Comparing Primary and Secondary Growth of Co-Occurring Deciduous and Evergreen Conifers in an Alpine Habitat
Abstract
:1. Introduction
2. Materials and Methods
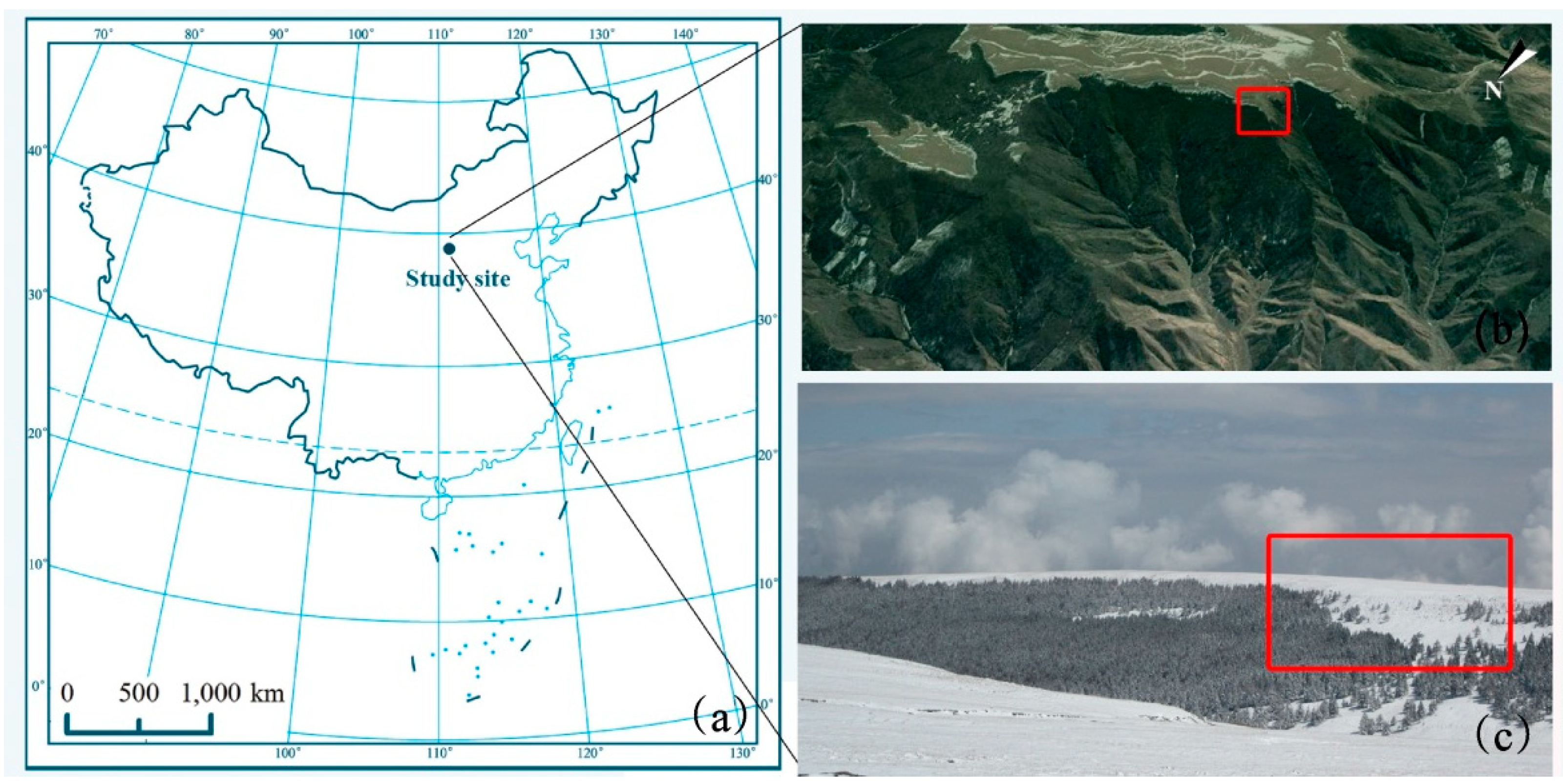
2.1. Study Site and Species Descriptions
2.2. Primary Growth
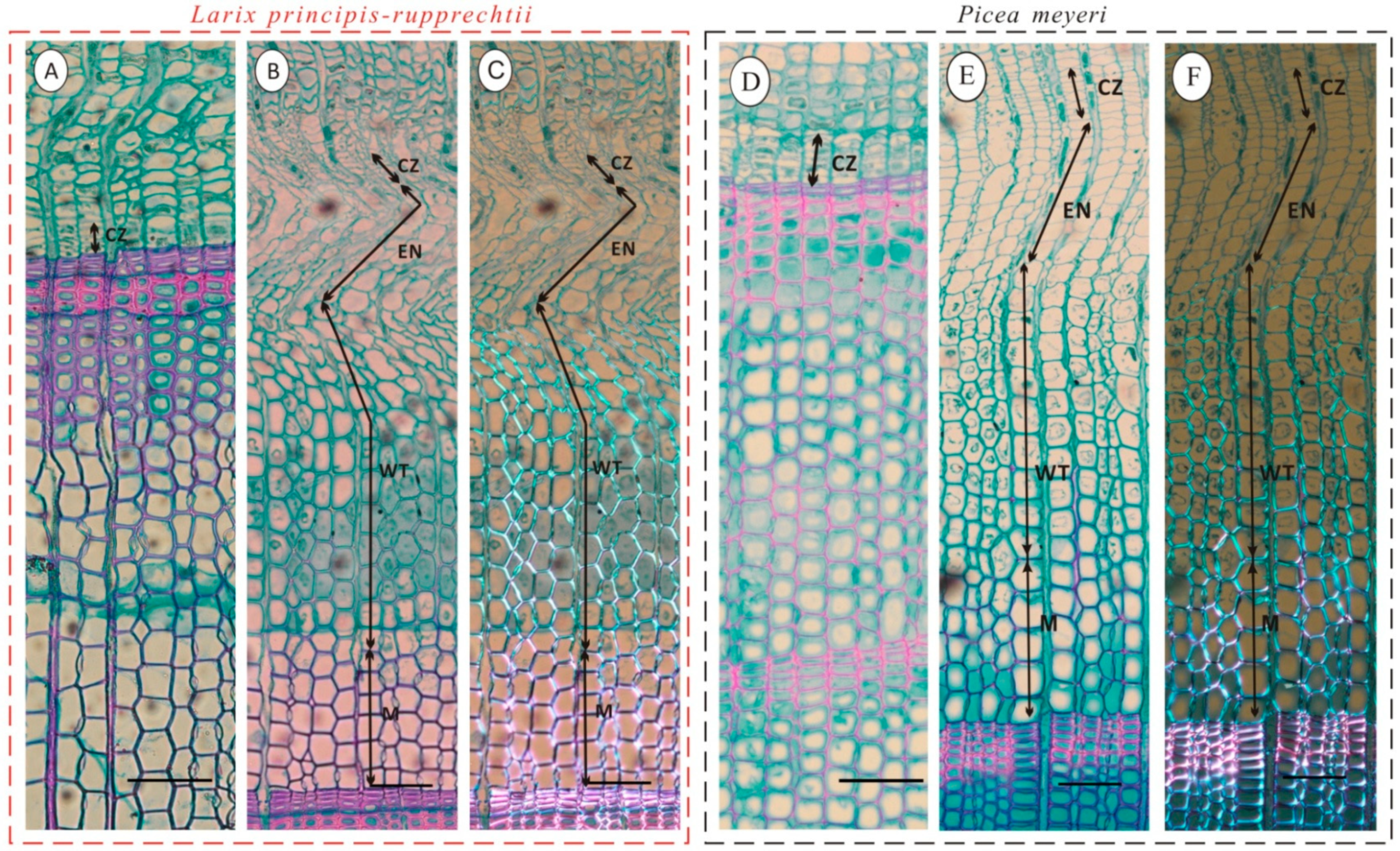
2.3. Secondary Growth
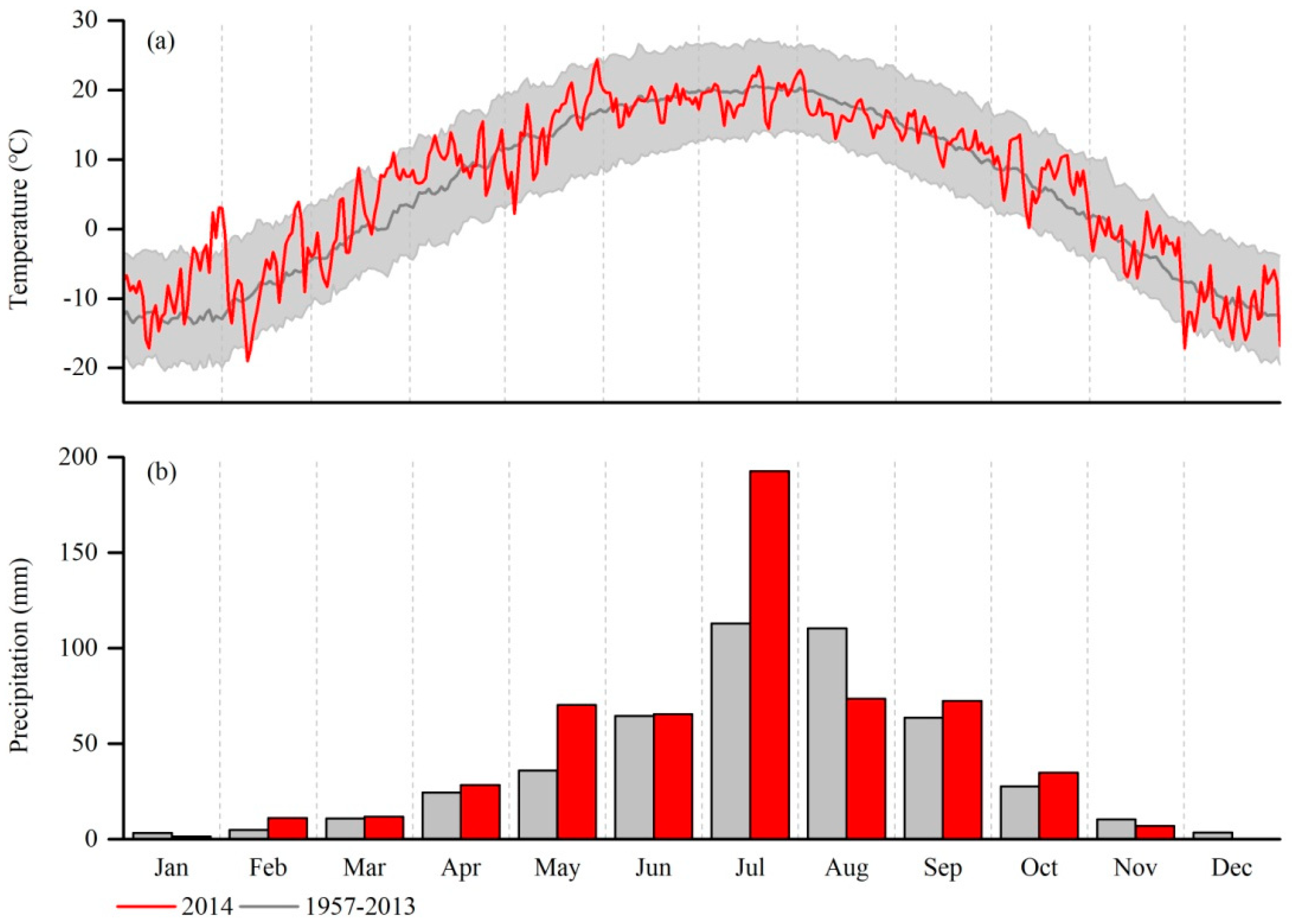
2.4. Weather Station
2.5. Data Analysis
3. Results
3.1. Primary Growth
3.2. Secondary Growth
3.3. Comparing Primary and Secondary Growth
3.4. Environment–Growth Relationship
4. Discussion
4.1. Coordination and Correlation between Primary and Secondary Growth
4.2. Synchronisms of Secondary Growth but Asynchronisms of Primary Growth between Species
4.3. Environmental Effect on Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Mäkinen, H.; Prislan, P.; Rossi, S.; del Castillo, E.M.; Campelo, F.; Vavrčík, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.H.; Zhao, H.F.; Piao, S.L.; Peaucelle, M.; Peng, S.S.; Zhou, G.Y.; Ciais, P.; Huang, M.T.; Menzel, A.; Uelas, J.P.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [PubMed] [Green Version]
- De Micco, V.; Carrer, M.; Rathgeber, C.B.K.; Julio Camarero, J.; Voltas, J.; Cherubini, P.; Battipaglia, G. From xylogenesis to tree rings: Wood traits to investigate tree response to environmental changes. New Phytol. 2019, 40, 2–29. [Google Scholar]
- Chuine, I.; Beaubien, E.G. Phenology is a major determinant of tree species range. Ecol. Lett. 2001, 4, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.G.; Bergeron, Y.; Zhai, L.H.; Denneler, B. Variation in intra-annual radial growth (xylem formation) of Picea mariana (pinaceae) along a latitudinal gradient in western Quebec, Canada. Am. J. Bot. 2011, 98, 792–800. [Google Scholar] [PubMed]
- Ren, P.; Rossi, S.; Camarero, J.J.; Ellison, A.M.; Liang, E.; Penuelas, J. Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan Plateau. Ann. Bot. 2018, 121, 617–624. [Google Scholar]
- Antonucci, S.; Rossi, S.; Deslauriers, A.; Lombardi, F.; Marchetti, M.; Tognetti, R. Synchronisms and correlations of spring phenology between apical and lateral meristems in two boreal conifers. Trees-Struct. Funct. 2015, 35, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, Y.; Guo, Y.; Kang, M.; Wang, M.; Wang, B. Intra-annual xylem growth of Larix principis-rupprechtii at its upper and lower distribution limits on the Luyashan mountain in north-central China. Forests 2015, 6, 3809–3827. [Google Scholar] [CrossRef]
- Huang, J.; Deslauriers, A.; Rossi, S. Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytol. 2014, 203, 831–841. [Google Scholar] [CrossRef]
- Perrin, M.; Rossi, S.; Isabel, N. Synchronisms between bud and cambium phenology in black spruce: Early-flushing provenances exhibit early xylem formation. Tree Physiol. 2017, 37, 593–603. [Google Scholar]
- Heinrich, S.; Dippold, M.A.; Werner, C.; Wiesenberg, G.L.B.; Kuzyakov, Y.; Glaser, B. Allocation of freshly assimilated carbon into primary and secondary metabolites after in situ 13C pulse labelling of Norway spruce (Picea abies). Tree Physiol. 2015, 35, 1176–1191. [Google Scholar]
- Fernández-de-Uña, L.; Rossi, S.; Aranda, I.; Fonti, P.; González-González, B.D.; Cañellas, I.; Gea-Izquierdo, G. Xylem and leaf functional adjustments to drought in Pinus sylvestris and Quercus pyrenaica at their elevational boundary. Front. Plant Sci. 2017, 8, 1200. [Google Scholar] [CrossRef]
- González-González, B.D.; García-González, I.; Vázquez-Ruiz, R.A. Comparative cambial dynamics and phenology of Quercus robur L. and Q. pyrenaica Willd. in an Atlantic forest of the northwestern Iberian Peninsula. Trees-Struct. Funct. 2013, 27, 1571–1585. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Xu, J.L.; Su, W.; Zhao, X.P.; Xu, X.L. Spring precipitation effects on formation of first row of earlywood vessels in Quercus variabilis at Qinling Mountain (China). Trees-Struct. Funct. 2019, 33, 469–480. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol Plant 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Čufar, K.; Prislan, P.; de Luis, M.; Gričar, J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees-Struct. Funct. 2008, 22, 749–758. [Google Scholar] [CrossRef]
- Rossi, S.; Rathgeber, C.B.K.; Deslauriers, A. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann. For. Sci. 2009, 66, 206–213. [Google Scholar] [CrossRef]
- Zhai, L.H.; Bergeron, Y.; Huang, J.G.; Berninger, F. Variation in intra-annual wood formation, and foliage and shoot developement of three major Candian boreal tree species. Am. J. Bot. 2012, 99, 827–837. [Google Scholar] [CrossRef]
- Moser, L.; Fonti, P.; Büentgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010, 30, 225–233. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.T.; Wang, M.C.; Kang, M.Y.; Dong, M.Y. Radial growth of two dominant montane conifer tree species in response to climate change in North-Central China. PLoS ONE 2014, 9, e112537. [Google Scholar] [CrossRef]
- Antonova, G.F.; Stasova, V.V. Effects of environmental factors on wood formation in larch (Larix sibirica Ldb.) stems. Trees-Struct. Funct. 1997, 11, 462–468. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. For. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Körner, C. Significance of temperature in plant life. In Plant Growth and Climate Change; Morison, J.I.L., Morecroft, M.D., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 48–69. [Google Scholar]
- Hänninen, H. Does climatic warming increase the risk of frost damage in northern trees? Plant Cell Environ. 1991, 14, 449–454. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.; Lebourgeois, F.; Fortin, M.; Fournier, M. Life strategies in intra-annual dynamics of wood formation: Example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012, 32, 612–625. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Pierrat, J.C.; Perez, V.; Piedallu, C.; Cecchini, S.; Ulrich, E. Simulating phenological shifts in French temperate forests under two climatic change scenarios and four driving global circulation models. Int. J. Biometeorol. 2010, 54, 563–581. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Gričar, J.; Seo, J.-W.; Rathgeber, C.B.K.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Swidrak, I.; Schuster, R.; Oberhuber, W. Comparing growth phenology of co-occurring deciduous and evergreen conifers exposed to drought. Flora 2013, 208, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z. Vegetation of Shanxi; China Science and Technology Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Migliavacca, M.; Cremonese, E.; Colombo, R.; Busetto, L.; Galvagno, M.; Ganis, L.; Meroni, M.; Pari, E.; Rossini, M.; Siniscalco, C.; et al. European larch phenology in the Alps can we grasp the role of ecological factors by combining field observations and inverse modelling. Int. J. Biometeorol. 2008, 52, 587–605. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Jiang, Y.; Wang, B.; Jiao, L.; Wang, M.C. Seasonal water use by Larix principis-rupprechtii in an alpine habitat. For. Ecol. Manag. 2018, 409, 47–55. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A new tool for sampling microcores from tree stems. New Phytol. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Minderlein, S.; Blodau, C. Humic-rich peat extracts inhibit sulfate reduction, methanogenesis, and anaerobic respiration but not acetogenesis in peat soils of a temperate bog. Soil Biol. Biochem. 2010, 42, 2078–2086. [Google Scholar] [CrossRef]
- Keel, S.G.; Schädel, C. Expanding leaves of mature deciduous forest trees rapidly become autotrophic. Tree Physiol. 2010, 30, 1253–1259. [Google Scholar] [Green Version]
- Matsumoto-Kitano, M.; Kusumoto, T.; Tarkowski, P.; Kinoshita-Tsujimura, K.; Vaclavikova, K.; Miyawaki, K.; Kakimoto, T. Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA 2008, 105, 20027–20031. [Google Scholar] [Green Version]
- Weinstein, D.A.; Beloin, R.M.; Yanai, R.D. Modeling changes in red spruce carbon balance and allocation in response to interacting ozone and nutrient stresses. Trees-Struct. Funct. 1991, 9, 127–146. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar]
- Klein, T.; Vitasse, Y.; Hoch, G. Coordination between growth, phenology and carbon storage in three coexisting deciduous tree species in a temperate forest. Tree Physiol. 2016, 36, 847–855. [Google Scholar] [Green Version]
- Palacio, S.; Camarero, J.J.; Maestro, M.; Alla, A.Q.; Lahoz, E.; Montserrat-Martí, G. Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees-Struct. Funct. 2018, 32, 777–790. [Google Scholar]
- Ide, R.; Oguma, H. Use of digital cameras for phenological observations. Ecol. Inform. 2010, 5, 339–347. [Google Scholar]
- Delpierre, N.; Lireux, S.; Hartig, F.; Camarero, J.J.; Cheaib, A.; Cufar, K.; Cuny, H.; Deslauriers, A.; Fonti, P.; Gricar, J.; et al. Chilling and forcing temperatures interact to predict the onset of wood formation in Northern Hemisphere conifers. Glob. Chang. Biol. 2019, 25, 1089–1105. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keefe, J.F.O. Phenological differences between understory and overstory: A case study using the longterm Harvard Forest records. In Phenology of Ecosystem Processes; Noormets, A., Ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Dong, M.Y.; Jiang, Y.; Zhang, W.T.; Yang, Y.G.; Yang, H.C. Effect of apline treeline conditions on the response of the stem radial variation of Picea meyeri Rebd. Et Wils to environmental factors. Pol. J. Ecol. 2011, 59, 729–739. [Google Scholar]
- Wu, X.C.; Li, X.Y.; Liu, H.Y.; Ciais, P.; Li, Y.Q.; Xu, C.Y.; Babst, F.; Guo, W.C.; Hao, B.Y.; Wang, P.; et al. Uneven winter snow influence on tree growth across temperate China. Glob. Chang. Biol. 2019, 25, 144–154. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Bayramzadeh, V.; Oribe, Y.; Kubo, T.; Funada, R. Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Trees-Struct. Funct. 2008, 28, 1813–1819. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef]




| Species | Height (m) | Diameter (cm) | Age (year) | Crown Breadth (m × m) |
|---|---|---|---|---|
| Larix principis-rupprechtii Mayr. | 7.8 (1.8) | 21.1 (2.9) | 57 (6) | 3 × 3 |
| Picea meyeri Rehd. et Wils. | 8.5 (1.5) | 21.5 (4.6) | 58 (9) | 4 × 3 |
| Species | Organ | Onset (DOY) | χ (p) | End (DOY) | χ (p) | tp (DOY) | χ (p) |
|---|---|---|---|---|---|---|---|
| Larch | Needle | 125 ± 1 aA | 8.889 (0.012) | 171 ± 2 aA | 9.143 (0.010) | 150 ± 3 aA | 5.778 (0.056) |
| Shoot | 142 ± 2 bA | 201 ± 4 bA | 185 ± 1 bA | ||||
| Stem | 155 ± 4 cA | 247 ± 3 cA | 183 ± 3 bA | ||||
| Spruce | Needle | 145 ± 2 aB | 8.848 (0.012) | 176 ± 4 aB | 8.434 (0.015) | 161 ± 5 aB | 8.727 (0.013) |
| Shoot | 153 ± 1 bB | 186 ± 6 aB | 169 ± 2 aB | ||||
| Stem | 160 ± 4 cA | 245 ± 4 bA | 183 ± 5 bA |
| Needle | Shoot | Stem | |
|---|---|---|---|
| Needle | - | −0.238 | −0.336 * |
| Shoot | 0.561 *** | - | 0.969 *** |
| Stem | −0.167 | 0.414 ** | - |
| Organ | Species | Ta | Ts | SWC | P | RH | GDDa | GDDs |
|---|---|---|---|---|---|---|---|---|
| Needle growth | Larch | 0.816 * | 0.661 | 0.769 * | 0.210 | 0.047 | 0.610 | 0.681 |
| Spruce | 0.796 | 0.633 | −0.771 | −0.765 | −0.137 | 0.795 | 0.827 | |
| Shoot growth | Larch | 0.312 | 0.946 ** | −0.07 | 0.445 | 0.454 | 0.621 * | 0.985 *** |
| Spruce | 0.467 | 0.841 * | −0.273 | 0.008 | -0.582 | 0.575 | 0.938 ** | |
| Stem growth | Larch | 0.636 | 0.931 ** | −0.016 | 0.427 | 0.158 | 0.874 * | 0.958 ** |
| Spruce | 0.335 | 0.904 ** | 0.590 | 0.543 | 0.670 | 0.746 | 0.955 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, Y.; Wen, Y.; Ding, X.; Wang, B.; Xu, J. Comparing Primary and Secondary Growth of Co-Occurring Deciduous and Evergreen Conifers in an Alpine Habitat. Forests 2019, 10, 574. https://doi.org/10.3390/f10070574
Zhang Y, Jiang Y, Wen Y, Ding X, Wang B, Xu J. Comparing Primary and Secondary Growth of Co-Occurring Deciduous and Evergreen Conifers in an Alpine Habitat. Forests. 2019; 10(7):574. https://doi.org/10.3390/f10070574
Chicago/Turabian StyleZhang, Yiping, Yuan Jiang, Yan Wen, Xinyuan Ding, Biao Wang, and Junliang Xu. 2019. "Comparing Primary and Secondary Growth of Co-Occurring Deciduous and Evergreen Conifers in an Alpine Habitat" Forests 10, no. 7: 574. https://doi.org/10.3390/f10070574
APA StyleZhang, Y., Jiang, Y., Wen, Y., Ding, X., Wang, B., & Xu, J. (2019). Comparing Primary and Secondary Growth of Co-Occurring Deciduous and Evergreen Conifers in an Alpine Habitat. Forests, 10(7), 574. https://doi.org/10.3390/f10070574





