Identification of RING-H2 Gene Candidates Related to Wood Formation in Poplar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Identification of the RING-H2 Genes in P. trichocarpa
2.3. Phylogenetic and Chromosomal Duplication Analyses
2.4. Microarray and AspWood RNAseq Analyses
2.5. qRT-PCR Analysis
2.6. Identification of Xylem Development-Related Cis-Elements in the Promoters
2.7. Plasmid Construction and Agrobacterium-Mediated Transformation
2.8. Histochemical GUS Assay
3. Results
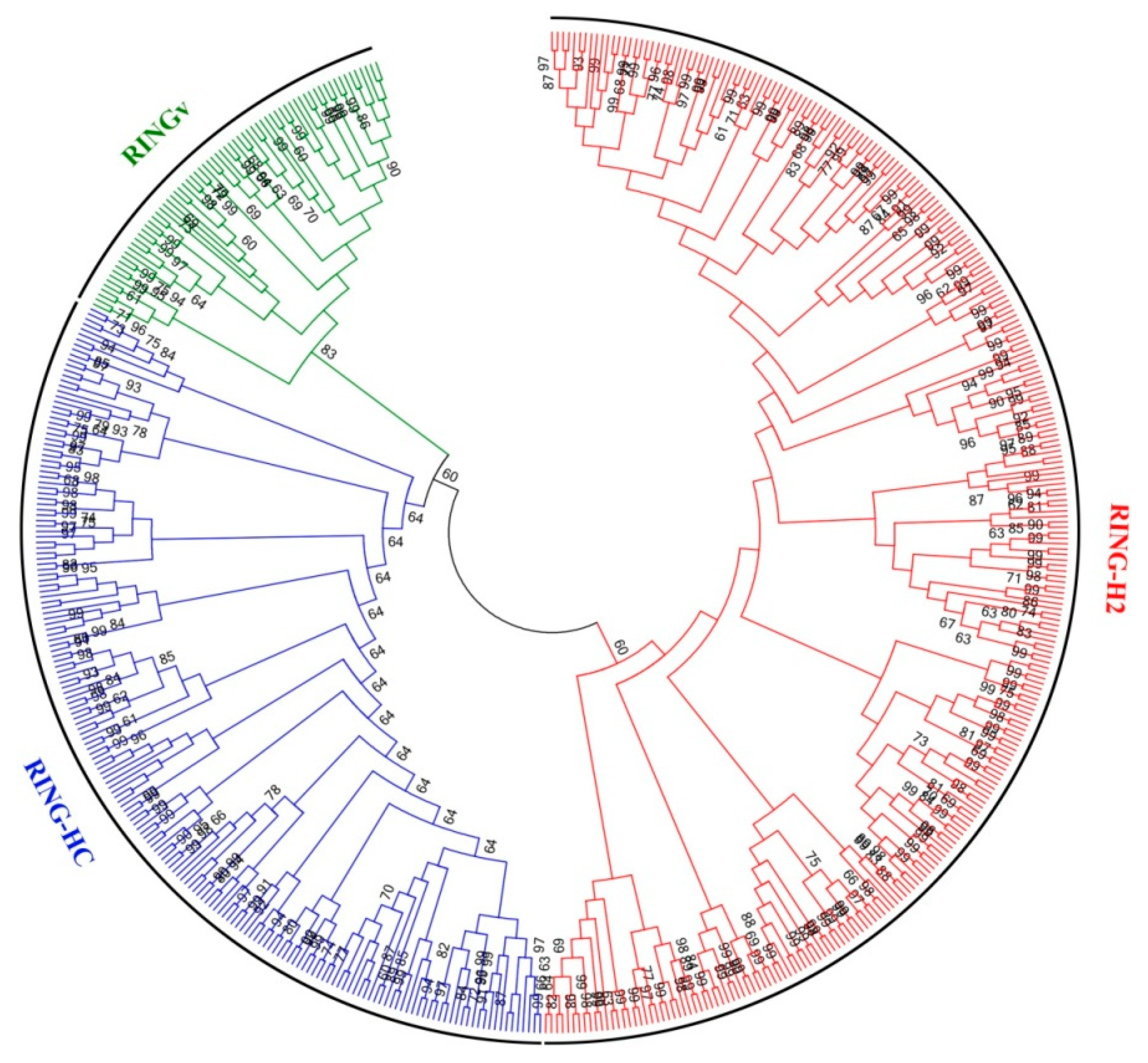
3.1. Identification of the RING-H2 Genes in P. trichocarpa
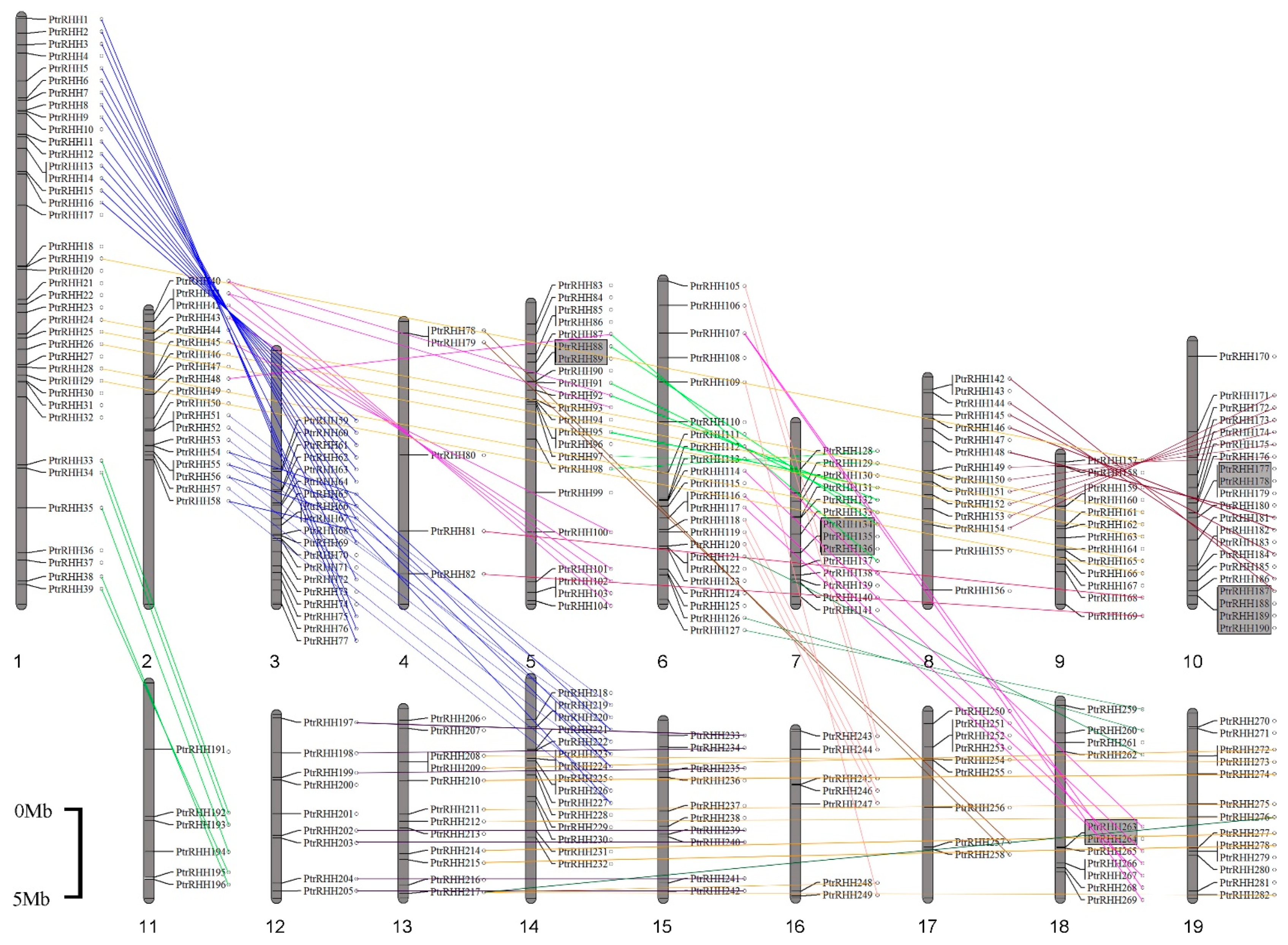
3.2. Gene Duplications, Gene Structures and Conserved Motifs in Populus RING-H2 Genes
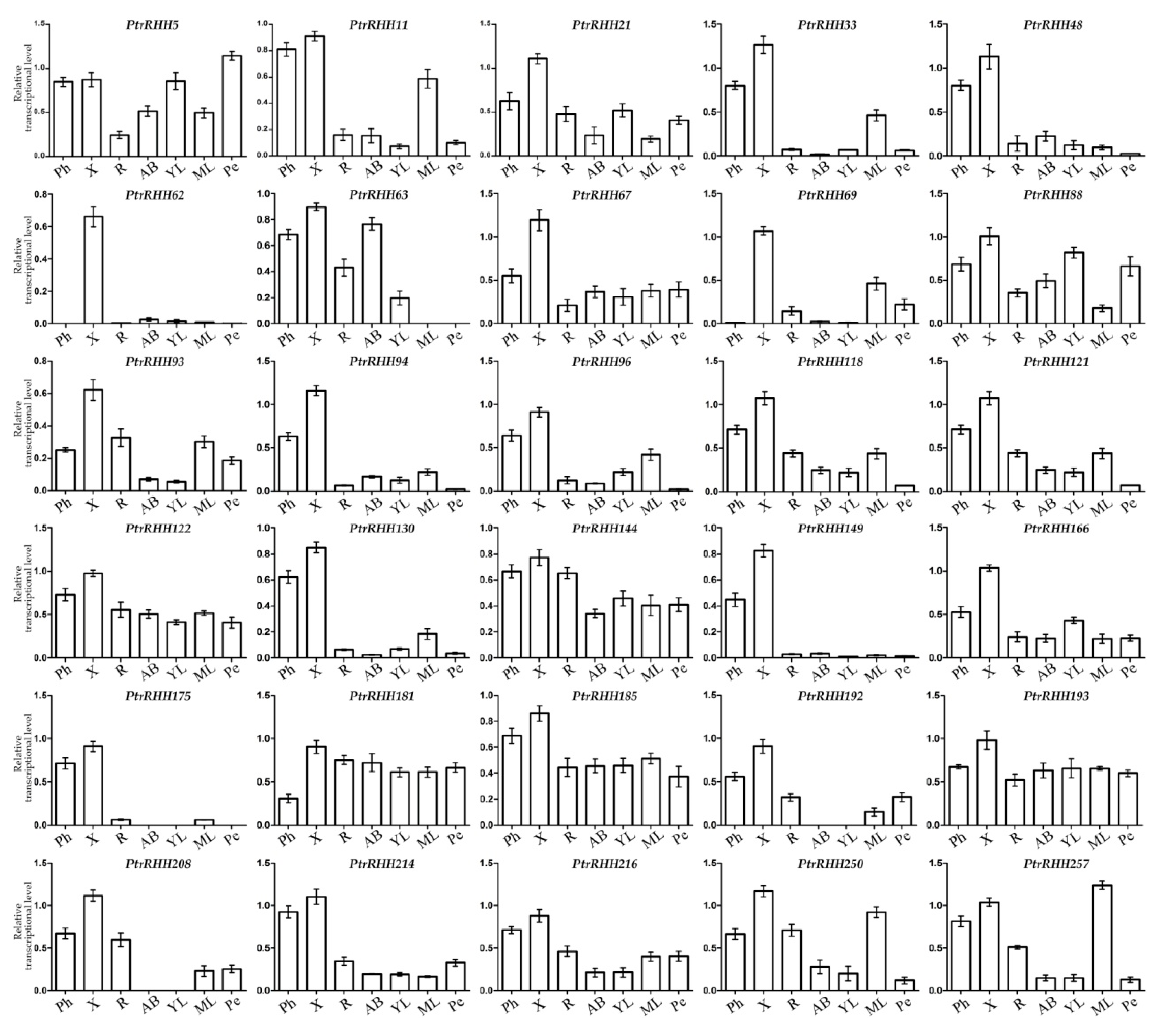
3.3. Identification of the RING-H2 Genes Highly or Preferentially Expressed in Populus Xylem
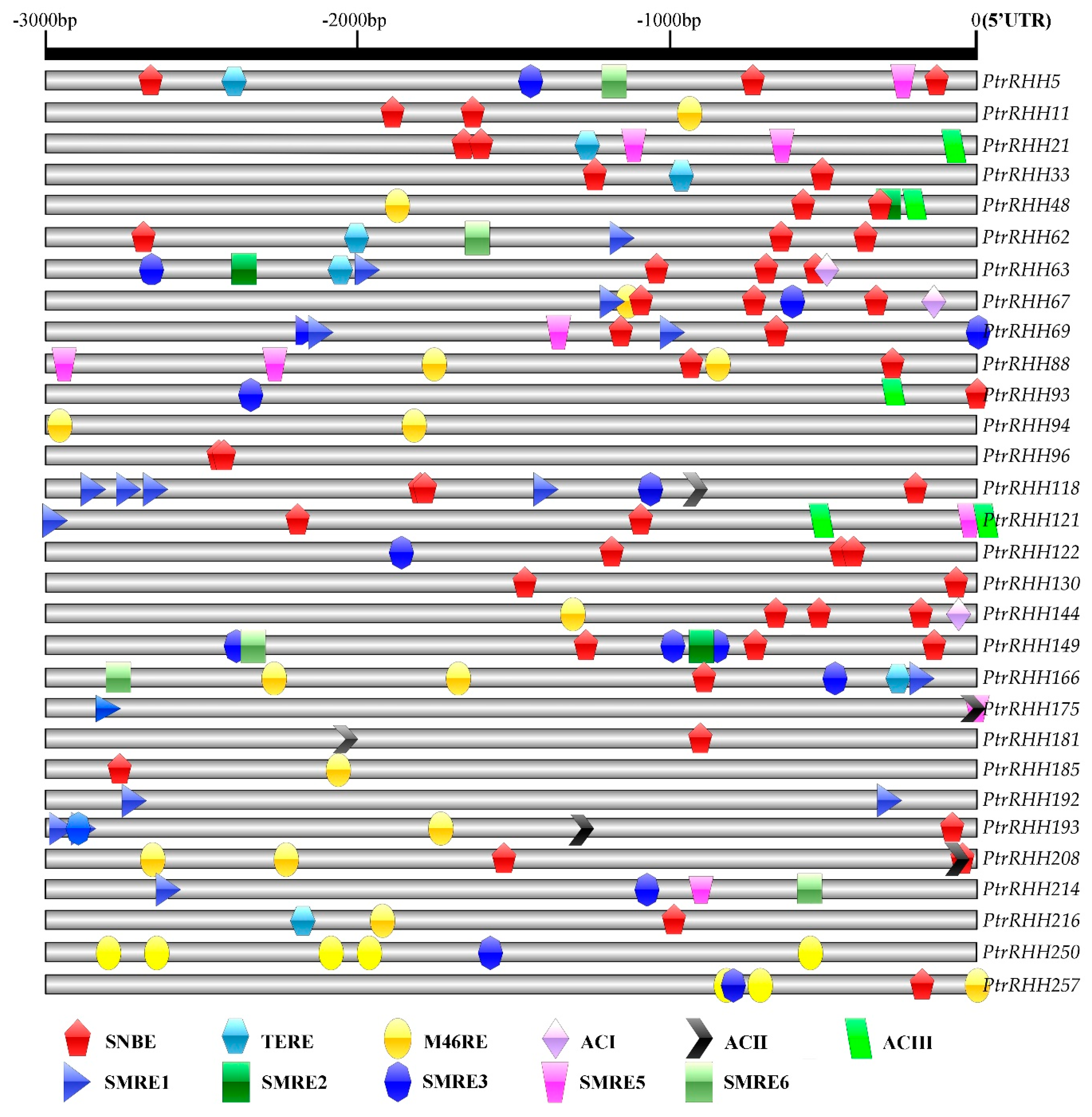
3.4. Analysis of Xylem Development-Related Cis-Elements in the Promoter Regions of Highly or Preferentially Xylem-Expressed RING-H2 Genes
3.5. Promoter: GUS-Based Analysis of PtrRHH94 Related to Wood Formation in Transgenic Populus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guerriero, G.; Sergeant, K.; Hausman, J.-F. Wood biosynthesis and typologies: A molecular rhapsody. Tree Physiol. 2014, 34, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nieminen, K.; Serra, J.A.A.; Helariutta, Y. The formation of wood and its control. Curr. Opin. Plant Biol. 2014, 17, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Baucher, M.; El Jaziri, M.; Vandeputte, O. From primary to secondary growth: Origin and development of the vascular system. J. Exp. Bot. 2007, 58, 3485–3501. [Google Scholar] [CrossRef] [PubMed]
- Déjardin, A.; Laurans, F.; Arnaud, D.; Breton, C.; Pilate, G.; Leplé, J.C. Wood formation in Angiosperms. Comptes Rendus Biol. 2010, 333, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-H.; Zhong, R. Molecular control of wood formation in trees. J. Exp. Bot. 2015, 66, 4119–4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellerowicz, E.J.; Sundberg, B. Wood cell walls: Biosynthesis, developmental dynamics and their implications for wood properties. Curr. Opin. Plant Biol. 2008, 11, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mc Farlane, H.; Döring, A.; Persson, S. The Cell Biology of Cellulose Synthesis. Annu. Rev. Plant Biol. 2014, 65, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Rennie, E.A.; Scheller, H.V. Xylan biosynthesis. Curr. Opin. Biotechnol. 2014, 26, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q. Lignification: Flexibility, Biosynthesis and Regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Demura, T.; Fukuda, H. Transcriptional regulation in wood formation. Trends Plant Sci. 2007, 12, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Groover, A. Transcriptional Regulation of Secondary Growth and Wood Formation. J. Integr. Plant Biol. 2010, 52, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Ye, Z.H. Secondary cell walls: Biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 2015, 56, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Gallois, P.; Brown, D. Tracheary element differentiation. Annu. Rev. Plant Biol. 2007, 58, 407–433. [Google Scholar] [CrossRef] [PubMed]
- Courtois-Moreau, C.L.; Pesquet, E.; Sjödin, A.; Muñiz, L.; Bollhöner, B.; Kaneda, M.; Samuels, L.; Jansson, S.; Tuominen, H. A unique program for cell death in xylem fibers of Populus stem. Plant J. 2009, 58, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Borden, K.L. RING domains: Master builders of molecular scaffolds? J. Mol. Biol. 2000, 295, 1103–1112. [Google Scholar] [CrossRef]
- Kosarev, P.; Mayer, K.F.X.; Hardtke, C.S. Evaluation and classification of RING-finger domains encoded by the Arabidopsis genome. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Freemont, P.S. The RING finger. A novel protein sequence motif related to the zinc finger. Ann. N. Y. Acad. Sci. 1993, 684, 174–192. [Google Scholar] [CrossRef]
- Freemont, P.S. RING for destruction? Curr. Biol. 2000, 10, 84–87. [Google Scholar] [CrossRef]
- Lovering, R.; Hanson, I.M.; Borden, K.L.; Martin, S.; O’Reilly, N.J.; Evan, G.I.; Rahman, D.; Pappin, D.J.; Trowsdale, J.; Freemont, P.S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc. Natl. Acad. Sci. USA 1993, 90, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Nemoto, K.; Seki, M.; Shinozaki, K.; Takeda, H.; Takahashi, H.; Sawasaki, T. Wheat germ-based protein libraries for the functional characterisation of the Arabidopsis E2 ubiquitin conjugating enzymes and the RING-type E3 ubiquitin ligase enzymes. BMC Plant Biol. 2015, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-J.; Lin, W.; Oda, Y.; Cui, K.-M.; Fukuda, H.; He, X.-Q. The proteasome is responsible for caspase-3-like activity during xylem development. Plant J. 2012, 72, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H. Xylogenesis: Initiation, progression, and cell death. Annu. Rev. Plant Biol. 1996, 47, 299–325. [Google Scholar] [CrossRef]
- Li, Y.; Wu, B.; Yu, Y.; Yang, G.; Wu, C.; Zheng, C. Genome-wide analysis of the RING finger gene family in apple. Mol. Genet. Genom. 2011, 286, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.D.; Yim, W.C.; Moon, J.C.; Kim, D.S.; Lee, B.M.; Jang, C.S. A gene family encoding RING finger proteins in rice: Their expansion, expression diversity, and co-expressed genes. Plant Mol. Biol. 2010, 72, 369–380. [Google Scholar] [CrossRef]
- Stone, S.L.; Hauksdóttir, H.; Troy, A.; Herschleb, J.; Kraft, E.; Callis, J. Functional Analysis of the RING-Type Ubiquitin Ligase Family of Arabidopsis. Plant Physiol. 2005, 137, 13–30. [Google Scholar] [CrossRef]
- Cheng, M.C.; Hsieh, E.J.; Chen, J.H.; Chen, H.Y.; Lin, T.P. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012, 158, 363–375. [Google Scholar] [CrossRef]
- Cho, S.K.; Ryu, M.Y.; Seo, D.H.; Kang, B.G.; Kim, W.T. The Arabidopsis RING E3 Ubiquitin Ligase AtAIRP2 Plays Combinatory Roles with AtAIRP1 in Abscisic Acid-Mediated Drought Stress Responses. Plant Physiol. 2011, 157, 2240–2257. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Wu, Y.; Zhang, Y.; Liu, L.; Ning, Y.; Wang, D.; Tong, H.; Chen, S.; Chu, C.; Xie, Q. OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 2011, 76, 145–156. [Google Scholar] [CrossRef]
- Lee, H.K.; Cho, S.K.; Son, O.; Xu, Z.; Hwang, I.; Kim, W.T. Drought Stress-Induced Rma1H1, a RING Membrane-Anchor E3 Ubiquitin Ligase Homolog, Regulates Aquaporin Levels via Ubiquitination in Transgenic Arabidopsis Plants. Plant Cell 2009, 21, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Choi, H.W.; Hwang, I.S.; Hwang, B.K. Role of a novel pathogen-induced pepper C3–H–C4 type RING-finger protein gene, CaRFP1, in disease susceptibility and osmotic stress tolerance. Plant Mol. Biol. 2007, 63, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ryu, M.Y.; Kim, W.T. Suppression of Arabidopsis RING-DUF1117 E3 ubiquitin ligases, AtRDUF1 and AtRDUF2, reduces tolerance to ABA-mediated drought stress. Biochem. Biophys. Res. Commun. 2012, 420, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Choi, J.Y.; Kim, S.J.; Kim, E.Y.; Shin, J.S.; Kim, W.T. Constitutive expression of CaRma1H1, a hot pepper ER-localized RING E3 ubiquitin ligase, increases tolerance to drought and salt stresses in transgenic tomato plants. Plant Cell Rep. 2012, 31, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liu, Q.; Wu, J.; Ding, J. ZmRFP1, the putative ortholog of SDIR1, encodes a RING-H2 E3 ubiquitin ligase and responds to drought stress in an ABA-dependent manner in maize. Gene 2012, 495, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lyzenga, W.J.; Booth, J.K.; Stone, S.L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 2012, 71, 23–34. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ecker, J.R. Plant responses to ethylene gas are mediated by SCF (EBF1/EBF2) dependent proteolysis of EIN3 transcription factor. Cell 2003, 115, 667–677. [Google Scholar] [CrossRef]
- Pan, J.; Fujioka, S.; Peng, J.; Chen, J.; Li, G.; Chen, R. The E3 Ubiquitin Ligase SCFTIR1/AFB and Membrane Sterols Play Key Roles in Auxin Regulation of Endocytosis, Recycling, and Plasma Membrane Accumulation of the Auxin Efflux Transporter PIN2 in Arabidopsis thaliana. Plant Cell 2009, 21, 568–580. [Google Scholar] [CrossRef]
- Christians, M.J.; Gingerich, D.J.; Hansen, M.; Binder, B.M.; Kieber, J.J.; Vierstra, R.D. The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 2009, 57, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.; Pedmale, U.V.; Morrow, J.; Sachdev, S.; Lechner, E.; Tang, X.; Zheng, N.; Hannink, M.; Genschik, P.; Liscum, E. Modulation of Phototropic Responsiveness in Arabidopsis through Ubiquitination of Phototropin 1 by the CUL3-Ring E3 Ubiquitin Ligase CRL3NPH3. Plant Cell 2011, 23, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, G.; Dai, X.; Zhao, Y. NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 18825–18829. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hellmann, H. Plant E3 Ligases: Flexible Enzymes in a Sessile World. Mol. Plant 2013, 6, 1388–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, T.; Mochizuki, S.; Haga, K.; Uehara, Y.; Suzuki, A.; Harada, A.; Wada, T.; Ishiguro, S.; Okada, K. The WAVY GROWTH 3 E3 ligase family controls the gravitropic response in Arabidopsis roots. Plant J. 2012, 70, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.-L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.-P.; et al. Genetic Characterization and Functional Analysis of the GID1 Gibberellin Receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef] [PubMed]
- Baldacci-Cresp, F.; Moussawi, J.; Leplé, J.-C.; Van Acker, R.; Kohler, A.; Candiracci, J.; Twyffels, L.; Spokevicius, A.V.; Bossinger, G.; Laurans, F.; et al. PtaRHE1, a Populus tremula × Populus alba RING-H2 protein of the ATL family, has a regulatory role in secondary phloem fibre development. Plant J. 2015, 82, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, J.; Baldacci-Cresp, F.; El Jaziri, M.; Baucher, M. Does PtaRHE1, a poplar RING-H2 protein, play a role in water conduction through ABA signaling? Plant Signal. Behav. 2014, 9, 27611. [Google Scholar] [CrossRef] [PubMed]
- Mukoko Bopopi, J.; Vandeputte, O.M.; Himanen, K.; Mol, A.; Vaessen, Q.; El Jaziri, M.; Baucher, M. Ectopic expression of PtaRHE1, encoding a poplar RING-H2 protein with E3 ligase activity, alters plant development and induces defence-related responses. J. Exp. Bot. 2010, 61, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Hai, G.H.; Jia, Z.G.; Xu, W.J.; Wang, C.; Cao, S.Q.; Liu, J.W.; Cheng, Y.X. Characterization of the Populus PtrCesA4 promoter in transgenic Populus alba × P. glandulosa. Plant Cell Tiss. Organ. Cult. 2016, 124, 495–505. [Google Scholar] [CrossRef]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam protein families database. Nucleic Acids Res. 2010, 38, 211–222. [Google Scholar] [CrossRef]
- Letunic, I.; Copley, R.R.; Schmidt, S.; Ciccarelli, F.D.; Doerks, T.; Schultz, J.; Ponting, C.P.; Bork, P. SMART 4.0: Towards genomic data integration. Nucleic Acids Res. 2004, 32, D142–D144. [Google Scholar] [CrossRef] [PubMed]
- Chenna, R. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa. Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Yang, Z.-L.; Yang, X.; Liu, Y.-J.; Wang, X.-R.; Zeng, Q.-Y. Extensive Functional Diversification of the Populus Glutathione S-Transferase Supergene Family. Plant Cell 2009, 21, 3749–3766. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.-Y.; Zhu, Q.H.; Chen, X.; Luo, J.C. GSDS: A gene structure display server. Hereditas 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A Toolkit for Illustrating Heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. DOG 1.0: Illustrator of protein domain structures. Cell Res. 2009, 19, 271–273. [Google Scholar] [CrossRef]
- Li, S.; Zhen, C.; Xu, W.; Wang, C.; Cheng, Y. Simple, rapid and efficient transformation of genotype Nisqually-1: A basic tool for the first sequenced model tree. Sci. Rep. 2017, 7, 2638. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Wilkins, O.; Nahal, H.; Foong, J.; Provart, N.J.; Campbell, M.M. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009, 149, 981–993. [Google Scholar] [CrossRef]
- Sundell, D.; Street, N.R.; Kumar, M.; Mellerowicz, E.J.; Kucukoglu, M.; Johnsson, C.; Kumar, V.; Mannapperuma, C.; Delhomme, N.; Nilsson, O.; et al. AspWood: High-spatial-resolution transcriptome profiles reveal uncharacterized modularity of wood formation in Populus tremula. Plant Cell 2017, 29, 1585–1604. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Zhong, R.; Ye, Z.-H. Secondary wall NAC binding element (SNBE), a key cis-acting element required for target gene activation by secondary wall NAC master switches. Plant Signal. Behav. 2011, 6, 1282–1285. [Google Scholar] [CrossRef] [Green Version]
- Pyo, H.; Demura, T.; Fukuda, H. TERE; a novel cis-element responsible for a coordinated expression of genes related to programmed cell death and secondary wall formation during differentiation of tracheary elements. Plant J. 2007, 51, 955–965. [Google Scholar] [CrossRef]
- Ohashi-Ito, K.; Oda, Y.; Fukuda, H. Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 Directly Regulates the Genes That Govern Programmed Cell Death and Secondary Wall Formation during Xylem Differentiation. Plant Cell 2010, 22, 3461–3473. [Google Scholar] [CrossRef]
- Kim, W.-C.; Ko, J.-H.; Han, K.-H. Identification of a cis-acting regulatory motif recognized by MYB46, a master transcriptional regulator of secondary wall biosynthesis. Plant Mol. Biol. 2012, 78, 489–501. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z.H. MYB46 and MYB83 Bind to the SMRE Sites and directly activate a suite of transcription factors and secondary wall biosynthetic genes. Plant Cell Physiol. 2012, 53, 368–380. [Google Scholar] [CrossRef]
- Park, G.-G.; Park, J.-J.; Yoon, J.; Yu, S.-N.; An, G. A RING finger E3 ligase gene, Oryza sativa Delayed Seed Germination 1 (OsDSG1), controls seed germination and stress responses in rice. Plant Mol. Biol. 2010, 74, 467–478. [Google Scholar] [CrossRef]
- Hervé, C.; Lefebvre, B.; Cullimore, J. How many E3 ubiquitin ligase are involved in the regulation of nodulation ? Plant Signal. Behav. 2011, 6, 660–664. [Google Scholar] [CrossRef]
- Liu, Q.G.; Yang, J.L.; Wang, Z.C.; Xu, X.M.; Mao, X.L.; Li, D.D.; Hu, X.Q.; Jin, D.C.; Li, C.H. Genome-wide classification, identification and expression profile of the C3HC4-type RING finger gene family in poplar (Populus trichocarpa). Plant Mol. Biol. Rep. 2015, 33, 1740–1754. [Google Scholar] [CrossRef]
- Kumar, M.; Campbell, L.; Turner, S. Secondary cell walls: Biosynthesis and manipulation. J. Exp. Bot. 2016, 67, 515–531. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, G.; Shen, H.; Cao, S.; Xu, W.; Ma, X.; Cheng, Y. Identification of RING-H2 Gene Candidates Related to Wood Formation in Poplar. Forests 2019, 10, 698. https://doi.org/10.3390/f10080698
Tong G, Shen H, Cao S, Xu W, Ma X, Cheng Y. Identification of RING-H2 Gene Candidates Related to Wood Formation in Poplar. Forests. 2019; 10(8):698. https://doi.org/10.3390/f10080698
Chicago/Turabian StyleTong, Guimin, Hongmei Shen, Shenquan Cao, Wenjing Xu, Xujun Ma, and Yuxiang Cheng. 2019. "Identification of RING-H2 Gene Candidates Related to Wood Formation in Poplar" Forests 10, no. 8: 698. https://doi.org/10.3390/f10080698
APA StyleTong, G., Shen, H., Cao, S., Xu, W., Ma, X., & Cheng, Y. (2019). Identification of RING-H2 Gene Candidates Related to Wood Formation in Poplar. Forests, 10(8), 698. https://doi.org/10.3390/f10080698




