Terrestrial Ecosystem Impacts of Sulfide Mining: Scope of Issues for the Boundary Waters Canoe Area Wilderness, Minnesota, USA
Abstract
1. Introduction
2. Potential Terrestrial Impacts in the Quetico–Superior Ecosystem
2.1. Baseline Impacts on Vegetation in the Primary Footprint
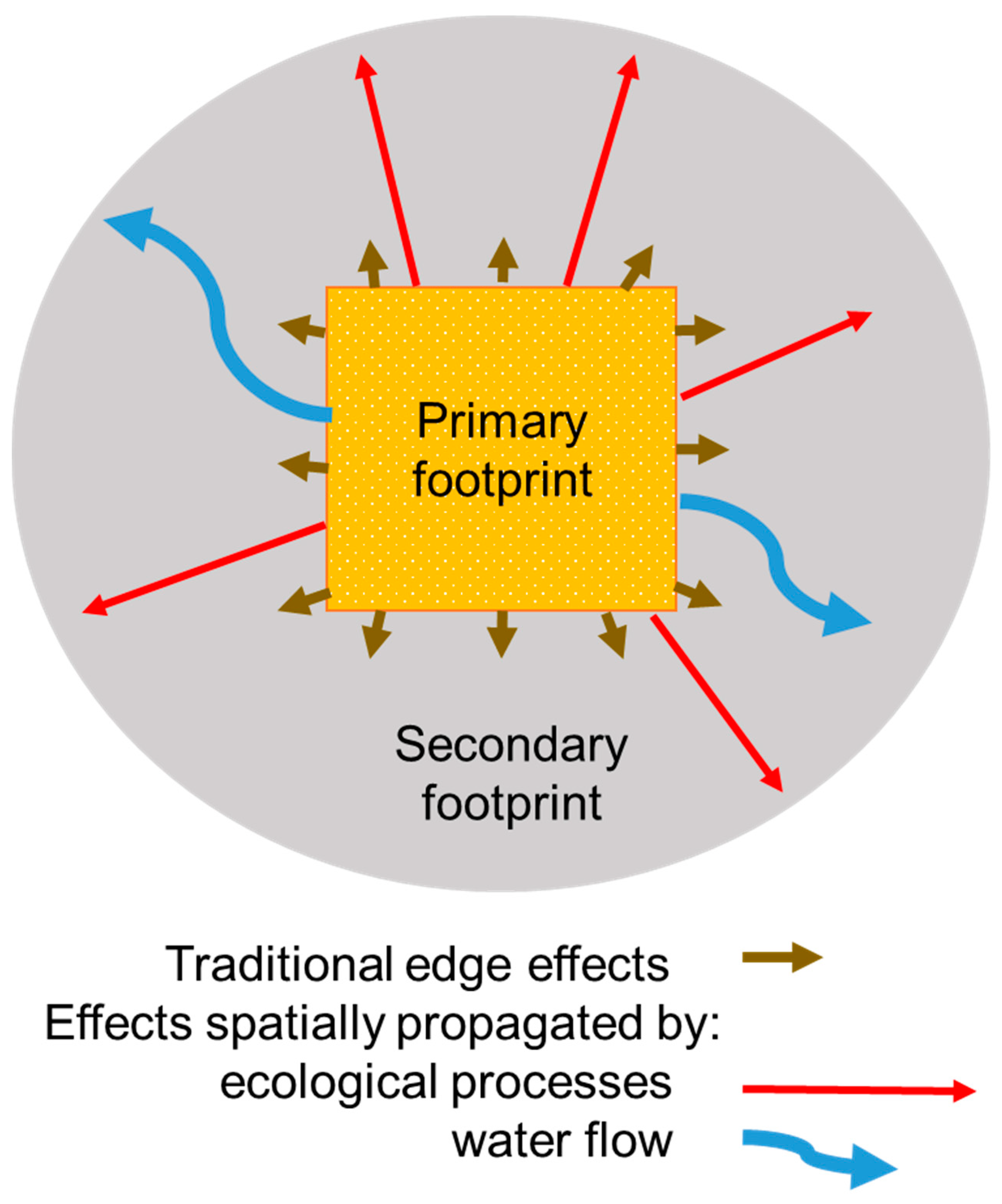
2.2. Fragmentation
2.3. Wildlife
2.4. Rare Species
2.5. Invasive Species
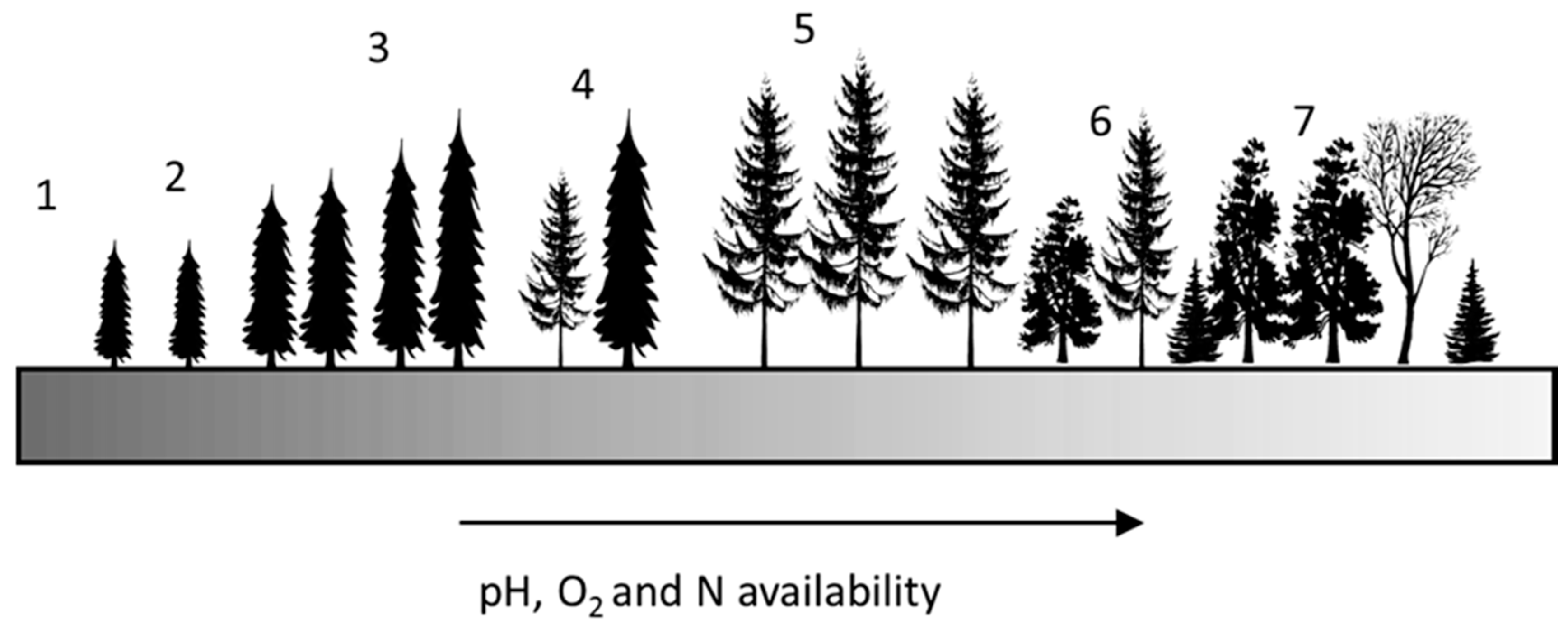
2.6. Soil Disruption and Ecosystem Recovery Time
2.7. Terrestrial-Aquatic Linkages
3. Cumulative Impacts
4. Conclusions and Summary of Wilderness Impacts
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schindler, D.W.; Lee, P.G. Comprehensive conservation planning to protect biodiversity and ecosystem services in Canadian boreal regions under a warming climate. Biol. Conserv. 2010, 143, 1571–1586. [Google Scholar] [CrossRef]
- Snyder, P.K.; Delire, C.; Foley, J.A. Evaluating the influence of different vegetation biomes on the global climate. Clim. Dyn. 2004, 23, 279–302. [Google Scholar] [CrossRef]
- Schmiegelow, F.K.; Machtans, C.S.; Hannon, S.J. Are boreal birds resilient to forest fragmentation? An experimental study of short-term community responses. Ecology 1997, 78, 1914–1932. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Wardle, D.A.; Jonsson, M.; Bansal, S.; Bardgett, R.D.; Gundale, M.J.; Metcalfe, D.B. Linking vegetation change, carbon sequestration and biodiversity: Insights from island ecosystems in a long-term natural experiment. J. Ecol. 2012, 100, 16–30. [Google Scholar] [CrossRef]
- Frelich, L.E. Wildland Fire: Understanding and maintaining an ecological baseline. Curr. For. Rep. 2017, 3, 188–201. [Google Scholar] [CrossRef]
- Frelich, L.E. Boreal Biome, Oxford Bibliographies in Ecology; Gibson, D., Ed.; Oxford University Press: New York, NY, USA, 2017; Available online: http://www.oxfordbibliographies.com/view/document/obo-9780199830060/obo-9780199830060-0085.xml?rskey=uXGPUZ&result=6 (accessed on 25 August 2018).
- Freedman, B.; Hutchinson, T.C. Long-term effects of smelter pollution at Sudbury, Ontario, on forest community composition. Can. J. Bot. 1980, 58, 2123–2140. [Google Scholar] [CrossRef]
- Salemaa, M.; Vanha-Majamaa, I.; Derome, J. Understorey vegetation along a heavy-metal pollution gradient in SW Finland. Environ. Pollut. 2001, 112, 339–350. [Google Scholar] [CrossRef]
- Rayfield, B.; Anand, M.; Laurence, S. Assessing simple versus complex restoration strategies for industrially disturbed forests. Restor. Ecol. 2005, 13, 639–650. [Google Scholar] [CrossRef]
- DeLong, C.; Skousen, J.; Pena-Yewtukhiw, E. Bulk density of rocky soils in forestry reclamation. Soil Sci. Soc. Am. J. 2012, 76, 1810–1815. [Google Scholar] [CrossRef]
- Anawar, H.M.; Canha, N.; Santa-Regina, I.; Freitas, M.C. Adaptation, tolerance, and evolution of plant species in a pyrite mine, in response to contamination level and properties of mine tailings: Sustainable rehabilitation. J. Soils Sediments 2013, 13, 730–741. [Google Scholar] [CrossRef]
- Limpitlaw, D.; Alsum, A.; Neale, D. Calculating ecological footprints for mining companies—an introduction to the methodology and assessment of the benefits. J. S. Afr. Inst. Min. Metall. 2017, 117, 13–18. [Google Scholar] [CrossRef][Green Version]
- Tarolli, P.; Sofia, G. Human topographic signatures and derived geomorphic processes across landscapes. Geomorphology 2016, 255, 140–161. [Google Scholar] [CrossRef]
- Shotyk, W.; Bicalho, B.; Cuss, C.W.; Duke, M.J.M.; Noernberg, T.; Pelletier, R.; Steinnes, E.; Zaccone, C. Dust is the source of “heavy metals” to peat moss (Sphagnum fuscum) in the bogs of the Athabasca bituminous sands region of northern Alberta. Environ. Int. 2016, 92, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Igondova, E.; Pavlickova, K.; Majzlan, O. The ecological impact assessment of a proposed road development (the Slovak approach). Environ. Impact Assess. 2016, 59, 43–54. [Google Scholar] [CrossRef]
- Phillips, J. Climate change and surface mining: A review of environment-human interactions & their spatial dynamics. Appl. Geogr. 2016, 74, 95–108. [Google Scholar]
- Heinselman, M.L. The Boundary Waters Wilderness Ecosystem; University of Minnesota Press: Minneapolis, MN, USA, 1996. [Google Scholar]
- Shrestha, R.K.; Lal, R. Changes in physical and chemical properties of soil after surface mining and reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Kałucka, I.; Horodecki, P.; Oleksyn, J. Aboveground biomass allocation and accumulation in a chronosequence of young Pinus sylvestris stands growing on a lignite mine spoil heap. Dendrobiology 2014, 72, 139–150. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Brabcová, V.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Vaněk, D.; Frouz, J.; Šantrůčková, H.; Baldrian, P. Litter decomposition along a primary post-mining chronosequence. Biol. Fertil. Soils 2014, 50, 827–837. [Google Scholar] [CrossRef]
- Jaakko Pöyry Consulting, Inc. Generic Environmental Impact Statement Study on Timber Harvesting and Forest Management in Minnesota; Jaakko Pöyry Consulting, Inc.: Tarrytown, NY, USA, 1994. [Google Scholar]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Frelich, L.E.; Reich, P.B. Will environmental changes reinforce the impact of global warming on the prairie-forest border of central North America? Front. Ecol. Environ. 2010, 8, 371–378. [Google Scholar] [CrossRef]
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Temperate tree expansion into adjacent boreal forest patches facilitated by warmer temperatures. Ecography 2014, 37, 152–161. [Google Scholar] [CrossRef]
- Jaakko Pöyry Consulting, Inc. Forest Wildlife: A Technical Paper for a Generic Environmental Impact Statement on Timber Harvesting and Forest Management in Minnesota; Jaakko Pöyry Consulting, Inc.: Tarrytown, NY, USA, 1992. [Google Scholar]
- Jaakko Pöyry Consulting, Inc. Biodiversity: A Technical Paper for a Generic Environmental Impact Statement on Timber Harvesting and Forest Management in Minnesota; Jaakko Pöyry Consulting, Inc.: Tarrytown, NY, USA, 1992. [Google Scholar]
- Minnesota Department of Natural Resources. Field Guide to the Native Plant Communities of Minnesota: The Laurentian Mixed Forest Province; Ecological Land Classification Program, Minnesota County Biological Survey, and Natural Heritage and Nongame Research Program: St. Paul, MN, USA, 2003. [Google Scholar]
- Frelich, L.E.; Reich, P.B. Perspectives on development of definitions and values related to old-growth forests. Environ. Rev. 2003, 11, S9–S22. [Google Scholar] [CrossRef]
- Anand, M.; Leithead, M.; Silva, L.C.R.; Wagner, C.; Ashiq, M.W.; Cecile, J.; Drobyshev, I.; Bergeron, Y.; Das, A.; Bulger, C. The scientific value of the largest remaining old-growth red pine forests in North America. Biodivers. Conserv. 2013, 22, 1847–1861. [Google Scholar] [CrossRef]
- Frelich, L.E. Old forest in the Lake States today and before European settlement. Nat. Areas J. 1995, 15, 157–167. [Google Scholar]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Robinson, C.; Duinker, P.N.; Beazley, K.F. A conceptual framework for understanding, assessing, and mitigating ecological effects of forest roads. Environ. Rev. 2010, 18, 61–86. [Google Scholar] [CrossRef]
- Cignac, L.D.; Dale, M.R.T. Effects of size, shape and edge on vegetation in remnants of the upland boreal mixed-wood forest in agro-environments of Alberta, Canada. Can. J. Bot. 2007, 85, 273–284. [Google Scholar]
- Hawbaker, T.J.; Radeloff, V.C. Roads and landscape pattern in northern Wisconsin based on a comparison of four road data sources. Conserv. Biol. 2004, 18, 1233–1244. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Groffman, P.M.; Likens, G.E.; Belt, K.T.; Stack, W.P.; Kelly, V.R.; Band, L.E.; Fisher, G.T. Increased salinization of fresh water in the northeastern United States. Proc. Natl. Acad. Sci. USA 2004, 102, 13517–13520. [Google Scholar] [CrossRef]
- Jull, L.G. Winter Salt Injury and Salt Tolerant Landscape Plants; University of Wisconsin Cooperative Extension: Madison, WI, USA, 2009. [Google Scholar]
- Bryson, G.M.; Barker, A.V. Sodium accumulation in soils and plants along Massachusetts roadsides. Commun. Soil Sci. Plant Anal. 2002, 33, 67–78. [Google Scholar] [CrossRef]
- Sasaki, K.; Lesbarrères, D.; Watson, G.; Litzgus, J. Mining-caused changes to habitat structure affect amphibian population ecology more than metal pollution. Ecol. Appl. 2015, 25, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgenburg, S.L.; Hobson, K.A.; Bayne, E.M.; Kopper, N. Estimated avian nest loss associated with oil and gas exploration and extraction in the Western Canadian Sedimentary Basin. Avian Conserv. Ecol. 2013, 8, 9. [Google Scholar] [CrossRef]
- Kociolek, A.V.; Clevenger, A.P.; Clair, C.C.S.; Proppe, D.S. Effects of road networks on bird populations. Conserv. Biol. 2011, 25, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Schorr, M.S.; Dyson, M.C.; Nelson, C.H.; Van Horn, G.S.; Collins, D.E.; Richards, S.M. Effects of stream acidification on lotic salamander assemblages in a coal-mined watershed in the Cumberland Plateau. J. Freshw. Ecol. 2013, 28, 339–353. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 114–137. [Google Scholar] [CrossRef]
- Lankester, M. Understanding the impact of meningeal worm, Paralaphostrongylus tenuis, on moose populations. Alces 2010, 46, 53–70. [Google Scholar]
- Mech, L.D.; Fritts, S.H.; Radde, G.L.; Paul, W.J. Wolf distribution and road density in Minnesota. Wildl. Soc. Bull. 1988, 16, 85–87. [Google Scholar]
- Frelich, L.E.; Peterson, R.O.; Dovciak, M.; Reich, P.B.; Vucetich, J.A.; Eisenhauer, N. Trophic cascades, invasive species, and body-size hierarchies interactively modulate climate change responses of ecotonal temperate-boreal forest. Philos. Trans. R. Soc. B 2012, 367, 2955–2961. [Google Scholar] [CrossRef]
- Callan, R.; Nebbelink, N.P.; Rooney, T.P.; Wiedenhoeft, J.E.; Wydeven, A.P. Recolonizing wolves trigger a trophic cascade in Wisconsin (USA). J. Ecol. 2013, 101, 837–845. [Google Scholar] [CrossRef]
- Burdett, C.L.; Moen, R.A.; Niemi, G.J.; Mech, L.D. Defining space and movements of Canada lynx with global positioning system telemetry. J. Mammal. 2007, 88, 457–467. [Google Scholar] [CrossRef]
- Bayne, E.M.; Boutin, S.; Moses, R.A. Ecological factors influencing the spatial pattern of Canada lynx relative to its southern range edge in Alberta, Canada. Can. J. Zool. 2008, 86, 1189–1197. [Google Scholar] [CrossRef]
- Minnesota Department of Natural Resources. NorthMet Mining Project and Land Exchange EIS—Record of Decision; Minnesota Department of Natural Resources: St. Paul, MN, USA, 2016.
- Moilanen, A.; Smith, A.T.; Hanski, I. Long-term dynamics in a metapopulation of the American pika. Am. Nat. 1998, 152, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Schlaghamersky, J.; Eisenhauer, N.; Frelich, L.E. Earthworm invasion alters enchytraied community composition and individual biomass in northern hardwood forests of North America. Appl. Soil Ecol. 2014, 83, 159–169. [Google Scholar] [CrossRef]
- Sverdrup-Thygeson, A.; Gustafsson, L.; Kouki, J. Spatial and temporal scales relevant for conservation of dead-wood associated species: Current status and perspectives. Biodivers. Conserv. 2014, 23, 513–535. [Google Scholar] [CrossRef]
- Petersen, H.; Luxton, M. A comparative analysis of sol fauna populations and their role in decomposition processes. Oikos 1982, 39, 288–388. [Google Scholar] [CrossRef]
- Sanderson, L.A.; McLaughlin, J.A.; Antunes, P.M. The last great forest: A review of the status of invasive species in the North American boreal forest. Forestry 2012, 85, 329–340. [Google Scholar] [CrossRef]
- Hansen, M.J.; Clevenger, A.P. The influence of disturbance and habitat on the presence of non-native plant species along transport corridors. Biol. Conserv. 2005, 125, 249–259. [Google Scholar] [CrossRef]
- Cameron, E.K.; Bayne, E.M.; Clapperton, M.J. Human-facilitated invasion of exotic earthworms into northern boreal forests. Ecoscience 2007, 14, 482–490. [Google Scholar] [CrossRef]
- Cameron, E.K.; Bayne, E.M. Road age and its importance in earthworm invasion of northern boreal forests. J. Appl. Ecol. 2009, 46, 28–36. [Google Scholar] [CrossRef]
- Knight, K.S.; Reich, P.B. Opposite relationships between invisibility and native species richness at patch versus landscape scales. Oikos 2005, 109, 81–88. [Google Scholar] [CrossRef]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- Knight, T.M.; Dunn, J.L.; Smith, L.A.; Davis, J.; Kalisz, S. Deer facilitate invasive plant success in a Pennsylvania forest. Nat. Areas J. 2009, 29, 110–116. [Google Scholar] [CrossRef]
- Roth, A.M.; Whitfeld, T.J.S.; Lodge, A.G.; Eisenhauer, N.; Frelich, L.E.; Reich, P.B. Invasive earthworms interact with abiotic conditions to influence invasion of common buckthorn (Rhamnus cathartica). Oecolgia 2015, 178, 219–230. [Google Scholar] [CrossRef] [PubMed]
- USDA Forest Service, Superior National Forest. Boundary Waters Canoe Area Wilderness Trip Planning Guide. 2011. Available online: ww.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5259796.pdf (accessed on 20 August 2018).
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.F.; Callaham, M.A., Jr.; Drake, J.M.; Huang, C.-Y.; James, S.W.; Snyder, B.A.; Zhang, W. Pandora’s box contained bait: The global problem of introduced earthworms. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 593–613. [Google Scholar] [CrossRef]
- Larson, E.; Frelich, L.E.; Reich, P.B.; Hale, C.M.; Kipfmueller, K. Tree rings detect earthworm invasions and their effects in northern hardwood forests. Biol. Invasions 2010, 12, 1053–1066. [Google Scholar] [CrossRef]
- Craven, D.; Thakur, M.P.; Cameron, E.K.; Frelich, L.E.; Beausejour, R.; Blair, R.B.; Blossey, B.; Burtis, J.; Choi, A.; Dávalos, A.; et al. The unseen invaders: Introduced earthworms as drivers of change in plant communities in North American forests (a meta analysis). Glob. Chang. Biol. 2017, 23, 1065–1074. [Google Scholar] [CrossRef]
- Mudrak, O.; Uteseny, K.; Frouz, J. Earthworms drive succession of both plant and Collembola communities in post-mining sites. Appl. Soil. Ecol. 2012, 62, 170–177. [Google Scholar] [CrossRef]
- Tardif, A.; Rodrigue-Morin, M.; Gagnon, V.; Shipley, B.; Roy, S.; Bellenger, J.-P. The relative importance of abiotic conditions and subsequent land use on the boreal primary succession of acidogenic mine tailings. Ecol. Eng. 2019, 217, 66–74. [Google Scholar] [CrossRef]
- Asensio, V.; Guala, S.D.; Vega, F.A.; Covelo, E.F. A soil quality index for reclaimed mine soils. Environ. Toxicol. Chem. 2013, 32, 2240–2248. [Google Scholar] [CrossRef]
- Frouz, J.; Jilkova, V.; Cajthami, T.; Pizl, V.; Tajovsky, K.; Hanel, L.; Buresova, A.; Simackova, H.; Kolarikova, K.; Franklin, J.; et al. Soil biota in post-mining sites along a climatic gradient in the USA: Simple communities in shortgrass prairie recover faster than complex communities in tallgrass prairie and forest. Soil. Biol. Biochem. 2013, 67, 212–225. [Google Scholar] [CrossRef]
- Bagatto, G.; Shorthouse, J.D. Biotic and abiotic characteristics of ecosystems on acid mettaliferous mine tailings near Sudbury, Ontario. Can. J. Bot. 1999, 77, 410–425. [Google Scholar]
- Bussinow, M.; Sarapathka, B.; Dlapa, P. Chemical degradation of forest soil as a result of polymetallic ore mining activities. Pol. J. Environ. Stud. 2012, 21, 1551–1561. [Google Scholar]
- Conestoga-Rovers and Associates. Expert Report Review of Air Permit Application and Draft Air Permit; Marquette Michigan, Kennecott Eagle Minerals, Eagle Project; Conestoga-Rovers and Associates: Waterloo, ON, Canada, 2007. [Google Scholar]
- Johnson, D.; MacDonald, W.; Hendershot, W.; Hale, B. Metals in northern forest ecosystems: Role of vegetation sequestration and cycling, and implications for ecological risk assessment. Hum. Ecol. Risk Assess. 2003, 9, 749–766. [Google Scholar] [CrossRef]
- Witt, E.L.; Kolka, R.K.; Nater, E.A.; Wickman, T.R. Forest fire effects on mercury deposition in the boreal forest. Environ. Sci. Technol. 2009, 43, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.B. Effects of air pollution on forests: A critical review. JAPCA 1985, 35, 516–534. [Google Scholar]
- Lorenc-Plucinska, G.; Walentynowicz, M.; Niewiadomska, A. Capabilities of alders (Alnus incana and A. glutinosa) to grow in metal-contaminated soil. Ecol. Eng. 2013, 58, 214–227. [Google Scholar] [CrossRef]
- Leyval, C.; Turnau, K.; Haselwandter, K. Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological and applied aspects. Mycorrhiza 1997, 7, 139–153. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Meharg, A.A. Infleunces of anthropogenic pollution on mycorrhizal fungal communities. Environ. Pollut. 1999, 106, 169–182. [Google Scholar] [CrossRef]
- Nadeau, M.B.; Laur, J.; Khasa, D.P. Mycorrhizae and rhizobacteria Precambrian rocky gold mine tailings: I. Mine-adapted symbionts promote white spruce health and growth. Front. Plant Sci. 2018, 9, 1267. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Gap disturbance, ground microtopography, and the regeneration dynamics of boreal coniferous forests in Finland. Ann. Zool. Fenn. 1994, 31, 35–51. [Google Scholar]
- Šamonil, P.; Schaetzl, R.J.; Valtera, M.; Goliáš, V.; Baldrian, P.; Vašičová, I.; Adam, D.; Janik, D.; Hort, L. Crossdating of disturbances by tree uprooting: Can treethrow microtopography persist for 6000 years? For. Ecol. Manag. 2013, 307, 123–135. [Google Scholar] [CrossRef]
- Brown, R.L.; Naeth, M.A. Woody debris amendment enhances reclamation after oil sands mining in Alberta, Canada. Restor. Ecol. 2014, 22, 40–48. [Google Scholar] [CrossRef]
- Norland, M.R.; Veith, D.L. Revegetation of coarse taconite iron ore tailing using municipal solid waste compost. J. Hazard. Mater. 1995, 41, 123–134. [Google Scholar] [CrossRef]
- Felleson, D.A. Iron ore and taconite mine reclamation and revegetation practices on the Mesabi Range in northeastern Minnesota. Restor. Reclam. Rev. 1999, 5, 5. [Google Scholar]
- Dhar, A.; Comeau, P.G.; Karst, J.; Pinno, B.D.; Chang, S.X.; Naeth, A.M.; Vassov, R.; Bampfylde, C. Community development following reclamation of oil sands mine sites in the boreal forest: A review. Environ. Rev. 2018, 26, 286–298. [Google Scholar] [CrossRef]
- Myers, T. Acid mine drainage risks—A modeling approach to siting mine facilities in northern Minnesota USA. J. Hydrol. 2016, 533, 277–290. [Google Scholar] [CrossRef]
- Berkowitz, J.F.; Summers, E.A.; Noble, C.V.; White, J.R.; DeLaune, R.D. Investigation of biogechemical functional proxies in headwater streams across a range of channel catchment alterations. Environ. Manag. 2014, 53, 534–548. [Google Scholar] [CrossRef]
- Stoeckeler, J.H. Drainage along swamp forest roads: Lessons from northern Europe. J. For. 1965, 63, 772–776. [Google Scholar]
- Nordstrom, D.K. Acid rock drainage and climate change. J. Geochem. Explor. 2009, 100, 97–104. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Kanae, S.; Seneviratne, S.I.; Handmer, J.; Nicholls, N.; Peduzzi, P.; Mechler, R.; Bouwer, L.M.; Arnell, N.; Mach, K.; et al. Flood risk and climate change: Global and regional perspectives. Hydrol. Sci. J. 2014, 59, 1–28. [Google Scholar] [CrossRef]
- Ford, M.S. A 10,000-yr history of natural ecosystem acidification. Ecol. Monogr. 1990, 60, 57–89. [Google Scholar] [CrossRef]
- Peltzer, D.A.; Wardle, D.A.; Allison, V.A.; Baisden, W.T.; Bardgett, R.D.; Chadwick, O.A.; Condron, L.M.; Parfitt, R.L.; Porder, S.; Richardson, S.J.; et al. Understanding ecosystem retrogression. Ecol. Monogr. 2010, 80, 509–529. [Google Scholar] [CrossRef]
- Sarica, J.; Amyot, M.; Hare, L.; Doyon, M.R.; Stanfield, L.W. Salmon-derived mercury and nutrients in a Lake Ontario spawning stream. Limnol. Oceanogr. 2004, 49, 891–899. [Google Scholar] [CrossRef]
- Mogren, C.L.; Walton, W.E.; Parker, D.R.; Trumble, J.T. Trophic transfer of arsenic from an aquatic insect to terrestrial insect predators. PLoS ONE 2013, 8, e67817. [Google Scholar] [CrossRef]
- Gabriel, M.; Kolka, R.; Wickman, T.; Woodruff, L.; Nater, E. Latent effect of soil organic matter oxidation on mercury cycling within a southern boreal ecosystem. J. Environ. Qual. 2012, 41, 495–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitchell, C.P.J.; Kolka, R.K.; Fraver, S. Singular and combined effects of blowdown, salvage logging, and wildfire on forest floor and soil mercury pools. Environ. Sci. Technol. 2012, 46, 7963–7970. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.W.; et al. Changing disturbance regimes, ecological memory and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Sapling growth responses to warmer temperatures ‘cooled’ by browse pressure. Glob. Chang. Biol. 2012, 18, 3455–3463. [Google Scholar] [CrossRef]
- McGraw, A.M.; Moen, R.; Wilson, G.; Edwards, A.; Peterson, R.; Cornicelli, L.; Schrage, M.; Frelich, L.E.; Lenarz, M.; Becker, D. An Advisory committee process to plan moose management in Minnesota. Alces 2010, 46, 189–200. [Google Scholar]

| Impact | Foot-Print | Explora-tion | Mining |
|---|---|---|---|
| Baseline vegetation impacts | |||
| Loss of forest acreage by type | 1 | × | × |
| Forest composition change by forest type | 1,2 | × | × |
| Loss of non-forest vegetation by type | 1 | × | × |
| Non-forest vegetation change by vegetation type | 1,2 | × | × |
| Loss of old-growth forest remnants, acres by forest type | 1 | × | × |
| Loss of old forest (80–120 years), acres by forest type | 1 | × | × |
| Loss of primary forest remnants, acres by forest type | 1 | × | × |
| Fragmentation (additional effects listed below under wildlife and rare species) | |||
| Edge to area ratio due to roads, transmission lines, parking, tailings, buildings, residential and commercial development | 1,2 | × | × |
| Environment effects in remaining forest within primary footprint | 1 | × | × |
| Changes in native edge versus interior plant and tree species | 1,2 | × | × |
| Road salt effects on trees and water | 1,2 | × | |
| Water flow effects on vegetation | 1,2 | × | × |
| Wildlife, all impacts are per species for the relevant species group | |||
| Area-sensitive mammals, marten, fisher (fragmentation effect) | 1 | × | × |
| Area-sensitive birds, warblers, etc. (fragmentation effect) | 1 | × | × |
| Loss of nesting habitat by forest type and bird species | 1 | × | × |
| Loss of habitat acres by wildlife species and vegetation/forest type | 1 | × | × |
| Effects on species sensitive to aquatic and aerial chemistry (amphibians) | 1,2 | × | × |
| Effects on wolves and trophic cascades (fragmentation effect) | 1,2 | × | |
| Effects on deer and deer-moose relationships (fragmentation effect) | 1,2 | × | |
| Roadkill effects (fragmentation effect) | 1,2 | × | |
| Road salt effects (fragmentation effect) | 1,2 | × | |
| Corridor disruption for mobile but non-flying species | 1,2 | × | |
| Loss of critical stopovers for migrating species | 1,2 | × | |
| Disruption of landscape pattern of vegetation/habitat | 1 | × | |
| Noise, light, and vibration effects | 1,2 | × | × |
| Rare species | |||
| Direct habitat loss per species | 1 | × | × |
| Impacts on local populations and regional stability of metapopulations per species (plants, wildlife, soil dwelling, and saproxylic species) | 1,2 | × | × |
| Invasive species | |||
| Transport by equipment and soil movement per species | 1 | × | × |
| Potential response to fragmentation per species | 1,2 | × | × |
| Soils and productivity | |||
| Acidification by water and air movement | 1,2 | × | |
| Movement and effects of heavy metals in the soil | 1,2 | × | |
| Loss of soil complexity | 1 | × | × |
| Terrestrial-aquatic linkages | |||
| Accelerated ecosystem aging | 1,2 | × | |
| Water chemistry effects on landscape arrangement of marshes, sedge meadows, peatlands, bogs, shrub carrs and wetland forests | 1,2 | × | |
| Changes in water flow effects on landscape arrangement of wetland vegetation types | 1,2 | × | |
| Heavy metal movement across aquatic-terrestrial boundaries | 1,2 | × | |
| Cumulative impacts | |||
| Spatial cascade of fragmentation effects including deer, moose, forest type, invasive species interactions | 2 | × | × |
| Sensitivity of future trajectory of forest and wildlife impacts to number of exploration sites and total size of primary footprint | 1,2 | × | × |
| Synergy among climate change, invasive species and mining impacts potential to overcome ecosystem resilience | 1,2 | × | × |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frelich, L.E. Terrestrial Ecosystem Impacts of Sulfide Mining: Scope of Issues for the Boundary Waters Canoe Area Wilderness, Minnesota, USA. Forests 2019, 10, 747. https://doi.org/10.3390/f10090747
Frelich LE. Terrestrial Ecosystem Impacts of Sulfide Mining: Scope of Issues for the Boundary Waters Canoe Area Wilderness, Minnesota, USA. Forests. 2019; 10(9):747. https://doi.org/10.3390/f10090747
Chicago/Turabian StyleFrelich, Lee E. 2019. "Terrestrial Ecosystem Impacts of Sulfide Mining: Scope of Issues for the Boundary Waters Canoe Area Wilderness, Minnesota, USA" Forests 10, no. 9: 747. https://doi.org/10.3390/f10090747
APA StyleFrelich, L. E. (2019). Terrestrial Ecosystem Impacts of Sulfide Mining: Scope of Issues for the Boundary Waters Canoe Area Wilderness, Minnesota, USA. Forests, 10(9), 747. https://doi.org/10.3390/f10090747




