Development and Application of EST-SSR Markers for DNA Fingerprinting and Genetic Diversity Analysis of the Main Cultivars of Black Locust (Robinia pseudoacacia L.) in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and DNA Extraction
2.2. SSR Locus Search and Primer Design
2.3. Polymorphism Analysis of EST-SSR Markers
2.4. SSR Analysis of the Main Black Locust Cultivars
2.5. Data Analyses
3. Results
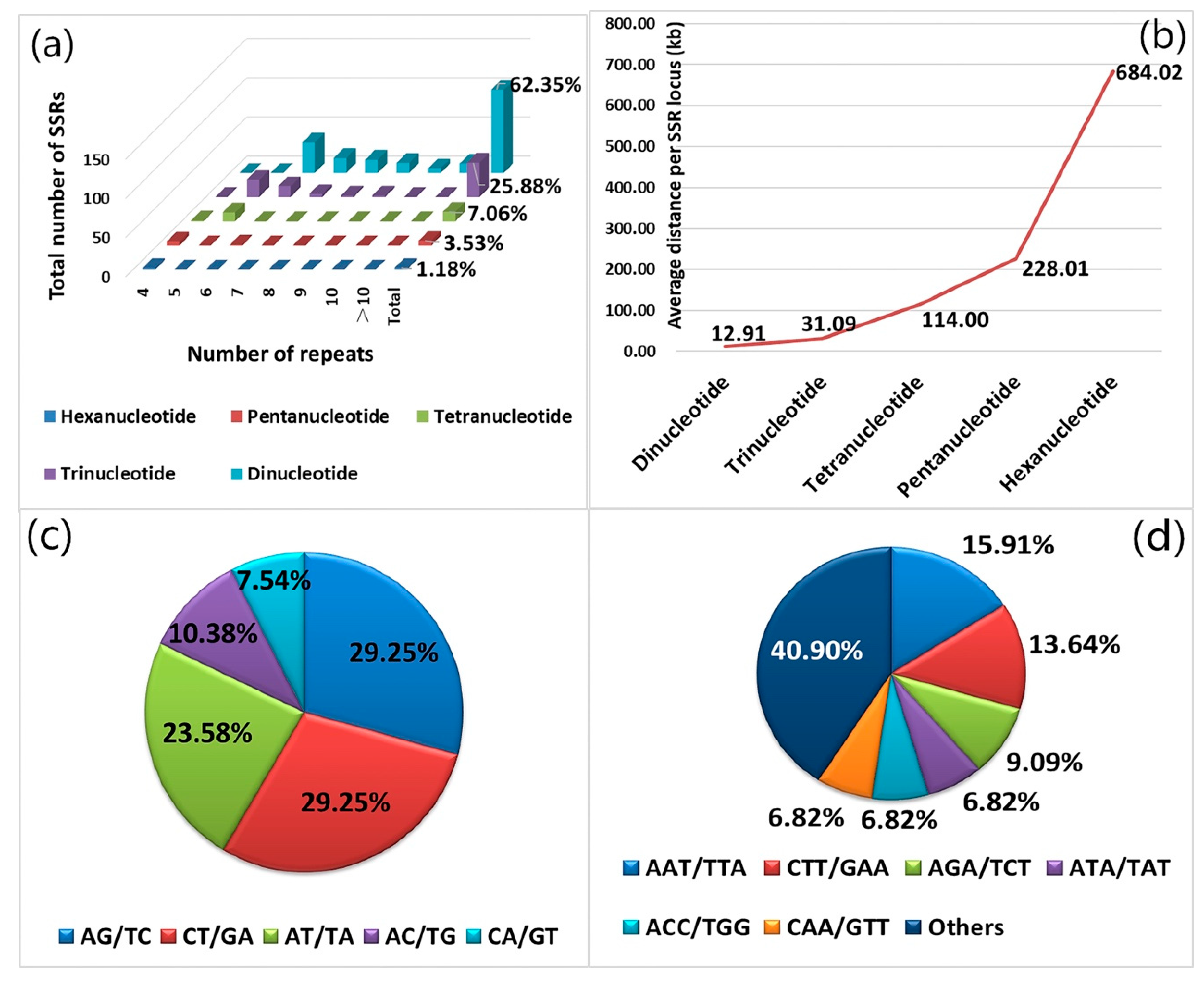
3.1. Distribution Characteristics of SSR Loci in Black Locust EST Sequences
3.2. Development of EST-SSR Markers
3.3. Genetic Diversity Analysis of the 123 Main Black Locust Cultivars in China
3.4. Construction of SSR Fingerprints
3.5. Similarity Coefficient and Clustering Analysis
4. Discussion
4.1. Frequency and Characteristics of SSRs in Black Locust EST Sequences
4.2. Validation of EST-SSR Loci
4.3. SSR Fingerprints and Cluster Analysis of Black Locust
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barrett, R.P.; Mebrahtu, T.; Hanover, J.W. Black locust: A multi-purpose tree species for temperate climates. In Advances in New Crops; Timber Press: Portland, OR, USA, 1990; pp. 278–283. [Google Scholar]
- Bongarten, B.C.; Huber, D.A.; Apsley, D.K. Environmental and genetic influences on short-rotation biomass production of black locust (Robinia pseudoacacia L.) in the Georgia Piedmont. For. Ecol. Manag. 1992, 55, 315–331. [Google Scholar] [CrossRef]
- Li, J. The introduction and development of black locust in Shandong. J. Shandong For. Sci. Technol. 1983, 04, 73–75. [Google Scholar]
- Kopp, R.; Smart, L.; Maynard, C.; Tuskan, G.; Abrahamson, L. Predicting within-family variability in juvenile height growth of Salix based upon similarity among parental AFLP fingerprints. Theor. Appl. Genet. 2002, 105, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zheng, Y.; Sun, B.; Zhang, Y.; Han, C.; Xiao, W.; Xun, S. Genetic Diversity and Fingerprints of Robinia pseudoacacia Clones Based on SSR Markers. Sci. Silvae Sin. 2017, 53, 80–89. [Google Scholar]
- Xiong, J.S.; Isik, F.; McKeand, S.E.; Whetten, R.W. Genetic variation of stem forking in loblolly pine. For. Sci. 2010, 56, 429–436. [Google Scholar]
- Ma, L.Y.; Kong, D.; Liu, H.; Wang, S.-Q.; Li, Y.-Y.; Pang, X.-M. Construction of SSR fingerprint on 36 Chinese jujube cultivars. Acta Hortic. Sin. 2012, 39, 647. [Google Scholar]
- Chen, C.; Zhang, X.; Cheng, F.; Zhuang, M.; Liu, Y.; Yang, L.; Zhang, Y.; Fang, Z. Establishment of SSR fingerprinting on autumn cabbage hybrids and their parents. Acta Hortic. Sin. 2011, 38, 159–164. [Google Scholar]
- Wang, Z.; Weber, J.L.; Zhong, G.; Tanksley, S.D. Survey of plant short tandem DNA repeats. Theor. Appl. Genet. 1994, 88, 6. [Google Scholar] [CrossRef]
- Bell, C.J.; Ecker, J.R. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 1994, 19, 137–144. [Google Scholar] [CrossRef]
- Morgante, M.; Olivieri, A.M. PCR-amplified microsatellites as markers in plant genetics. Plant J. 1993, 3, 175–182. [Google Scholar] [CrossRef]
- Tautz, D.; Renz, M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984, 12, 4127–4138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röder, M.S.; Korzun, V.; Wendehake, K.; Plaschke, J.; Tixier, M.-H.; Leroy, P.; Ganal, M.W. A microsatellite map of wheat. Genetics 1998, 149, 2007–2023. [Google Scholar] [PubMed]
- Ayres, N.; McClung, A.; Larkin, P.; Bligh, H.; Jones, C.; Park, W. Microsatellites and a single-nucleotide polymorphism differentiate apparentamylose classes in an extended pedigree of US rice germ plasm. Theor. Appl. Genet. 1997, 94, 773–781. [Google Scholar] [CrossRef]
- Lian, C.; Oishi, R.; Miyashita, N.; Hogetsu, T. High somatic instability of a microsatellite locus in a clonal tree, Robinia pseudoacacia. Theor. Appl. Genet. 2004, 108, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Hogetsu, T. Development of microsatellite markers in black locust (Robinia pseudoacacia) using a dual-supression-PCR technique. Mol. Ecol. Notes 2002, 2, 211–213. [Google Scholar]
- Mishima, K.; Hirao, T.; Urano, S.; Watanabe, A.; Takata, K. Isolation and characterization of microsatellite markers from Robinia pseudoacacia L. Mol. Ecol. Resour. 2009, 9, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, C.; Yuan, C.; Cui, B.; Qiu, Q.; Sun, P.; Hu, R.; Wu, D.; Sun, Y.; Li, Y. Characterization of ESTs from black locust for gene discovery and marker development. Genet. Mol. Res 2015, 14, 12684–12691. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, J.-X.; Su, L.-Z.; Lv, W.; Sun, Y.-H.; Li, Y. Development and evaluation of a novel set of EST-SSR markers based on transcriptome sequences of black locust (Robinia pseudoacacia L.). Genes 2017, 8, 177. [Google Scholar] [CrossRef]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Don, R.; Cox, P.; Wainwright, B.; Baker, K.; Mattick, J. ‘Touchdown’PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991, 19, 4008. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Xiong, F.; Duan, X.B.; Chen, D.Q. Isolation and characterization of polymorphic microsatellite loci from elongate loach (Leptobotia elongate), a threatened fish species endemic to the Yangtze River. Conserv. Genet. Resour. 2012, 4, 129–131. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2010, 6, 288–295. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [PubMed]
- Rohlf, F. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System, Ver. 2.1; Applied Biostatistics: New York, NY, USA, 2000. [Google Scholar]
- Yang, X.; Sun, X.; Zhang, S.; Xie, Y.; Han, H. Development of EST-SSR markers and genetic diversity analysis of second cycle elite population in Larix Kaempferi. Sci. Silvae Sin. 2011, 47, 52–58. [Google Scholar]
- Sheng, Z.; Huang, H.; Lin, E.; Tong, Z. Development and Application of EST-SSR Markers for Cunninghamia lanceolata and Taiwania cryptomerioides. Sci. Silvae Sin. 2013, 49, 173–180. [Google Scholar]
- Wang, Y. Data Mining for SSRs in ESTs and EST-SSR Marker Development in Betula platyphylla. Sci. Silvae Sin. 2008, 44, 78–84. [Google Scholar]
- Varshney, R.K.; Andreas, G.; Sorrells, M.E. Genic microsatellite markers in plants: Features and applications. Trends Biotechnol. 2005, 23, 48–55. [Google Scholar] [CrossRef]
- Nicot, N.; Chiquet, V.; Gandon, B.; Amilhat, L.; Legeai, F.; Leroy, P.; Bernard, M.; Sourdille, P. Study of simple sequence repeat (SSR) markers from wheat expressed sequence tags (ESTs). Theor. Appl. Genet. 2004, 109, 800–805. [Google Scholar] [CrossRef]
- Kantety, R.V.; Rota, M.L.; Matthews, D.E.; Sorrells, M.E. Data mining for simple sequence repeats in expressed sequence tags from barley, maize, rice, sorghum and wheat. Plant Mol. Biol. 2002, 48, 501–510. [Google Scholar] [CrossRef]
- Bérubé, Y.; Zhuang, J.; Rungis, D.; Ralph, S.; Bohlmann, J.; Ritland, K. Characterization of EST-SSRs in loblolly pine and spruce. Tree Genet. Genomes 2007, 3, 251–259. [Google Scholar] [CrossRef]
- Zhang, X. Development of EST-SSR in Populus deltoides and P. euramericana. Sci. Silvae Sin. 2009, 45, 53–59. [Google Scholar]
- Gupta, P.K.; Rustgi, S.; Sharma, S.; Singh, R.; Kumar, N.; Balyan, H.S. Transferable EST-SSR markers for the study of polymorphism and genetic diversity in bread wheat. Mol. Genet. Genom. 2003, 270, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Temnykh, S.; Park, W.D.; Ayres, N.; Cartinhour, S.; Hauck, N.; Lipovich, L.; Cho, Y.G.; Ishii, T.; Mccouch, S.R. Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L.). Theor. Appl. Genet. 2000, 100, 697–712. [Google Scholar] [CrossRef]
- Metzgar, D.; Bytof, J.; Wills, C. Selection against frameshift mutations limits microsatellite expansion in coding DNA. Genome Res. 2000, 10, 72–80. [Google Scholar] [PubMed]
- Kumpatla, S.P.; Mukhopadhyay, S. Mining and survey of simple sequence repeats in expressed sequence tags of dicotyledonous species. Genome 2005, 48, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Qi, X.; Wang, L.; Zhang, Y.; Hua, W.; Li, D.; Lv, H.; Zhang, X. Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genom. 2011, 12, 451. [Google Scholar] [CrossRef]
- Martienssen, R.A.; Colot, V. DNA methylation and epigenetic inheritance in plants and filamentous fungi. Science 2001, 293, 1070–1074. [Google Scholar] [CrossRef]
- Saha, M.C.; Mian, M.R.; Eujayl, I.; Zwonitzer, J.C.; Wang, L.; May, G.D. Tall fescue EST-SSR markers with transferability across several grass species. Theor. Appl. Genet. 2004, 109, 783–791. [Google Scholar] [CrossRef]
- Xun, S.H.; Zhou, J.R.; Huang, F.J.; Qiao, Y.L.; Zhang, L.J.; Mao, X.H. Guideline for the tests of DUS for new varieties of genus Robinia. J. Beijing For. Univ. 2013. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, Y. Analysis of Genetic Relationship of Robinia among Species Using RAPD Marker. J. Liaocheng Univ. 2009, 22, 68–70. [Google Scholar]
- Wang, D.; Zhou, J.; Xie, H. AFLP Genetic Diversity Analysis of Robinia pseudoacacia Clones. J. Southwest For. Univ. 2012, 32, 25–29. [Google Scholar]
- Devos, K.; Gale, M.D. The use of random amplified polymorphic DNA markers in wheat. Theor. Appl. Genet. 1992, 84, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Liesebach, H.; Ewald, E. Optimisation of a multiplex PCR assay of nuclear microsatellite markers for population genetics and clone identification in Robinia pseudoacacia L. Silvae Genet. 2012, 61, 142–148. [Google Scholar] [CrossRef]



| Code | Cultivar | Code | Cultivar | Code | Cultivar | Code | Cultivar |
|---|---|---|---|---|---|---|---|
| 1(c) | Lei 01 | 32(c) | Yu 8030 | 63(b) | 42 | 94(b) | II-7 |
| 2(c) | Mei 1 | 33(c) | Yu 8031 | 64(b) | 63 | 95(b) | III-21 |
| 3(c) | Lei 02 | 34(c) | Yu 8032 | 65(b) | 68 | 96(b) | 3-I |
| 4(c) | Lei 03 | 35(c) | Yu 8033 | 66(b) | 78 | 97(c) | X 2 |
| 5(c) | Lei 04 | 36(c) | Yu 8034 | 67(b) | 80 | 98(c) | X 3 |
| 6(c) | Lei 05 | 37(c) | Yu 8035 | 68(b) | 84 | 99(c) | X 4 |
| 7(c) | Lei 06 | 38(c) | Yu 8037 | 69(b) | 86 | 100(c) | X 5 |
| 8(b) | Lei 07 | 39(c) | Yu 8038 | 70(b) | 87 | 101(c) | X 6 |
| 9(c) | Lei 08 | 40(c) | Yu 8039 | 71(b) | 90 | 102(c) | X 7 |
| 10(c) | Lei 09 | 41(c) | Yu 8042 | 72(b) | 103 | 103(c) | X 8 |
| 11(a) | Xing1 | 42(c) | Yu 8043 | 73(b) | 165 | 104(c) | X 9 |
| 12(c) | Xing 2 | 43(c) | Yu 8044 | 74(b) | K 1 | 105(c) | X Daye |
| 13(a) | Xing 08 | 44(c) | Yu 8047 | 75(b) | K 2 | 106(a) | Jiangan 1 |
| 14(c) | Xing 11 | 45(c) | Yu 8048 | 76(b) | K 3 | 107(b) | Danye |
| 15(c) | Xing 14 | 46(c) | Yu 8054 | 77(b) | K 4 | 108(a) | Honghuahuai |
| 16(c) | Xing 16 | 47(c) | Yu 8057 | 78(b) | K 5 | 109(c) | Chuihuai |
| 17(a) | Xing 20 | 48(c) | Yu 8062 | 79(c) | Yuci 83002 | 110(b) | Changye |
| 18(c) | Xing 23 | 49(a) | Lu 038 | 80(c) | Yuci 84023 | 111(b) | Xiongyali |
| 19(c) | Xing 24 | 50(a) | Lu 042 | 81(c) | Yu 8402 | 112(b) | Xiaoye |
| 20(c) | Xing 32 | 51(a) | Lu 50 | 82(c) | Yu 8095 | 113(b) | Beilin |
| 21(c) | Yu 8001 | 52(a) | Lu 068 | 83(b) | Xinzheng 1 | 114(c) | Shilin |
| 22(c) | Yu 8004 | 53(a) | Lu 86 | 84(b) | Xinzheng 3 | 115(b) | Hebei |
| 23(c) | Yu 8005 | 54(a) | Lu 90 | 85(b) | A | 116(c) | Minquan |
| 24(c) | Yu 8006 | 55(a) | Lu 87 | 86(b) | B | 117(c) | Henanjiangan |
| 25(c) | Yu 8007 | 56(b) | No.4 | 87(b) | F | 118(b) | YQ 2 |
| 26(c) | Yu 8008 | 57(b) | No.9 | 88(b) | G | 119(b) | YQ 13 |
| 27(c) | Yu 8009 | 58(c) | No.10 | 89(b) | H | 120(b) | 1 XYL |
| 28(c) | Yu 8014 | 59(b) | 7 | 90(b) | Q | 121(b) | ZYDL |
| 29(c) | Yu 8023 | 60(b) | 10 | 91(b) | V-23 | 122(b) | HY 2-4 |
| 30(c) | Yu 8025 | 61(b) | 13 | 92(b) | V-31 | 123(b) | HY 101 |
| 31(c) | Yu 8027 | 62(b) | 14 | 93(b) | VI-37 |
| Accession No. | Locus | Repeat Motif | Primer Sequence (5′ to 3′) | Size (bp) | Annealing Temperature (℃) | Na. |
|---|---|---|---|---|---|---|
| gi|15630411|gb|BI677504.1|BI677504-2 | Rply1 | (AAG)6 | F: AGTTCGCAAAGGAAGGAG | 247–274 | 54 | 3 |
| R: GGTAACAAGCACCAGCAA | ||||||
| gi|15544384|gb|BI642174.1|BI642174-5 | Rply2 | (CCA)6 | F: TGTGAATGGTTGGTGGACAT | 148–160 | 58 | 4 |
| R: CGTTGCTTGGAGGAGAATAA | ||||||
| gi|15544478|gb|BI642268.1|BI642268-5 | Rply3 | (GTGGTA)4 | F: GCCTCATAAATAAAAGGAACG | 138–164 | 59 | 11 |
| R: CTGCCATTGGTAACTGGTAAA | ||||||
| gi|15544752|gb|BI642542.1|BI642542-4 | Rply5 | (ATG)8 | F: GAGTCATGCCCTTTGTATGTT | 122–143 | 59 | 6 |
| R: TGTCACCTTCAAGTCCCTATT | ||||||
| gi|15545024|gb|BI642814.1|BI642814-1 | Rply8 | (CT)14 | F: TCCCTACATAAAACTCCAAA | 239–271 | 55 | 15 |
| R: TCATTAAGTCAGCACTCACAG | ||||||
| gi|15544811|gb|BI642601.1|BI642601-1 | Rply11 | (AG)10 | F: ATAGCAGTGGGTGAACAAG | 106–128 | 53 | 3 |
| R: GAAACCGAAGCTCAAGTAG | ||||||
| gi|15630549|gb|BI677642.1|BI677642-3 | Rply15 | (CAT)7 | F: GGCACAAGAACAGAACAAA | 88–112 | 53 | 6 |
| R: GTGGTGGATAAGGATAAGC | ||||||
| gi|15630659|gb|BI677752.1|BI677752-6 | Rply16 | (GAA)7 | F: CGTCGGAGTGCTGTTATG | 239–245 | 55 | 3 |
| R: AGCTTATGGCAAAGAGGG | ||||||
| gi|15630701|gb|BI677794.1|BI677794-2 | Rply17 | (GAT)6 | F: TCTGATCCTGATGGTGCTG | 252–276 | 56 | 2 |
| R: GTCGTCGTGTCCTTCTGTC | ||||||
| gi|15630787|gb|BI677880.1|BI677880-2 | Rply18 | (TGCAG)4 | F: GCTCGTCAATATGGCAAAC | 181–221 | 58 | 3 |
| R: CAACATTATCACCCAACCC | ||||||
| gi|15632039|gb|BI679132.1|BI679132-4 | Rply21 | (TGTA)5 | F: TCAGTTGAACCAGCAGAGT | 177–185 | 53 | 2 |
| R: ATATCGCCATGTAAAGCAG | ||||||
| gi|15630346|gb|BI677439.1|BI677439-6 | Rply22 | (ACCTG)4 | F: ATCACCATCTGTTCCTCCAC | 115–130 | 58 | 4 |
| R: TTCTCCTCAGCCACTTCTTT | ||||||
| gi|15631600|gb|BI678693.1|BI678693-4 | Rply27 | (TAT)6 | F: CATTTAGTTGGAGGCATTG | 145–154 | 54 | 4 |
| R: AAGATGGAAGTGACGAGGA | ||||||
| gi|391233429|gb|JK974151.1|JK974151-1 | Rply28 | (TGAGC)4 | F: TGGAGGGTTAGATTTGGATGT | 152–172 | 58 | 3 |
| R: AGGATGGAGAGTAAAGGGTTG | ||||||
| gi|391233401|gb|JK974123.1|JK974123-4 | Rply31 | (TCC)5 | F: CATTCTTCCCAATCTCCTTT | 155–158 | 56 | 2 |
| R: GTTTCATTTTCGCATTCTGT | ||||||
| gi|15631784|gb|BI678877.1|BI678877-5 | Rply32 | (GTT)5 | F: TGGGATCTTTGGAGGTAAT | 247–251 | 54 | 3 |
| R: TAGAGGCTCAATGGTTTCA | ||||||
| gi|15631654|gb|BI678747.1|BI678747-6 | Rply33 | (ACT)7 | F: GCCCATTCTGTTGGTTCT | 273–279 | 55 | 2 |
| R: GGTTATGTGCCCTCGTGT | ||||||
| gi|15631164|gb|BI678257.1|BI678257-1 | Rply36 | (CT)7 | F: CGTTGAAAGAGGGAAAGCAG | 268–270 | 62 | 2 |
| R: GAAATCGACGCCAATGGTAC | ||||||
| gi|15630348|gb|BI677441.1|BI677441-2 | Rply43 | (AT)6 | F: GTTTACGGCGTTGTTGAGTT | 144–178 | 57 | 4 |
| R: CTAATTGCTTGCATTTCCCT | ||||||
| gi|15631529|gb|BI678622.1|BI678622-3 | Rply44 | (CT)7 | F: AAAGTGAGACATCCAAGTTC | 167–171 | 53 | 2 |
| R: CAACCTTCCATTGTTTACAC | ||||||
| gi|15631101|gb|BI678194.1|BI678194-3 | Rply45 | (TA)8 | F: CAACTCGTTTTCCTTCTTCA | 173–185 | 55 | 4 |
| R: ACCGAATCAGATCACATCAA | ||||||
| gi|15630467|gb|BI677560.1|BI677560-1 | Rply49 | (CT)8 | F: CCCCGTACAGTTCCATCT | 118–144 | 60 | 13 |
| R: GACCTCGTAAAAGCCACC | ||||||
| gi|15630465|gb|BI677558.1|BI677558-3 | Rply50 | (TC)8 | F: GCGAAAACGACAAGGAGATA | 292–300 | 58 | 4 |
| R: TTTGAGGAGCAACAAAGAGC | ||||||
| gi|15544793|gb|BI642583.1|BI642583-2 | Rply53 | (GA)6 | F: TGTTCTCCTCACCTTCCCTT | 183–211 | 57 | 3 |
| R: GTTCATGGCTCTTGTCTTCC | ||||||
| gi|15544810|gb|BI642600.1|BI642600-2 | Rply60 | (TC)6 | F: ATCTTTGACGCTTCTCGC | 271–281 | 55 | 4 |
| R: CATTTCTTCTTCAGCCTGTG |
| Locus | Repeat Motif | Primer Sequences (5′-3′) | Allele Size (bp) | Accession No. | Origin |
|---|---|---|---|---|---|
| Rops08 | (CA)8TA(CA)3 | F: TTCTGAGGAAGGGTTCCGTGG | 191–205 | AB075033 | Lian et al., 2002 [16] |
| R: GTTAAAGCAACAGGCACATGG | |||||
| Rp109 | (AG)17 | F: GAGGAATCACAAAACCGTTTGG | 136–160 | AB353930 | Mishima et al., 2009 [17] |
| R: TGGGATTTGAGAGAGTGGTGGTG | |||||
| Rp200 | (AG)23 | F: GGTTTCTTTGTTCACCTGCTCTGG | 160–198 | AB353933 | Mishima et al., 2009 [17] |
| R: ACCTACGTGTCCACGGCTCT | |||||
| Rp206 | (GT)9 | F: GCCAAATCCCATTAGATCACAGTTGA | 222–246 | AB353932 | Mishima et al., 2009 [17] |
| R: AGAAGTTAGACTTACGTGCTGC |
| Locus | Na | Ne | I | Ho | He | PIC |
|---|---|---|---|---|---|---|
| Rply2 | 4 | 2.686 | 1.129 | 0.610 | 0.628 | 0.573 |
| Rply5 | 6 | 3.152 | 1.331 | 0.732 | 0.683 | 0.648 |
| Rply3 | 11 | 7.896 | 2.210 | 0.881 | 0.873 | 0.805 |
| Rply60 | 4 | 1.744 | 0.782 | 0.174 | 0.427 | 0.402 |
| Rply27 | 4 | 2.610 | 1.125 | 0.517 | 0.617 | 0.564 |
| Rply49 | 13 | 5.771 | 2.023 | 0.787 | 0.827 | 0.806 |
| Rops08 | 8 | 2.799 | 1.360 | 0.567 | 0.643 | 0.584 |
| Rp109 | 9 | 4.106 | 1.710 | 0.737 | 0.756 | 0.726 |
| Rp200 | 13 | 8.395 | 2.307 | 0.929 | 0.881 | 0.857 |
| Rp206 | 9 | 3.080 | 1.484 | 0.400 | 0.675 | 0.629 |
| Rply8 | 15 | 7.422 | 2.246 | 0.757 | 0.865 | 0.842 |
| Rply15 | 6 | 3.023 | 1.363 | 0.719 | 0.669 | 0.609 |
| Mean | 8.5 | 4.390 | 1.589 | 0.651 | 0.712 | 0.670 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Sun, Y.; Zhao, K.; Zhang, J.; Zhang, Y.; Li, X.; Xun, S.; Zhang, J.; Wang, S.; Li, Y. Development and Application of EST-SSR Markers for DNA Fingerprinting and Genetic Diversity Analysis of the Main Cultivars of Black Locust (Robinia pseudoacacia L.) in China. Forests 2019, 10, 644. https://doi.org/10.3390/f10080644
Dong L, Sun Y, Zhao K, Zhang J, Zhang Y, Li X, Xun S, Zhang J, Wang S, Li Y. Development and Application of EST-SSR Markers for DNA Fingerprinting and Genetic Diversity Analysis of the Main Cultivars of Black Locust (Robinia pseudoacacia L.) in China. Forests. 2019; 10(8):644. https://doi.org/10.3390/f10080644
Chicago/Turabian StyleDong, Li, Yuhan Sun, Keqi Zhao, Jing Zhang, Yuwei Zhang, Xiuyu Li, Shouhua Xun, Jiangtao Zhang, Shaoming Wang, and Yun Li. 2019. "Development and Application of EST-SSR Markers for DNA Fingerprinting and Genetic Diversity Analysis of the Main Cultivars of Black Locust (Robinia pseudoacacia L.) in China" Forests 10, no. 8: 644. https://doi.org/10.3390/f10080644





