Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites
2.2. Site History
2.3. Soil Analysis and Statistical Analysis
3. Results
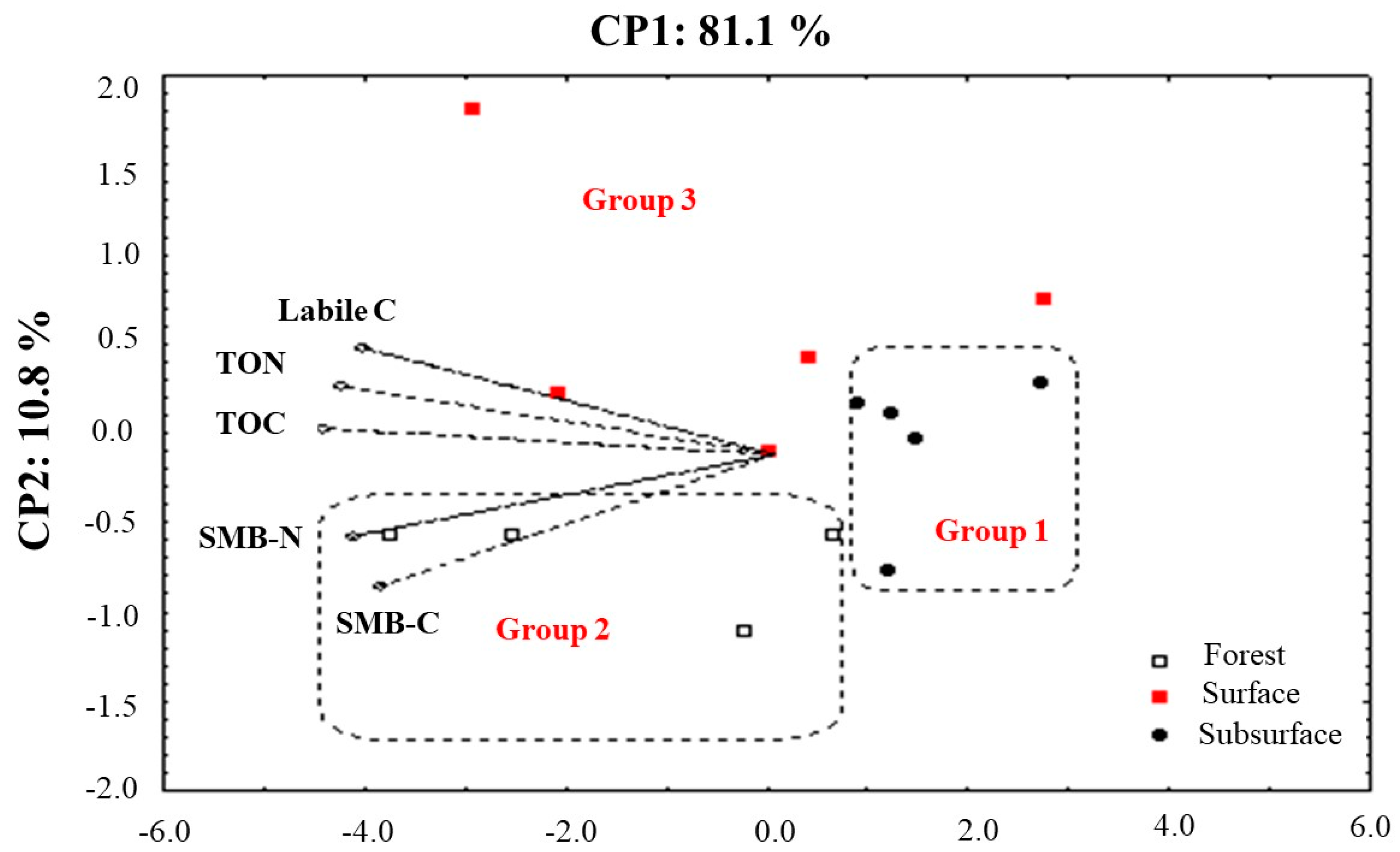
3.1. Carbon and Nitrogen Pools (Surface and Subsurface)
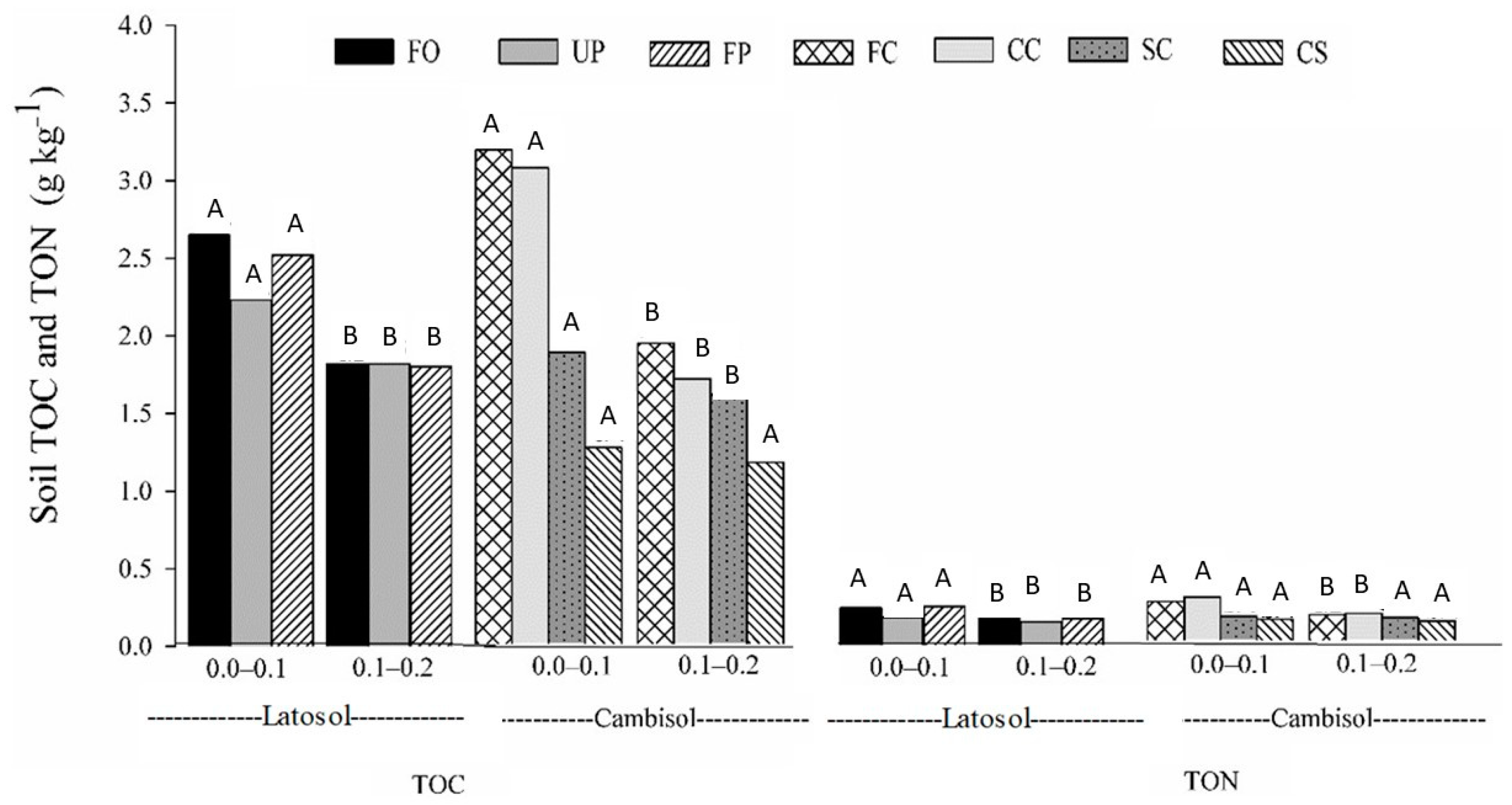
3.2. Total Organic Carbon
3.3. Soil Recalcitrant Carbon
3.4. Labile Carbon
3.5. Effect of Soil Use and Management on Carbon and Nitrogen Losses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994; p. 496. [Google Scholar]
- Scherer, E.E.; Baldissera, I.T.; Nesi, C.N. Propriedades químicas de um Latossolo Vermelho sob plantio direto e adubação com esterco de suínos. Rev. Bras. Cienc. Solo 2007, 31, 123–131. [Google Scholar] [CrossRef]
- Balota, E.L.; Calegari, A.; Nakatani, A.S.; Coyne, M.S. Benefits of winter cover crops and no-tillage for microbial parameters in a Brazilian Oxisol: A long-term study. Agric. Ecosyst. Environ. 2014, 197, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Mielniczuk, J.; Bayer, C.; Vezzani, F.M.; Lovato, T.; Fernandes, F.F.; Debarba, L. Manejo de solo e culturas e sua relação com os estoques de carbono e nitrogênio do solo. Tópicos Ciência Solo 2003, 3, 209–248. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Almeida, R.F.; Machado, H.A.; Martins, F.P.; Queiroz, I.D.S.; Teixeira, W.G.; Mikhael, J.E.R.; Borges, E.N. Correlação do tamanho e distribuição dos agregados em Latossolos amarelo da região do triângulo mineiro em diferentes ambientes. Biosci. J. 2014, 30, 1325–1334. [Google Scholar]
- Almeida, R.F.; Silveira, C.H.; Mikhael, J.E.R.; Franco, F.O.; Ribeiro, B.T.; Ferreira, A.S.; Mendonça, E.S.; Wendling, B. CO2 emissions from soil incubated with sugarcane straw and nitrogen fertilizer. Afr. J. Biotechnol. 2014, 13, 3376–3384. [Google Scholar] [CrossRef]
- Loss, A.; Pereira, M.G.; Schultz, N.; Anjos, L.H.C.; Silva, E.M.R. Carbono e frações granulométricas da matéria orgânica do solo sob sistemas de produção. Rev. Ciênc. Rural 2009, 39, 1067–1072. [Google Scholar] [CrossRef]
- Panosso, A.R.; Marques, J.; Pereira, G.T.; La Scala, N. Spatial and temporal variability of soil CO2 emission in a sugarcane area under green and slash-and-burn managements. Soil Tillage Res. 2009, 105, 275–282. [Google Scholar] [CrossRef]
- Theng, B.K.G.; Tate, K.R.; Sollins, P. Constituents of organic matter in temperate and tropical soils. In Dynamics of Soil Organic Matter in Tropical Ecosystems; Coleman, D.C., Oades, J.M., Uehara, G., Eds.; NiftTAL Project; University of Hawaii: Honolulu, HI, USA, 1989. [Google Scholar]
- Silva, I.R.; Mendonça, E.S. Matéria orgânica do solo. In Fertilidade do Solo; Novais, R.F., Alvarez, V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007. [Google Scholar]
- Lundquist, E.J.; Jackson, L.E.; Scow, K.M.; Hsu, C. Changes in microbial biomass and community composition, and soil carbon and nitrogen pools after incorporation of Rye into three California agricultural soils. Soil Biol. Biochem. 1999, 31, 221–236. [Google Scholar] [CrossRef]
- Catellan, A.J.; Vidor, C. Flutuações na biomassa, atividade e população microbiana do solo, em função de variações ambientais. Rev. Bras. Ciênc. Solo 1990, 14, 133–142. [Google Scholar]
- Embrapa—Empresa Brasileira de Pesquisa Agropecuária. Serviço Nacional de Levantamento e Conservação de Solos; Levantamento de reconhecimento de meia intensidade dos solos e avaliação da aptidão agrícola das terras do Triângulo Mineiro; Embrapa-Snlcs: Rio de Janeiro, Brazil, 1982; p. 562. [Google Scholar]
- Embrapa—Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos, 2nd ed.; Embrapa: Rio de Janeiro, Brazil, 2006; p. 412. [Google Scholar]
- Buol, S.W.; Southard, R.J.; Graham, R.C. Soil Genesis and Classification, 5th ed.; Blackwell Publishing: Ames, IA, USA, 2003. [Google Scholar]
- Wendling, B. Carbono e Nitrogênio no Solo Sob Diferentes Usos e Manejos e Sua Modelagem Pelo Century. Universidade de Viçosa, Viçosa-MG, Brazil. Available online: http://www.locus.ufv.br/bitstream/handle/123456789/1645/texto%20completo.pdf?sequence=1&isAllowed=y (accessed on 6 June 2018).
- Yeomans, J.C.; Bremner, L.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Bohnem, H.; Gianello, C.; Bissani, C.A.; Volkweiss, S.J. Análise de Solo, Plantas e Outros Materiais, 2nd ed.; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995; p. 174. [Google Scholar]
- Swift, R.S. Organic Matter Characterization. In Methods of Soil Analysis: Chimical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series 5; Soil Science Society of America; American Society of Agronomy: Madison, WI, USA, 1996; Volume 3, pp. 1011–1020. [Google Scholar]
- Shang, C.; Tiessen, H. Organic matter lability in a tropical oxisol: Evidence from shifting cultivation, chemical oxidation, particle size, density and magnetic fractionations. Soil Sci. 1997, 162, 795–807. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 1998, 27, 408–416. [Google Scholar] [CrossRef]
- Sparling, G.P.; West, A.W. A direct extraction method to estimate soil microbial C: Calibration in situ using microbial and 14C labelled cells. Soil Biol. Biochem. 1988, 20, 337–343. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure soil microbial biomass nitrogen in soil. Rev. Bras. Ciênc. Solo 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Tormena, C.A.; Friedrich, R.; Pintro, J.C.; Costa, A.C.S.; Fidalski, J. Propriedades físicas e taxa de estratificação de carbono orgânico num Latossolo Vermelho após dez anos sob dois sistemas de manejo. Rev. Bras. Ciênc. Solo 2004, 28, 1023–1031. [Google Scholar] [CrossRef]
- Canellas, L.P.; Velloso, A.C.X.; Marciano, C.R.; Ramalho, J.F.G.P.; Rumjanek, V.M.; Rezende, C.E.; Santos, G.A. Propriedades químicas de um cambissolo cultivado com cana-de-açúcar, com preservação do palhiço e adição de vinhaça por longo tempo. Rev. Bras. Ciênc. Solo 2003, 27, 935–944. [Google Scholar] [CrossRef]
- Monteiro, A.C.G.; Ponciano, N.J. Índice da qualidade do solo com cana-deaçúcar colhida crua e queimada. Rev. Cient. Int. 2012, 1, 58–70. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in a paleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Mcgill, W.B.; Cannon, K.R.; Robertson, J.A.; Cook, F.D. Dynamics of soil microbial biomass and water-soluble organic C in Breton L after 50 years of cropping to two rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Silva, I.F.; Mielniczuk, J. Ação do sistema radicular de plantas na formação e estabilização de agregados do solo. Rev. Bras. Ciênc. Solo 1997, 20, 113–117. [Google Scholar]
- Mielniczuk, J. Matéria orgânica e a sustentabilidade de sistemas agrícolas. In Fundamentos da Matéria Orgânica do Solo; Santos, G.A., Camargo, F.A.O., Eds.; Ecossistemas Tropicais e Subtropicais: Porto Alegre, Brazil, 1999; pp. 1–8. [Google Scholar]


| Treatment | Management and Usage of Soils |
|---|---|
| Latosol | |
| Forest (FO) | Secondary Atlantic forest |
| Unfertilized Braquiaria Pasture (UF) | 1950—Deforestation 1950 until 1985—Angola grass 1986 until 2011—Braquiaria grass |
| Fertilized Braquiaria Pasture (FP) | 1950—Deforestation 1950 until 1988—Angola grass 1989 until 2002—Elephant grass 2003 until 2011—Braquiaria grass |
| Cambisol | |
| Forest (FC) | Secondary Atlantic forest |
| Coast Cross (CC) | 1935—Deforestation 1935 until 1979—Angola grass 1980 until 1982—Oat pasture 1983 until 1991—Summer corn grain 1992 until 2011—Coast cross grass |
| Sugarcane (SC) | 1935—Deforestation 1935 until 1989—Angola grass 1990 until 2011—Sugarcane cultivation |
| Corn Silage (CS) | 1935—Deforestation 1935 until 1973—Horticultural crops 1974 until 1985—Angola grass 1986 until 2011—Corn silage |
| CP1 (81.1%) | CP2 (10.8%) | Mean | |
|---|---|---|---|
| TOC (g kg−1) | −0.98 | 0.09 | 2.10 ± 0.64 |
| TON (g kg−1) | −0.94 | 0.25 | 0.18 ± 0.05 |
| SMB-C (mg kg−1) | −0.85 | −0.47 | 465.57 ± 231.66 |
| SMB-N (mg kg−1) | −0.92 | −0.29 | 49.07 ± 16.39 |
| Labile-C (g kg−1) | −0.89 | 0.38 | 1.86 ± 0.55 |
| Soil | HU (g kg−1) | HA (g kg−1) | FA (g kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface | Subsurface | Average | Surface | Subsurface | Average | Surface | Subsurface | Average | |
| Latosol | |||||||||
| FO | 16.65 | 11.33 | 13.99 | 4.25 | 1.33 | 2.79 | 4.21 | 3.60 | 3.9 |
| UP | 13.35 | 10.05 | 11.70 | 3.21 | 1.45 | 2.33 | 3.47 | 2.91 | 3.18 |
| FP | 16.12 | 9.68 | 12.90 | 4.73 | 2.30 | 3.52 | 3.39 | 3.45 | 3.42 |
| Average | 16.37A | 10.35B | 4.06A | 1.69B | 3.69A | 3.32A | |||
| Cambisol | |||||||||
| FC | 21.90A | 12.30B | 17.10 | 5.69A | 2.54B | 4.12 | 4.53 | 3.79 | 4.16 |
| CC | 23.10A | 12.75B | 17.93 | 4.56A | 2.14B | 3.35 | 3.62 | 2.77 | 3.20 |
| SC | 23.28A | 9.75B | 11.51 | 3.07A | 2.39A | 2.73 | 3.07 | 2.77 | 2.92 |
| CS | 10.88A | 9.83A | 10.35 | 0.99A | 0.74A | 0.87 | 1.83 | 1.56 | 1.69 |
| Average | 17.29 | 11.16 | 3.58 | 1.95 | 3.26A | 2.72B | |||
| Soil Management | Carbon | Nitrogen | |||
|---|---|---|---|---|---|
| Recalcitrant | Labile | Recalcitrant | Labile | ||
| Latosol | UP | −9% | −23% | −19% | −24% |
| FP | +16% | −7% | +2% | −25% | |
| Cambisol | CC | −7% | −7% | +8% | −25% |
| SC | −32% | −26% | −28% | −28% | |
| CS | −53% | −49% | −37% | −62% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz de Almeida, R.; Rodrigues Mikhael, J.E.; Oliveira Franco, F.; Fonseca Santana, L.M.; Wendling, B. Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions. Forests 2019, 10, 544. https://doi.org/10.3390/f10070544
Ferraz de Almeida R, Rodrigues Mikhael JE, Oliveira Franco F, Fonseca Santana LM, Wendling B. Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions. Forests. 2019; 10(7):544. https://doi.org/10.3390/f10070544
Chicago/Turabian StyleFerraz de Almeida, Risely, Joseph Elias Rodrigues Mikhael, Fernando Oliveira Franco, Luna Monique Fonseca Santana, and Beno Wendling. 2019. "Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions" Forests 10, no. 7: 544. https://doi.org/10.3390/f10070544
APA StyleFerraz de Almeida, R., Rodrigues Mikhael, J. E., Oliveira Franco, F., Fonseca Santana, L. M., & Wendling, B. (2019). Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions. Forests, 10(7), 544. https://doi.org/10.3390/f10070544






