). Asterisks indicate a significant difference between treatments within the same provenance, according to the LSD value for the interaction (p = 0.05).
3.5. Accumulation of Compatible Solutes
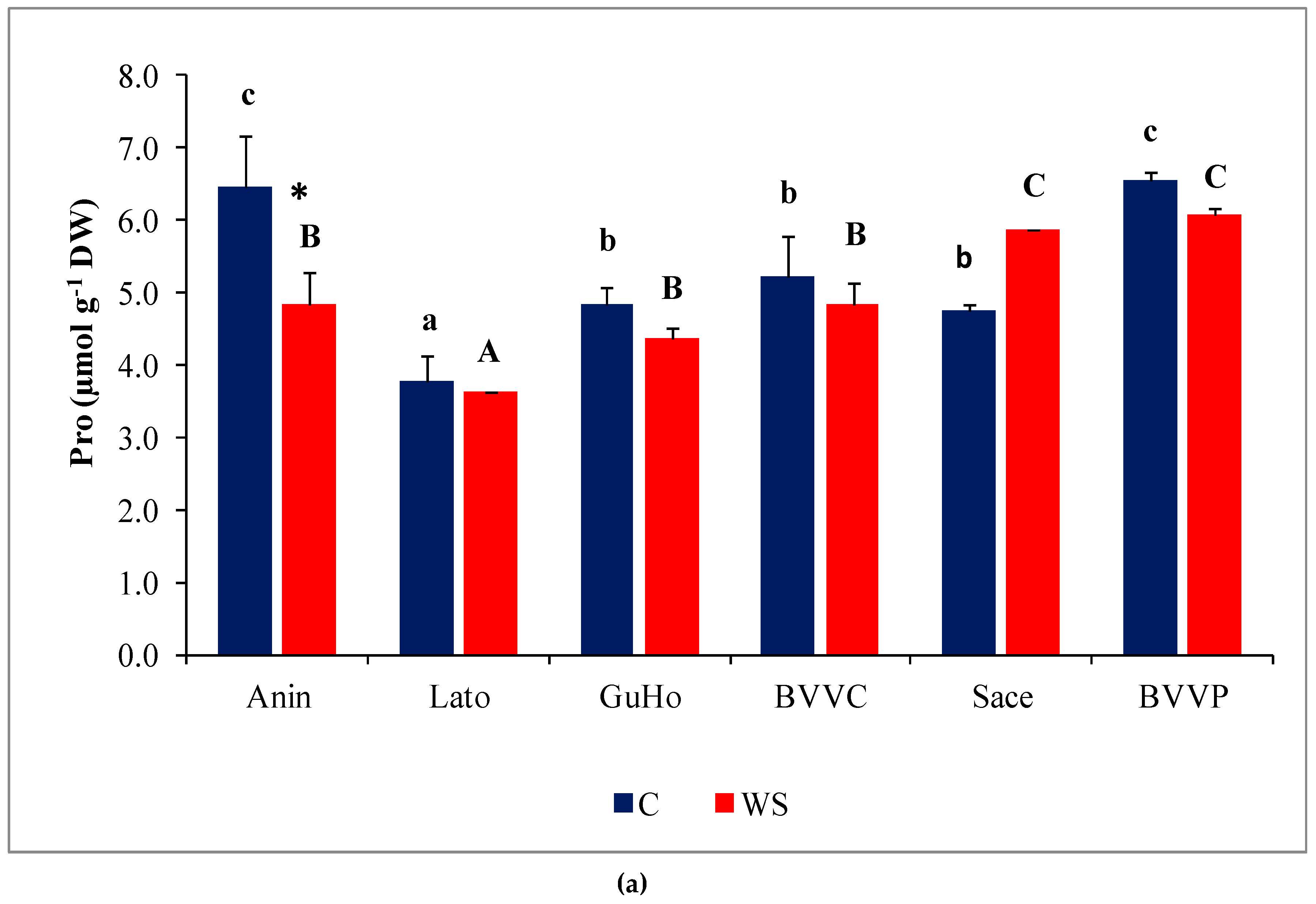
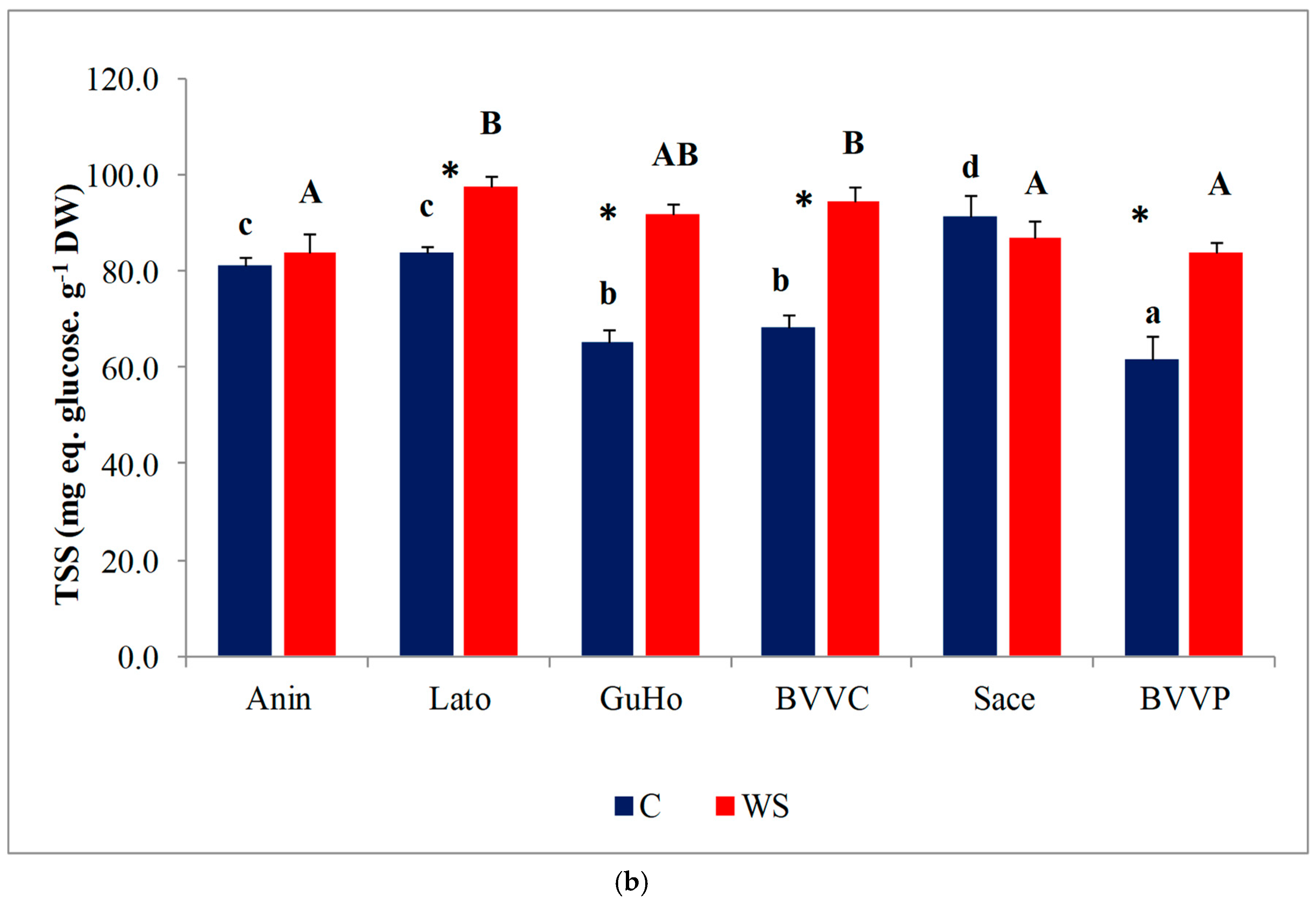
Pro concentrations varied significantly between provenances, both in control and stressed seedlings; within each provenance, however, Pro contents remained nearly unchanged in response to the water deficit treatment, except for a significant decrease in stressed plantlets of provenance Anin (Figure 6a). On the other hand, TSS contents in the needles of water-stressed plantlets recorded a significant increase, as compared to the corresponding non-stressed controls, in all studied provenances except Anin and Sace (Figure 6b). Provenances BVVC, BVVP, and GuHo showed the largest relative increase of TSS, nearly 40%, in comparison to their controls.
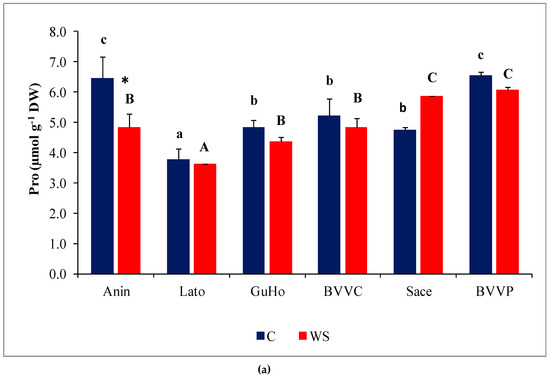
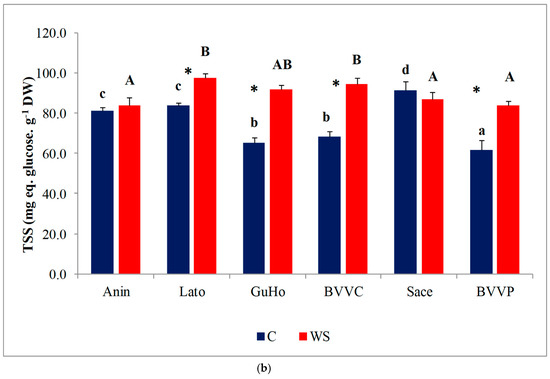
Figure 6.
Needles osmolytes contents of six Romanian provenances of L. decidua after one month of applied drought stress (Mean ± SE, n = 5): (a) Proline (Pro) expressed in µmol g−1 DW; (b) total soluble sugars (TSS) expressed in mg eq. glucose g−1 DW. Comparing provenances, different lowercase letters indicate significant differences between control plants, and different uppercase letters indicate significant differences between drought affected plants, according to Tukey’s test (α = 0.05). Asterisks indicate a significant difference between treatments within the same provenance, according to the LSD value for the interaction (p = 0.05).
3.6. MDA and Non-Enzymatic Antioxidants
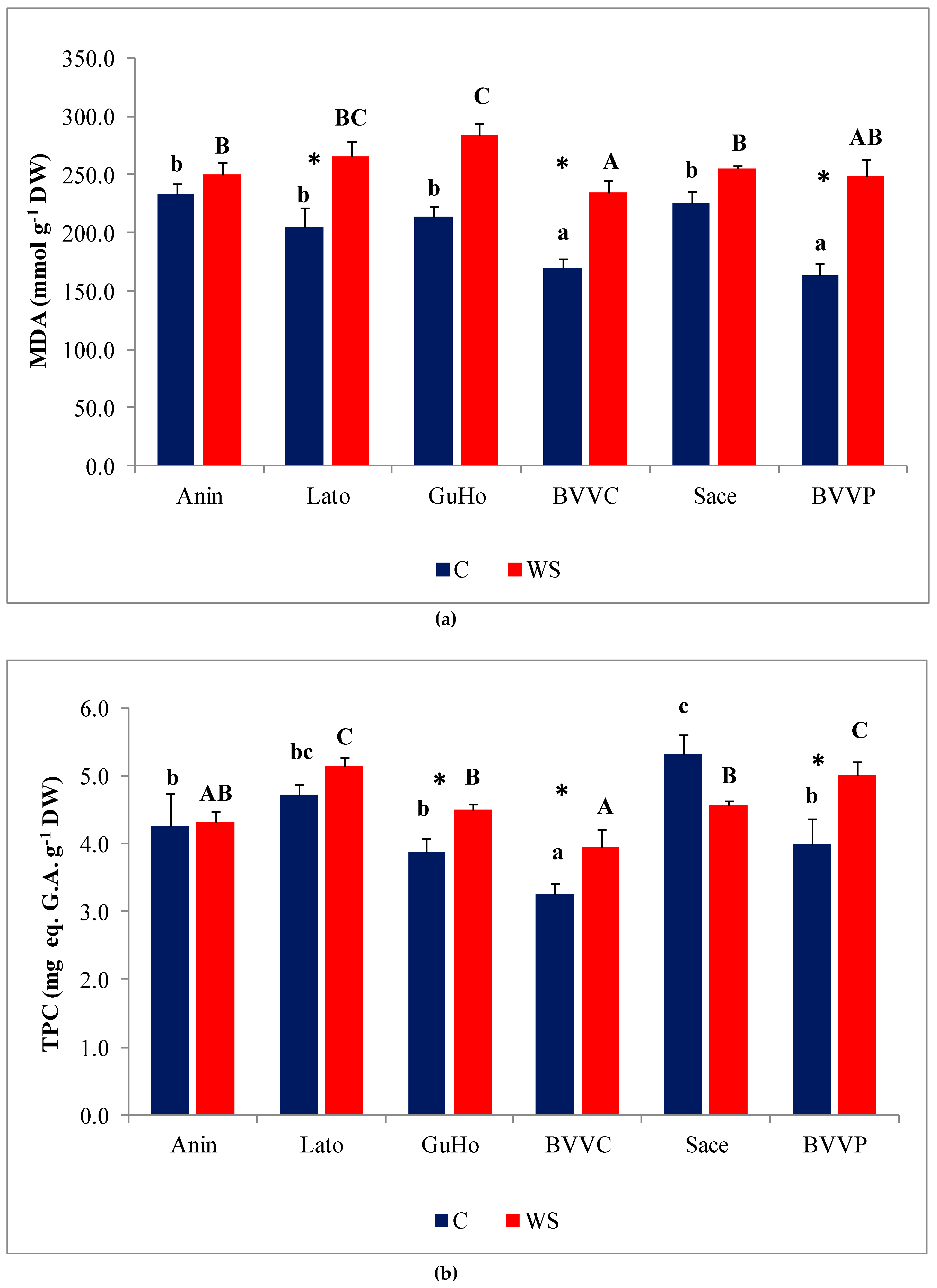
MDA, a reliable oxidative stress biomarker, showed a significant increase in all studied provenances of European larch except in Anin and Sace, in response to the applied drought treatments (Figure 7a), with BVVP recording the largest increase (ca. 53%) over its control levels (Figure 7a).
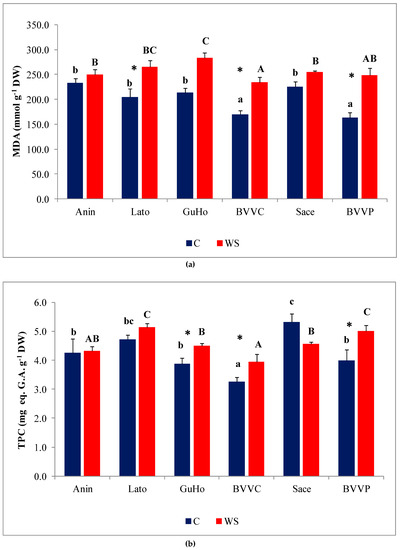
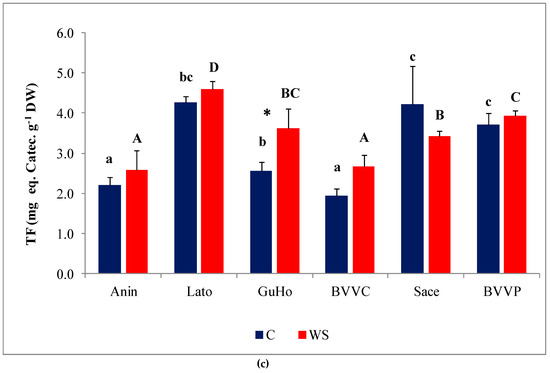
Figure 7.
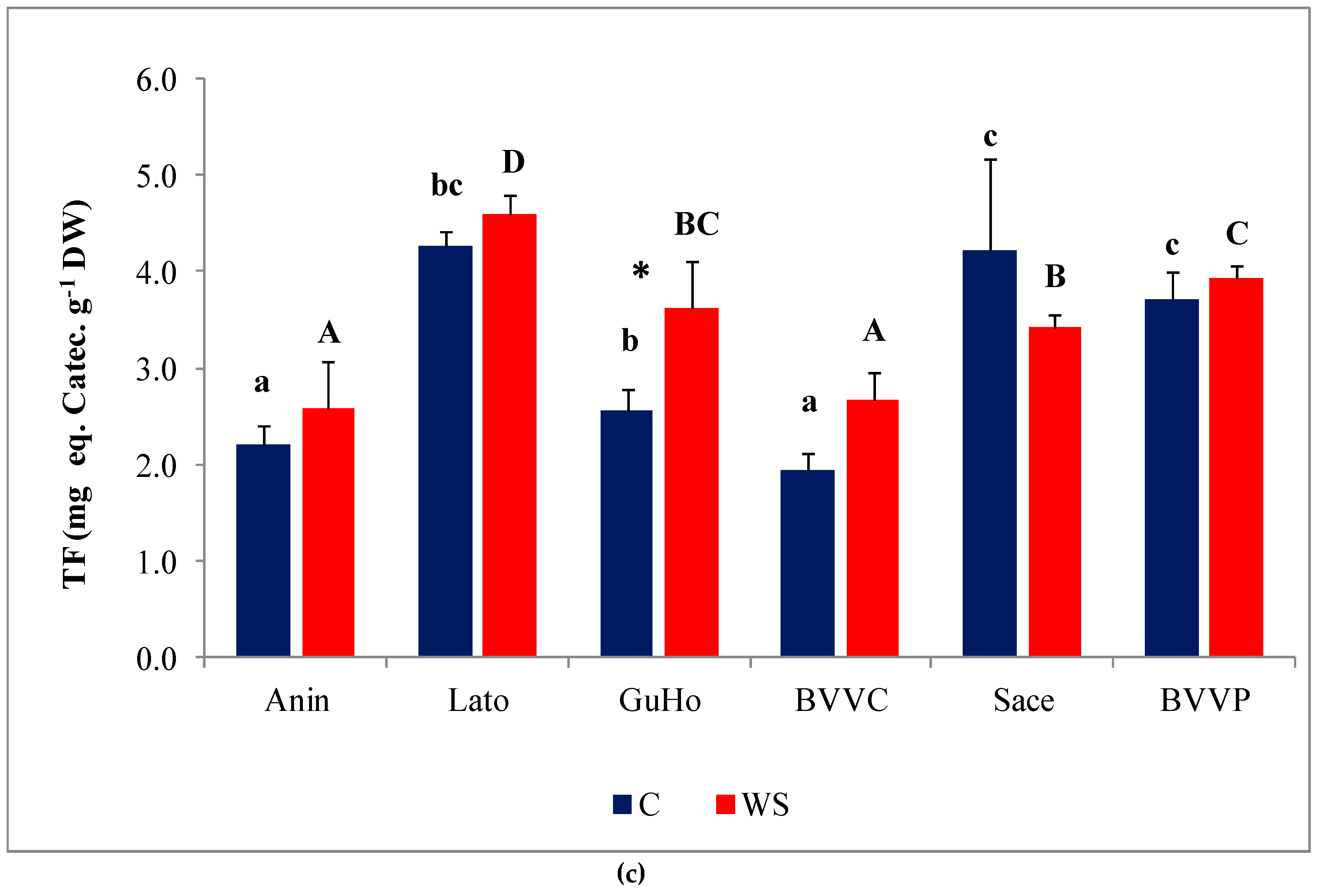
Oxidative stress and non-enzymatic antioxidants in six Romanian provenances of L. decidua after one month of applied drought stress (Mean ± SE, n = 5): (a) Malondialdehyde (MDA) expressed in nmol g−1 DW; (b) total phenolic compounds (TPC) expressed in mg eq. G.A. g−1 DW; (c) total flavonoids (TF) expressed in mg eq. C. g−1 DW. Comparing provenances, different lowercase letters indicate significant differences between control plants, and different uppercase letters indicate significant differences between drought-affected plants, according to Tukey’s test (α = 0.05). Asterisks indicate a significant difference between treatments within the same provenance, according to the LSD value for the interaction (p = 0.05).
Phenolic compounds, and especially the subgroup of flavonoids, include a large number of antioxidant metabolites, which plants can use to counteract the deleterious effects of oxidative stress. Total phenolic compounds (TPC) levels increased slightly, but significantly, under water deficit conditions in three of the studied provenances of European larch (GuHo, BVVC, and BVVP (Figure 7b)). Regarding total flavonoid (TF) concentrations in the needles, they increased significantly, in response to the drought treatment, only in seedlings of the GuHo provenance, (Figure 7c).
3.7. Multivariate Analyses
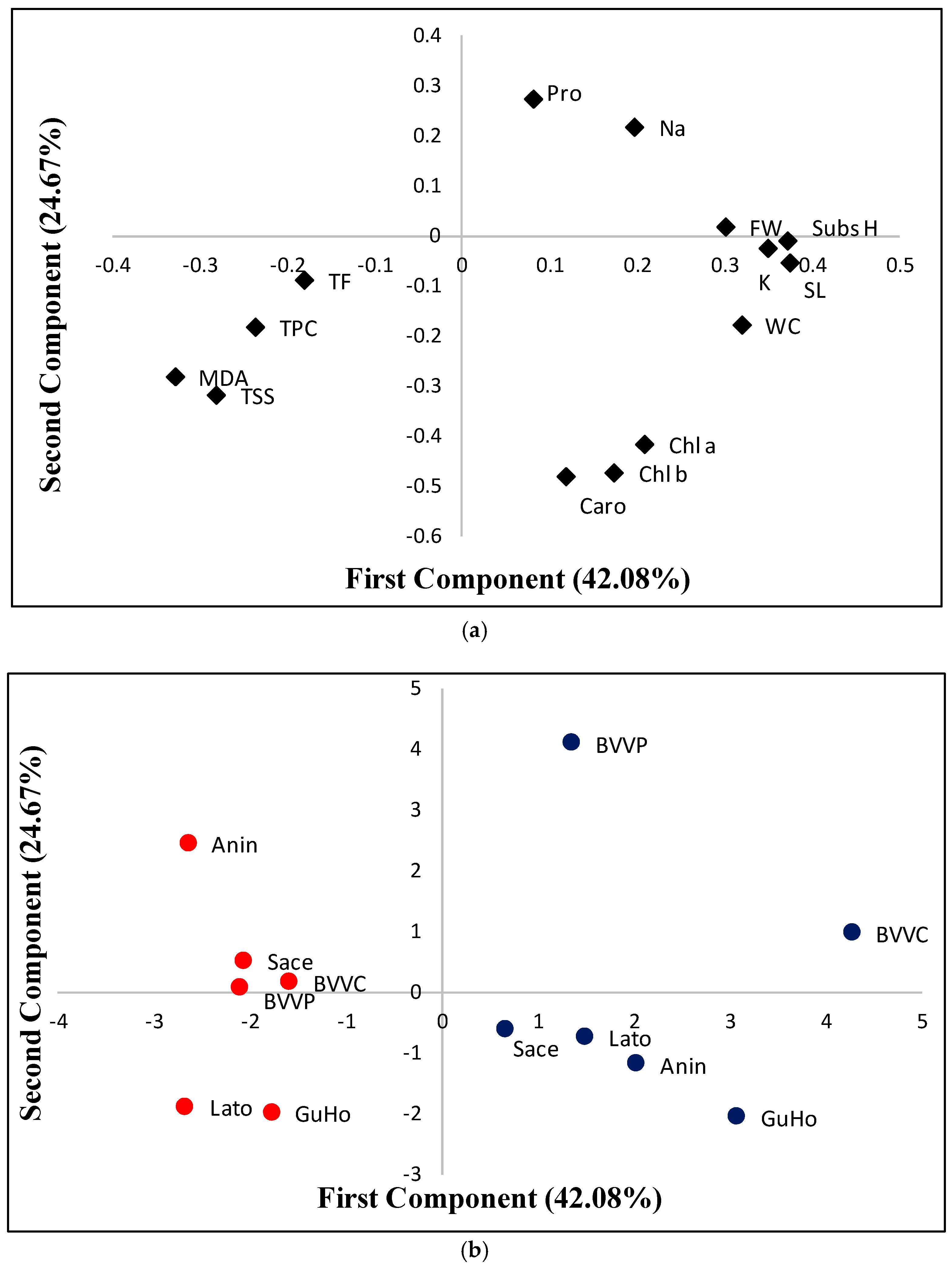
A principal component analysis (PCA) was performed using all traits analyzed. The biplot of the two main principal components, is shown in Figure 8a. The first component accounts for 42.08% of the total variability and displayed positive correlations with substrate humidity; growth parameters (stem length, fresh weight, and water content); and with K+ in needles with values above 0.25 for the correlation; as well as with the three photosynthetic pigments, Na+, and Pro, which displayed lower positive correlation values. This first component was negatively correlated with MDA, TSS, TPC, and TF (Figure 8a). The second component, which explains a 26.67% of the variation is positively correlated (values above 0.1) with Pro and Na+, and negatively correlated with the photosynthetic pigments (Chl a, Chl b, and Caro), TSS, MDA, TPC, and WC. The rest of traits display absolute correlation values below 0.1 (Figure 8a). The first component of the PCA clearly separated the control from the drought stressed plants, which respectively displayed positive and negative values for this first component (Figure 8b). However, a larger dispersion was observed in this first component for the control plants than for those subjected to the drought treatment. In this way, the highest value for control plants in the first component was observed for provenance BVVC and the lowest for provenance Sace. When considering drought stressed plants, BVVC also displayed the highest values for this first component, and Anin and Lato the lowest (Figure 8b). Regarding the second component, the highest and lowest values for the control treatment are those of BVVP and GuHo, respectively, whereas for the drought stressed plants the highest value is found in provenance Anin and the lowest again in provenance GuHo, but also in provenance Lato (Figure 8b).
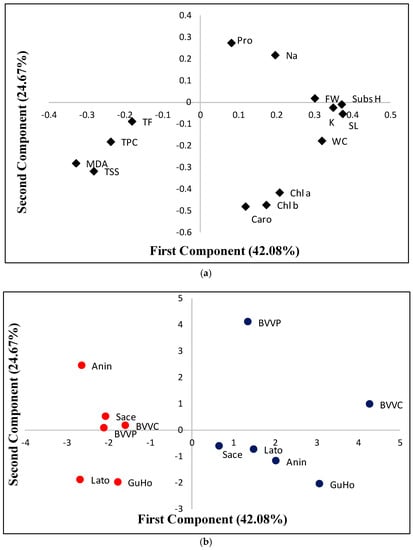
Figure 8.
Loading (a) and score (b) plots for a principal component analysis (PCA) based on the first and second component, which respectively account for 42.08% and 24.67% of the total variability observed in six Romanian provenances of L. decidua from control plants (blue dots) or submitted to drought stress(red dots) after one month of initiation of the treatments. Subs H, substrate humidity; SL, stem length; FW, fresh weight; WC, water content; Pro, proline; TSS, total soluble sugars; Na, sodium; Chl a, chlorohyll a; chl b, chlorohyll b; caro, carotenoids; MDA, malondialdehyde; TPC, total phenolic compounds; TF, total flavonoids.
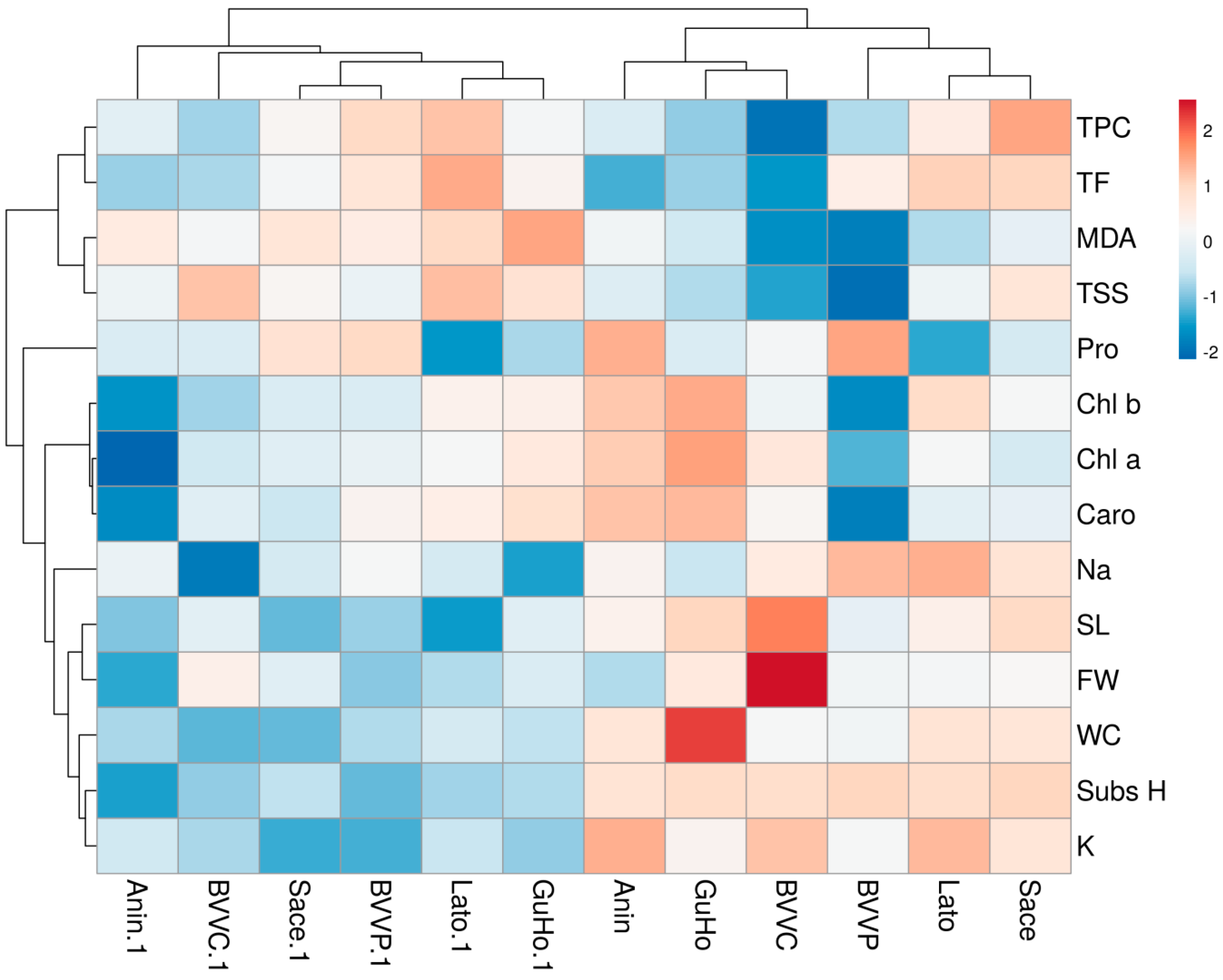
The hierarchical cluster analysis (HCA) performed together with the heatmap (Figure 9) confirmed the PCA results and revealed a clear separation of the control and drought-stressed plants in two main clusters. In addition, the cluster topology was similar under the two conditions (Figure 9). For the traits measured, the HCA separated two main clusters: one which included TPC, TF, MDA, and TSS, and the other which included the rest of traits. TPC and TF were highly correlated, and the same occurred for MDA and TSS. In the other cluster, high correlations were observed between the three photosynthetic pigments, and also between stem length (SL) and fresh weight (FW), as well as between WC, substrate humidity, and K+ contents (Figure 9).
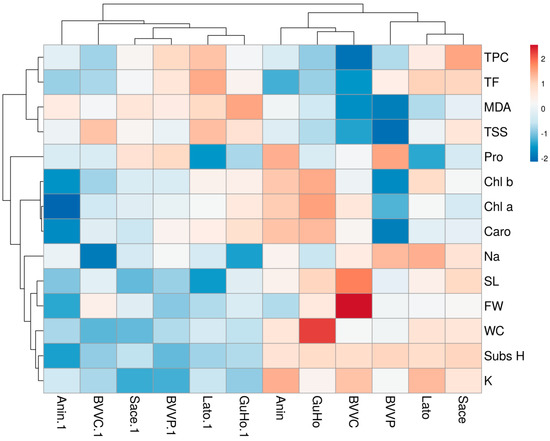
Figure 9.
Hierarchical cluster analysis and heatmap of physiological and biochemical parameters in relation to substrate humidity measured in six Romanian provenances of L. decidua. SubsH, substrate humidity; SL, stem length; FW, fresh weight; WC, water content; Pro, proline; TSS, total soluble sugars; Na, sodium; Chl a, chlorohyll a; Chl b, chlorohyll b; Caro, carotenoids; MDA, malondialdehyde; TPC, total phenloc compounds; TF, total flavonoids.
4. Discussion
Drought is a major threat for the European forests that will affect practically all of Europe except the Scandinavian region, extending from 13 to 26 percent of the total area of the continent, compared to the reference period of 1971 to 2000; drought episodes will also last three to four times longer than in the past [34]. Forestry is at present demanding a good knowledge of the genetic and phenotypic traits of the plant material used in reforestation and afforestation programs, and clever management will represent an important tool in adjusting to the effects of global warming [35]. A shift towards more drought-tolerant species, or the use of drought-resistant provenances to enrich the local gene pools of forest stands are highly recommended in areas exposed to global warming [36]. European larch is a pioneer species, considered by some authors as more drought tolerant than other conifers [37], due to its ability to maintain a high stomatal conductance at low water potential, whereas other reports indicate that L. europea is more sensitive due to its deciduous character and high transpiration rate [38,39]. The results presented here indicate that the six provenances responded in a similar way to one month of lack of irrigation, and no correlation with the latitude or altitude of parental populations could be established, as neither in seedlings from the control treatment. On the other hand, the effects of drought were less pronounced than those detected when submitting seedlings with the same origin to salt stress [40] or those reported in spruce [41].
Under artificial experimental conditions, the effects of abiotic stress, including drought, are generally assessed by measuring the degree of inhibition of plant growth. Faced with a situation of stress, plants divert resources (energy and metabolic precursors) from normal metabolism and biomass accumulation, to the activation of defense mechanisms, which causes the arrest or drastic reduction of growth [42,43]. For this reason, differences in stem length, fresh weight, or dry weight of plants submitted to stress treatments—in comparison to those from control—are optimal for ranking genotypes according to their degree of stress tolerance [44,45], but they are not always obvious when dealing with slow-growth woody species. However, European larch has a fast growing rate compared to other conifers [46] and effects of one month of drought stress could be noticed in the reduction of stem length growth, as also reported under salt stress [47]. Early diagnosis of stress suffered by plants is of great importance to minimize deleterious effects of prolonged droughts [48], but growth inhibition in a short time is difficult to assess in natural forest stands. On the other hand, conclusions based exclusively on growth parameters of young plants under greenhouse conditions cannot be extrapolated to the behavior of the adult trees in their natural environments. Therefore, the development of a set of biochemical markers of stress can be of great utility for early diagnosis [40,41,49,50,51].
Photosynthetic pigments are pivotal for the vegetative growth and reproductive success of plants. They are diverse, however, and some like carotenoids and anthocyanins are known to play a role in photo-protection from oxidative stress resulting from various forms of abiotic stresses. There is a variation of photosynthetic pigments related to plants’ phenology [52], but their reduction under water deficit conditions is common due to the reduction of chlorophyll synthesis, the inhibition of primary enzymes involved in photosynthesis, but also due to their degradation by chlorophyllase [53,54], and has been reported in several coniferous species under drought stress [41,55,56,57,58]. The levels of photosynthetic pigments proved to be a reliable salt stress marker in European larch [40]. Under drought, pigment degradation of European larch was not accentuated and, in some populations, their levels even increased, but concentrations were correlated with the water content of needles.
In our previous work, in addition to photosynthetic pigments, several other biochemical parameters could be associated with the observed salt-induced inhibition of growth, increasing concentrations of Na+ and Cl−, maintenance of K+ levels, and accumulation of Pro and MDA in needles [40]. In this study, Na+ did not increase under drought, but on the contrary decreased, as the plants reduced their absorption rate. K+ was positively correlated with growth parameters and the humidity of the substrate, but its level decreased in the stressed plants, as under drought its diffusion towards the shoots is restricted.
Proline is one of the most common compatible solutes in plants, accumulated under different types of abiotic and biotic stresses. Besides its direct role in osmoregulation, Pro is involved in the protection of enzymes against denaturation and stabilization of protein synthesis, or may act as low-molecular weight chaperone, reactive oxygen species (ROS) scavenger or signaling molecule [59,60,61]. A strong increase in Pro under drought has been reported in many coniferous species, such as spruce [41,62], or pine [58,63], and fir [64]. In the genus Larix, a three-fold Pro increase has been reported in the hybrid L. x eurolepis when exposed to cadmium, or four- to seven-fold increase in L. decidua when exposed to salinity [65]. Exogenous Pro application improved tolerance to cold and salt stress in European larch [65]. However, in the present study, Pro concentrations measured in water-stressed plants were very low and practically did not change as we previously reported [47], indicating that this osmolyte probably does not play a prominent role in the mechanisms of drought tolerance in plantlets of L. decidua or that the drought stress applied was very mild. Consequently, our data do not indicate that Pro is a suitable marker of mild drought-induced stress in this species.
Soluble sugars are primary products of photosynthesis, but their levels can also increase by remobilization from storage reserves in stem and roots and translocation to the leaves [66]. Different soluble sugars are common osmolytes in plants and an increment in their concentration in response to drought has been reported in the needles, sapwood, and inner bark of coniferous species [67]. Only a small significant increase was found in European larch seedlings submitted to salt, and not in all provenances [40]. The present findings indicate that they significantly increased in four of the studied provenances, but not in Anin and Sace. In the early stages or under mild drought stress, the concentration of TSS usually increases because growth declines earlier than photosynthesis [66].
MDA, a reactive aldehyde generated by increased free-radical production is considered as a suitable biomarker of cellular oxidative stress [68] and was reported to increase under salt and drought stress in European larch [40,47]. The results presented here indicate that it could be considered as a marker of mild drought stress in L. decidua, as it increased in all provenances except the two that were not so much affected by stress. Phenolics, especially flavonoids, are strong antioxidants in plants, belonging to the secondary ROS scavenging systems that are activated only when the activity of the antioxidant enzymes decline under severe stress [69]. In the provenances more affected by stress, total phenolics levels increased, whereas they were constant in Anin and decreased in Lato. Flavonoid variation showed the same pattern but differences were statistically significant only in the GuHo provenance.
As in other forestry species [70], the separation of the control and stressed plants in the multivariate analyses indicates a clear effect of the drought stress treatment on the growth and biochemical parameters of the European larch. Both PCA and heat map proved to be reliable tools, indicating strong correlations between photosynthetic pigments (chlorophyll a, b and carotenoids), or between total phenolics and flavonoids, as flavonoids belong to the first larger category. MDA, a good indicator of oxidative stress, was mostly correlated with TSS, which appears to play a more important role than Pro in this species. On the other hand, growth parameters were related with each other and also with the levels of K+ in the needles.
5. Conclusions
The six Romanian European larch provenances analyzed in this work withstood mild water stress, and only minor differences between provenances were found. The fresh weight practically did not vary and the reduction of the water content in the needles was not so strong, indicating a relative tolerance to dehydration of this species. Among the biochemical characteristics analyzed, photosynthetic pigments suffered only a slight variation, not related with the degree of tolerance of provenances. Proline also showed only a small variation and its concentrations were very low. The only suitable markers of mild drought stress in European larch could be the increase in total soluble sugars, MDA, and total phenolic compounds and reduction of K+. The variation among provenances suggests that some Carpathian populations of European larch may be promising for selection of materials with increased tolerance to drought.
Author Contributions
Conceptualization, M.B. and R.E.S.; Methodology, O.V. and M.A.H.; Validation, J.P., O.V., and R.E.S.; Formal analysis, J.P. and A.F.S.; Investigation, I.M.P., S.G.-O., and M.A.H.; Resources, R.E.S.; Data curation, A.F.S.; Writing—original draft preparation, I.M.P., M.A.H., J.P., and M.B.; Writing—review and editing, O.V. and R.E.S.; Visualization, A.F.S.; Supervision, O.V.; Project administration, R.E.S.; Funding acquisition, R.E.S.
Funding
This research received no external funding.
Acknowledgments
We are indebted to the personnel of the IBMCP greenhouse for their help in plant handling and maintenance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Dale, V.H.; Joyce, L.A.; McNulty, S.; Neilson, R.P.; Ayres, M.P.; Flannigan, M.D.; Hanson, P.J.; Irland, L.C.; Lugo, A.E.; Peterson, C.J.; et al. Climate change and forest disturbances. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Gilliam, F.S. Forest ecosystems of temperate climatic regions: From ancient use to climate change. New Phytol. 2016, 212, 871–887. [Google Scholar] [CrossRef]
- Eilmann, B.; de Vries, E.; Ouden, S.M.G.; Godefridus, J.; Mohren, G.M.J.; Sauren, P.; Sass-Klaassen, U. Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. For. Ecol. Manage. 2013, 302, 133–143. [Google Scholar] [CrossRef]
- Gao, R.; Shi, X.; Wang, J.R. Comparative studies of the response of larch and birch seedlings from two origins to water deficit. New Zeal. J. For. Sci. 2017, 47, 14. [Google Scholar] [CrossRef]
- Koskela, J.; Buck, A.; du Cros, E.T. (Eds.) EUFORGEN Climate Change and Forest Genetic Diversity: Implications for Sustainable Forest Management in Europe; Biodiversity International: Rome, Italy, 2007; Available online: https://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1216.pdf (accessed on 15 June 2019).
- Savolainen, O.; Bokma, F.; Knürr, T.; Kärkkäinen, K.; Pyhäjärvi, T.; Wachowiak, W. Adaptation of forest trees to climate change. In Climate Change and Forest Genetic Diversity: Implications for Sustainable Forest Management in Europe; Biodiversity International: Rome, Italy, 2007; pp. 19–29. Available online: https://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1216.pdf (accessed on 15 June 2019).
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manage. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Lautner, S. Wood formation under drought stress and salinity. In Cellular Aspects of Wood Formation; Fromm, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 187–202. [Google Scholar]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.J.; Nabuurs, G.J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Change 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Sala, A.; Hoch, G. Height-related growth declines in ponderosa pine are not due to carbon limitation. lant. Cell Environ. 2009, 32, 22–30. [Google Scholar] [CrossRef]
- Milad, M.; Schaich, H.; Bürgi, M.; Konold, W. Climate change and nature conservation in Central European forests: A review of consequences, concepts and challenges. For. Ecol. Manage. 2011, 261, 829–843. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Lof, M.; Madsen, P.; Nabuurs, G.J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. Forest Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Xiang, W.; Lei, X.; Zhang, X. Modelling tree recruitment in relation to climate and competition in semi-natural Larix-Picea-Abies forests in northeast China. For. Ecol. Manage. 2016, 382, 100–109. [Google Scholar] [CrossRef]
- Sánchez-Gómez, D.; Robson, T.M.; Gascó, A.; Gil-Pelegrín, E.; Aranda, I. Differences in the leaf functional traits of six beech (Fagus sylvatica L.) populations are reflected in their response to water limitation. Environ. Exp. Bot. 2013, 87, 110–119. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Da Ronch, F.; Caudullo, G.; Tinner, W.; de Rigo, D. Larix decidua and other larches in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 108–110. [Google Scholar]
- Mihai, G.; Teodosiu, M. Genetic diversity and breeding of larch (Larix decidua Mill.) in Romania. Ann. For. Res. 2009, 52, 97–108. [Google Scholar]
- Pàques, L.E.; Foffová, E.; Hienze, B.; Lelu-Walter, M.A.; Liesebach, M.; Phillipe, G. Larches (Larix sp.). In Forest Tree Breeding in Europe: Current State-of-the-art and Perspectives, Managing Forest Ecosystems; Pàques, L.E., Ed.; Series 25; Springer: Dordrecht, The Netherlands, 2013; pp. 13–123. [Google Scholar]
- Farcas, S.; Tantau, I.; Turtureanu, P.D. Larix decidua Mill. in Romania: Current and past distributions, coenotic preferences and conservation status. Contrib. Bot. 2013, 48, 39–50. [Google Scholar]
- Gramazio, P.; Plesa, I.M.; Truta, A.M.; Sestras, A.F.; Vilanova, S.; Plazas, M.; Vicente, O.; Boscaiu, M.; Prohens, J.; Sestras, R.E. Highly informative SSR genotyping reveals large genetic diversity and limited differentiation in European larch (Larix decidua) populations from Romania. Turk. J. Agric. For. 2018, 42, 165–175. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; California Agriculture Experiment Station Publications: Berkeley, CA, USA, 1950. [Google Scholar]
- Al Hassan, M.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-invasive plants vs. halophytes: Stress tolerance mechanisms in two related species. Front. Plant. Sci. 2016, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.W. On the computation of saturation vapor pressure. J. Appl. Meteorol. 1967, 6, 203–204. [Google Scholar] [CrossRef]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; 422p. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plantarum. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for drought studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Reberd, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Palazzodemello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, L.; Thober, S.; Kumar, R.; Wanders, N.; Rakovec, O.; Pan, M.; Zink, M.; Sheffield, J.; Wood, E.F.; Marx, A. Anthropogenic warming exacerbates European soil moisture droughts. Nat. Clim. Change 2018, 8, 421. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manage. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Hlásny, T.; Mátyás, C.; Seidl, R.; Kulla, L.; Merganičová, K.; Trombik, J.; Dobor, L.; Barcza, Z.; Konôpka, B. Climate change increases the drought risk in Central European forests: What are the options for adaptation? Lesn Cas For. J. 2014, 60, 5–18. [Google Scholar] [CrossRef]
- Badalotti, A.; Anfodillo, T.; Grace, J. Evidence of osmoregulation in Larix decidua at Alpine treeline and comparative responses to water availability of two co-occurring evergreen species. Ann. Forest Sci. 2000, 57, 623–633. [Google Scholar] [CrossRef]
- Eilmann, B.; Rigling, A. Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol. 2012, 32, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Oberhuber, W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees 2013, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Plesa, I.; Al Hassan, M.; Sestras, A.F.; Vicente, O.; Boscaiu, M.; Sestras, R.E. Biochemical markers of salt stress in European larch (Larix decidua). Not. Sci. Biol. 2018, 10, 430–438. [Google Scholar] [CrossRef]
- Schiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, E.; Vicente, O. Biochemical responses to drought, at the seedling stage, of several Romanian Carpathian populations of Norway spruce (Picea abies L. Karst). Trees 2017, 31, 1479–1490. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Funct. Plant. Biol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant. Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Arteaga, S.; Al Hassan, M.; Chaminda Bandara, W.M.; Yabor, L.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Screening for salt tolerance in four local varieties of Phaseolus lunatus from Spain. Agriculture 2018, 8, 201. [Google Scholar] [CrossRef]
- Cicevan, R.; Al Hassan, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E.; Boscaiu, M. Screening for drought tolerance in cultivars of the ornamental genus Tagetes (Asteraceae). PeerJ 2016, 4, e2133. [Google Scholar] [CrossRef]
- Matras, J.; Pâques, L. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Larch (Larix decidua); Biodiversity International: Rome, Italy, 2008; p. 6. [Google Scholar]
- Plesa, I.M.; González-Orenga, S.; Al Hassan, M.; Sestras, A.F.; Vicente, O.; Prohens, J.; Sestras, R.E.; Boscaiu, M. Effects of drought and salinity on European Larch (Larix decidua Mill.) seedlings. Forests 2018, 9, 320. [Google Scholar] [CrossRef]
- Toldi, O.; Tuba, Z.; Scott, P. Vegetative desiccation tolerance: Is it a goldmine for bioengineering crops? Plant. Sci. 2009, 176, 187–199. [Google Scholar] [CrossRef]
- Corcuera, L.; Pelegrín, E.; Notivol, E. Aridity promotes differences in proline and phytohormone levels in Pinus pinaster populations from contrasting environments. Trees 2012, 26, 799–808. [Google Scholar] [CrossRef]
- Alonso, M.; Rozados, M.J.; Vega, J.A.; Bará, S.; Cuiñas, P. Parámetros químicos indicadores de daños producidos por fuego en Pinus pinaster Ait: Nutrientes, capacidad buffer, iminoacidos y compuestos fenolicos. Invest. Agr. Sist. Recur. For. 1998, 7, 5–26. [Google Scholar]
- Schiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Identification of salt stress biomarkers in Romanian Carpathian populations of Picea abies (L.) Karst. PLoS ONE 2015, 10, e0135419. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.V.; Ensminger, I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revised. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Munné Bosch, S.; Allegre, I. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant. Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Alonso, R.; Elvira, S.; Castillo, F.J.; Gimeno, B.S. Interactive effects of ozone and drought stress on pigments and activities of antioxidative enzymes in Pinus halepensis. Plant. Cell Environ. 2001, 24, 905–916. [Google Scholar] [CrossRef]
- Croser, C.; Renault, S.; Franklin, J.; Zwiazek, J. The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca and Pinus banksiana. Environ. Pollut. 2001, 115, 9–16. [Google Scholar] [CrossRef]
- Miron, M.S.; Sumalan, R.L. Physiological responses of Norway spruce (Picea abies [L.] Karst) seedlings to drought and overheating stress conditions. J. Hortic. Biotechnol. 2015, 19, 146–151. [Google Scholar]
- Taïbi, K.; del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought Tolerance in Pinus halepensis seed sources as identified by distinctive physiological and molecular markers. Front. Plant. Sci. 2017, 8, 202. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant. Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ditmarová, L.; Kurjak, D.; Palmroth, S.; Kmet, J.; Strelcová, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2010, 30, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Ding, G.; Zhao, X. Responses to continuous drought stress and drought resistance comprehensive evaluation of different Masson pine families. Sci. Silvae Sin. 2017, 53, 21–29. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Wang, G.; Yang, L.; Sun, X. Ecophysiological responses of Abies fabri seedlings to drought stress and nitrogen supply. Physiol. Plant. 2010, 139, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, D.; Lelu-Walter, M.-A.; Parkinson, M. Influence of exogenous L-proline onembryogenic cultures of larch (Larix leptoeuropaea Dengler), sitka spruce (Picea sitchensis (Bong.) Carr.) and oak (Quercus robur L.) subjected to cold and salt stress. Ann. For. Sci. 2004, 61, 125–128. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of non-structural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Clancy, K.M.; Wagner, M.R.; Reich, P.B. Ecophysiology and insect herbivory. In Ecophysiology of Coniferous Forests; Smith, W.K., Hinckley, T.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 125–180. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant. Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Granda, V.; Delatorre, C.; Cuesta, C.; Centeno, M.L.; Fernández, B.; Rodríguez, A.; Feito, I. Physiological and biochemical responses to severe drought stress of nine Eucalyptus globulus clones: A multivariate approach. Tree Physiol. 2014, 34, 778–786. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).