The Impact of Moth Migration on Apparent Fecundity Overwhelms Mating Disruption as a Method to Manage Spruce Budworm Populations
Abstract
:1. Introduction
2. Materials and Methods
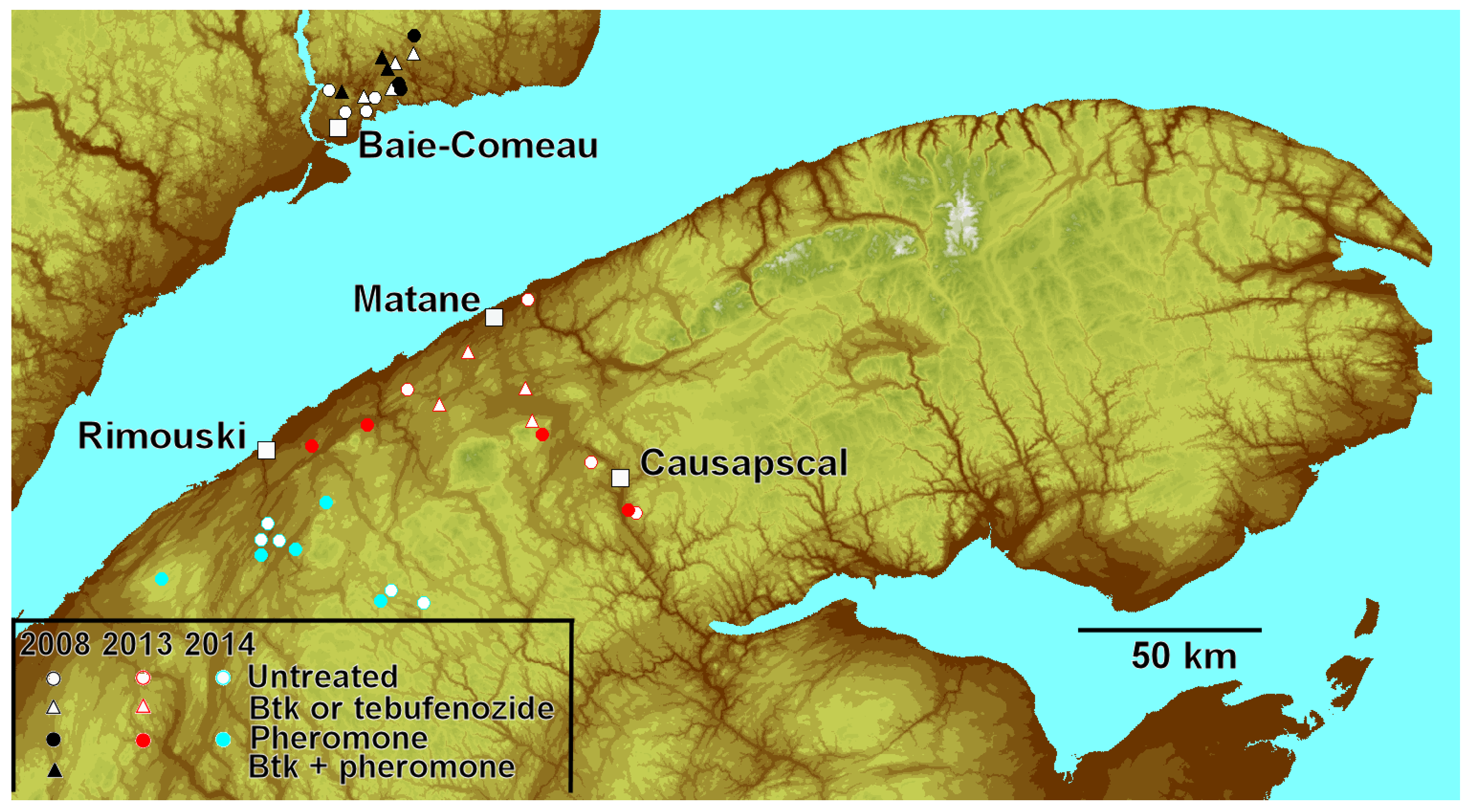
2.1. Sites
2.2. Treatments
2.3. Pheromone Traps
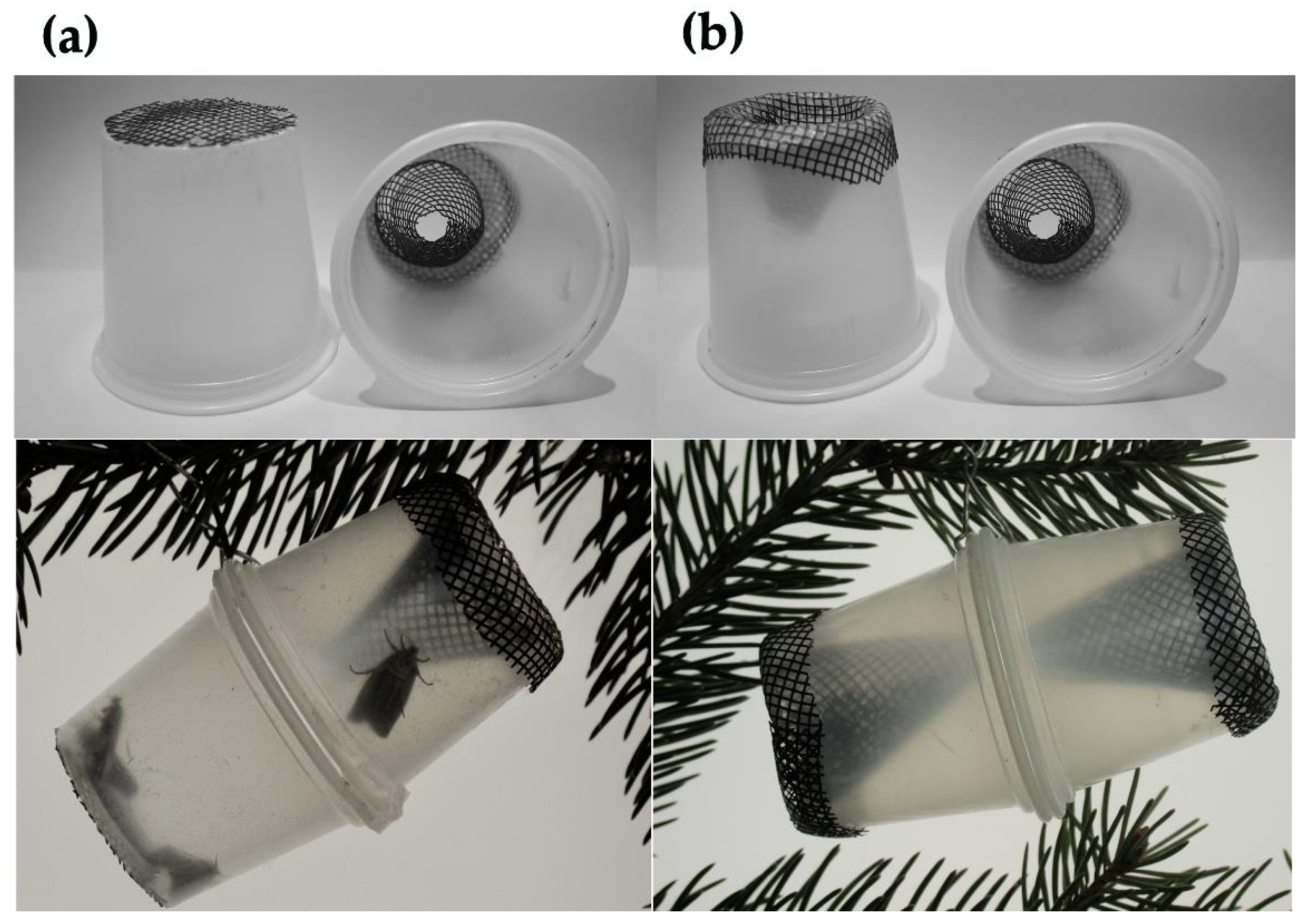
2.4. Mating Success
2.5. Foliage Sampling
2.6. Analysis
3. Results
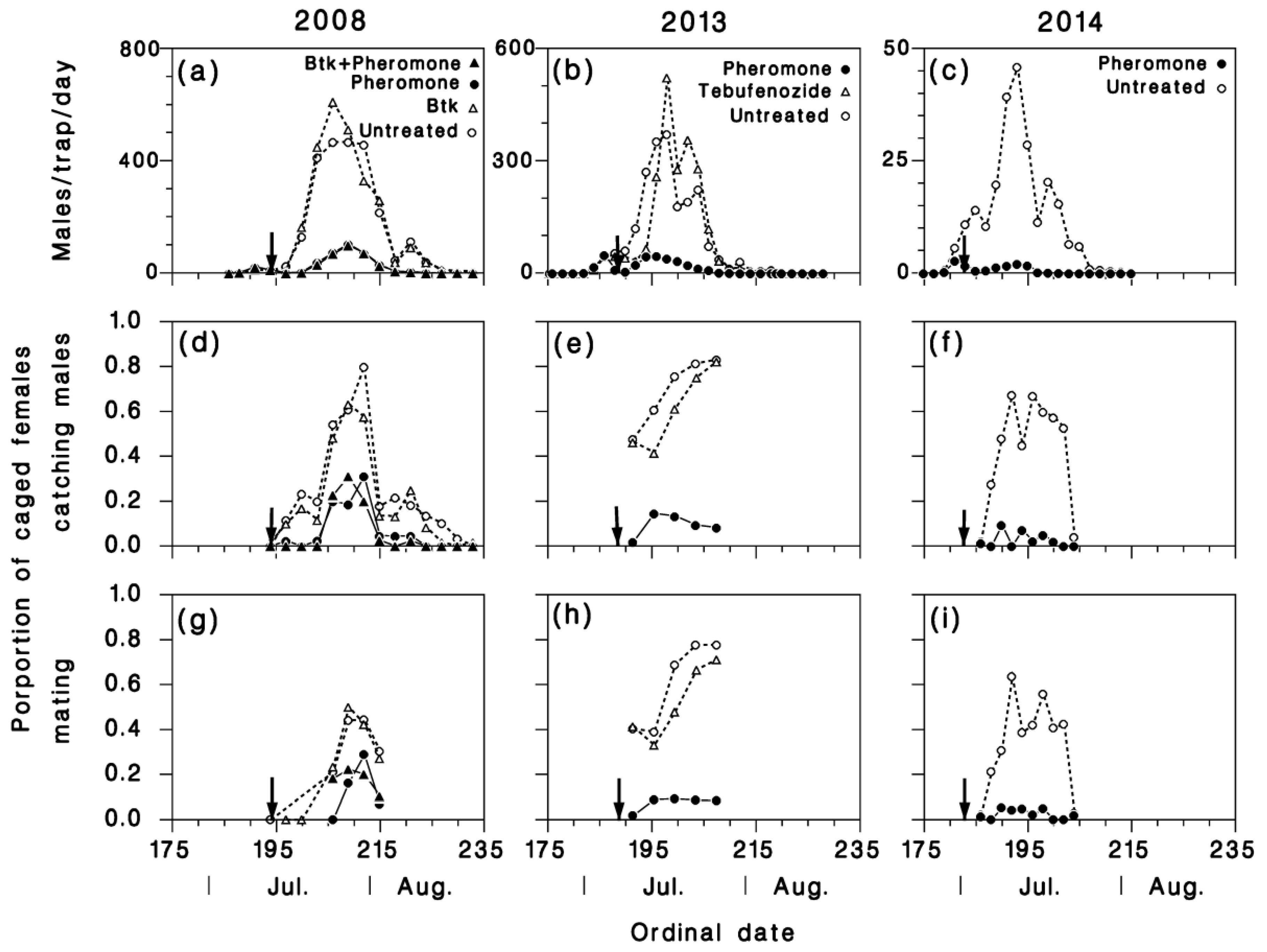
3.1. Mating Success of Caged and Tethered Females
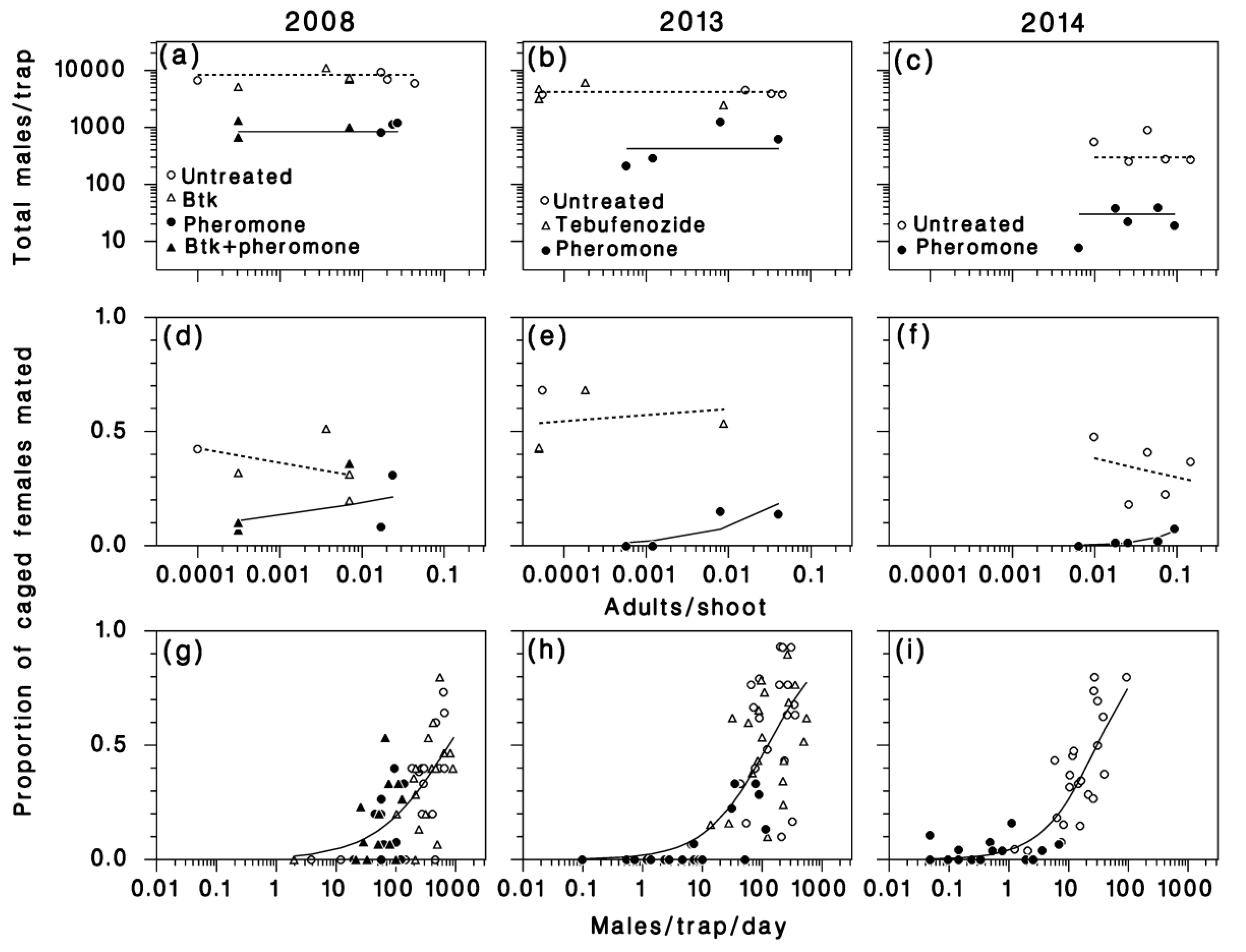
3.2. Pheromone Traps
3.3. Mating Success
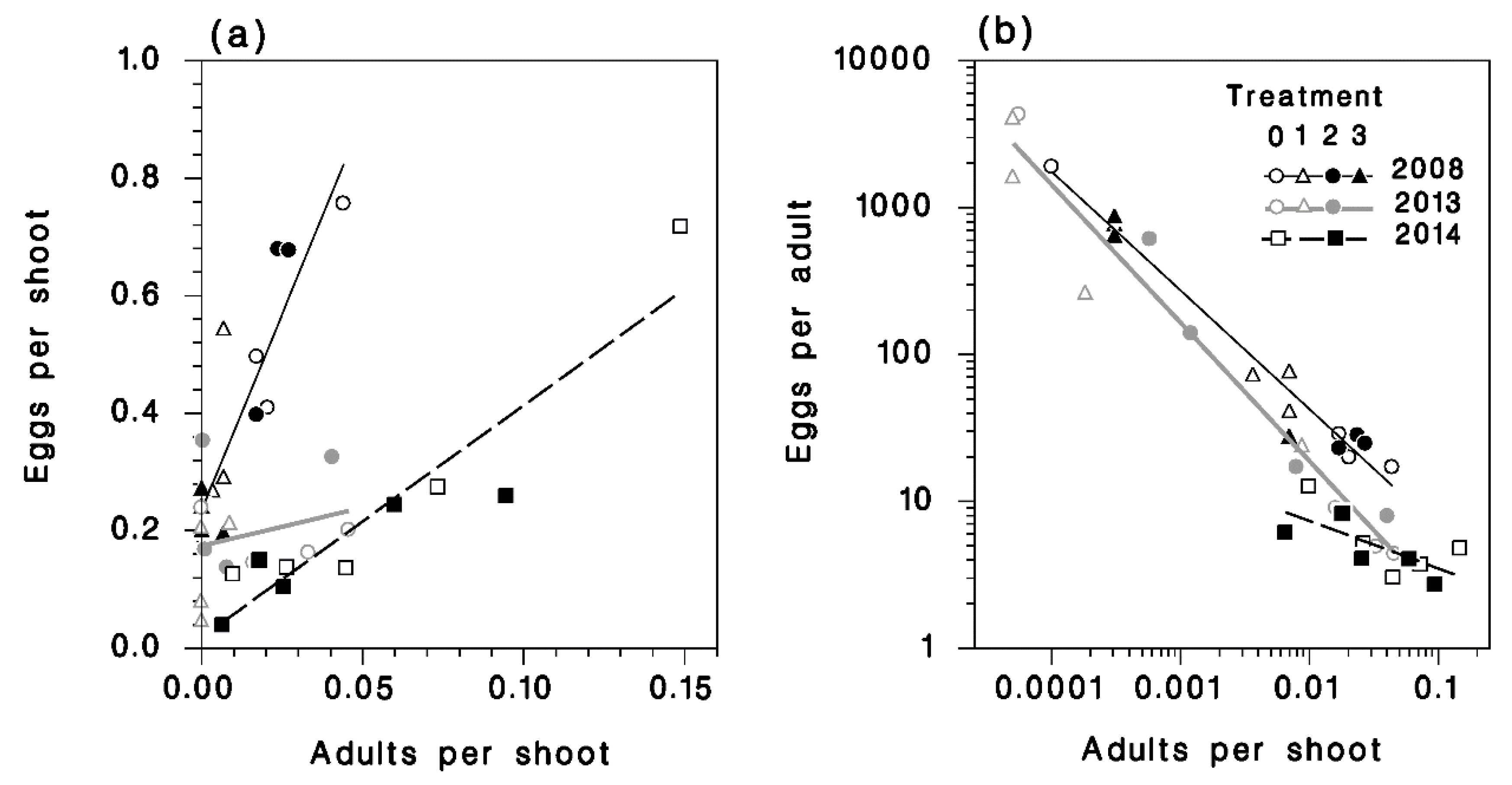
3.4. Egg Density
3.5. Apparent Fecundity
3.6. L2 Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blais, J.R. Effects of the destruction of the current year’s foliage of balsam fir on the fecundity and habits of flight of the spruce budworm. Can. Entomol. 1953, 85, 446–448. [Google Scholar] [CrossRef]
- Sanders, C.J. Biology of North American spruce budworms. In Tortricid Pests, Their Biology, Natural Enemies and Control; van der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier Science Publishers BV: Amsterdam, The Netherlands, 1991; pp. 579–620. [Google Scholar]
- Royama, T. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef]
- Jardon, Y.; Morin, H.; Dutilleul, P. Périodicité et synchronisme des épidémies de la tordeuse des bourgeons de l’épinette au Québec. Can. J. For. Res. 2003, 33, 1947–1961. [Google Scholar] [CrossRef]
- Rhainds, M.; Kettela, E.G.; Silk, P.J. Thirty-five years of pheromone-based mating disruption studies with Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 2012, 144, 379–395. [Google Scholar] [CrossRef]
- Byers, J.A. Simulation of mating disruption and mass trapping with competitive attraction and camouflage. Environ. Entomol. 2007, 36, 1328–1338. [Google Scholar] [CrossRef]
- Shorey, H.H. Manipulation of insect pests of agricultural crops. In Chemical Control of Insect Behaviour: Theory and Application; Shorey, H.H., McKelvey, J.J., Jr., Eds.; Wiley: New York, NY, USA, 1977; pp. 353–367. [Google Scholar]
- Bartell, R.J. Mechanisms of communication disruption by pheromones in control of Lepidoptera: A review. Physiol. Entomol. 1982, 7, 353–364. [Google Scholar] [CrossRef]
- Cardé, R.T. Principles of mating disruption. In Behavior Modifying Chemicals for Pest Management: Applications of Pheromones and Other Attractants; Ridgway, R.L., Silverstein, R.M., Eds.; Marcel Dekker: New York, NY, USA, 1990; pp. 47–71. [Google Scholar]
- Valeur, P.G.; Löfstedt, C. Behaviour of male oriental fruit moth, Grapholita molesta, in overlapping sex pheromone plumes in a wind tunnel. Entomol. Exp. Appl. 1996, 79, 51–59. [Google Scholar] [CrossRef]
- Cardé, R.T.; Staten, R.T.; Mafra-Neto, A. Behaviour of pink bollworm males near high-dose, point sources of pheromone in Þeld wind tunnels: Insights into mechanisms of mating disruption. Entomol. Exp. Appl. 1998, 89, 35–46. [Google Scholar] [CrossRef]
- Evenden, M.L.; Judd, G.J.R.; Borden, J.H. Investigations of mechanisms of pheromone communication disruption of Choristoneura rosaceana (Harris) in a wind tunnel. J. Insect Behav. 2000, 13, 499–510. [Google Scholar] [CrossRef]
- Gut, L.J.; Stelinski, L.L.; Thompson, D.R.; Miller, J.R. Behaviour-modifying chemicals: Prospects and constraints in IPM. In Integrated Pest Management: Potential, Constraints, and Challenges; Koul, O., Dhaliwal, G.S., Cuperus, G.W., Eds.; CABI Publishing: Cambridge, MA, USA, 2004; pp. 73–121. [Google Scholar]
- Miller, J.R.; Gut, L.J.; de Lame, F.M.; Stelinski, L.L. Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone (part 1): Theory. J. Chem. Ecol. 2006, 32, 2089–2114. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J.; de Lame, F.M.; Stelinski, L.L. Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone (part 2): Case studies. J. Chem. Ecol. 2006, 32, 2115–2143. [Google Scholar] [CrossRef]
- Daterman, G.E.; Sower, L.L.; Sartwell, C. Challenges in the use of pheromones for managing western forest Lepidoptera. In Insect Pheromone Technology: Chemistry and Applications; Leonhardt, B., Beroza, M., Eds.; American Chemical Society: Washington, DC, USA, 1982; pp. 243–254. [Google Scholar]
- Mani, E.; Schwaller, R. Results of 12 years experience to control codling moth, Cydia pomonella L. by mating disruption. IOBC/WPRS Bull. 1992, 15, 76–80. [Google Scholar]
- Stelinski, L.L.; Gut, L.J.; Pierzchala, A.V.; Miller, J.R. Field observations quantifying attraction of four tortricid moths to high-dosage pheromone dispensers in untreated and pheromone-treated orchards. Entomol. Exp. Appl. 2004, 113, 187–196. [Google Scholar] [CrossRef]
- Bartell, R.J.; Roelofs, W.L. Inhibition of sexual response in males of the moth Argyrotaenia velutinana by brief exposures to synthetic pheromone or its geometric isomer. J. Insect Physiol. 1973, 19, 655–661. [Google Scholar] [CrossRef]
- Kuenen, L.P.S.; Baker, T.C. Habituation versus sensory adaptation as the cause of reduced attraction following pulsed and constant sex pheromone pre-exposure in Trichoplusia ni. J. Insect Physiol. 1981, 27, 721–726. [Google Scholar] [CrossRef]
- Figueredo, A.J.; Baker, T.C. Reduction of the response to sex pheromone in the oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae) following successive heromonal exposures. J. Insect Behav. 1992, 5, 347–362. [Google Scholar] [CrossRef]
- Rumbo, E.R.; Vickers, R.A. Prolonged adaptation as possible mating disruption mechanism in oriental fruit moth, Cydia (Grapholita) molesta. J. Chem. Ecol. 1997, 23, 445–457. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Miller, J.R.; Gut, L.J. Presence of long-lasting peripheral adaptation in the oblique-banded leafroller, Choristoneura rosaceana and absence of such adaptation in redbanded leafroller, Argyrotaenia velutinana. J. Chem. Ecol. 2003, 29, 405–423. [Google Scholar] [CrossRef]
- Judd, G.J.R.; Gardiner, M.G.T.; Delury, N.C.; Karg, G. Reduced antennal sensitivity, behavioural response, and attraction of male codling moths, Cydia pomonella, to their pheromone (E,E)-8,10-dodecadien-1-ol following various pre-exposure regimes. Entomol. Exp. Appl. 2005, 114, 65–78. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Matching technology with mechanism. Environ. Entomol. 2005, 44, 427–453. [Google Scholar] [CrossRef]
- Shorey, H.H.; Gaston, L.K.; Saario, C.K. Sex pheromones of noctuid moths. XIV. Feasibility of behavioral control by disrupting pheromone communication in cabbage loopers. J. Econ. Entomol. 1967, 60, 1541–1545. [Google Scholar] [CrossRef]
- Cardé, R.T.; Minks, A.K. Control of moth pests by mating disruption: Successes and constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Thomson, D.; Brunner, J.; Gut, L.; Judd, G.; Knight, A. Ten years implementing codling moth mating disruption in the orchards of Washington and British Columbia: Starting right and managing for success. IOBC/WPRS Bull. 2001, 24, 23–30. [Google Scholar]
- Van Frankeyhuyzen, K.; Régnière, J. Multiple effects of tebufenozide on the survival and performance of the spruce budworm (Lepidoptera: Tortricidae). Can. Entomol. 2017, 149, 227–240. [Google Scholar] [CrossRef]
- McMorran, A. A synthetic diet for the spruce budworm, Chroristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. Entomol. 1965, 97, 58–62. [Google Scholar] [CrossRef]
- Régnière, J.; Delisle, J.; Pureswaran, D.; Trudel, R. Mate-finding Allee effect in spruce budworm population dynamics. Entomol. Exp. Appl. 2012, 146, 112–122. [Google Scholar] [CrossRef]
- Delisle, J. A Tool for Evaluating Mating Disruption in SBW: CFS Forges Ahead; Canadian Forest Service: Quebec, QC, Canada, 2008; p. 43. [Google Scholar]
- Sanders, C.J. A Summary of Current Techniques Used for Sampling Spruce Budworm Populations and Estimating Defoliation in Eastern Canada; Canadian Forest Service: Quebec, QC, Canada, 1980. [Google Scholar]
- Régnière, J.; Nealis, V.G. Moth dispersal, egg recruitment and spruce budworms: Measurement and interpretation. Forests 2019, 10, 706. [Google Scholar] [CrossRef]
- Anderson, T.W.; Darling, D.A. Asymptotic theory of certain “goodness-of-fit” criteria based on stochastic processes. Ann. Math. Stat. 1952, 23, 193–212. [Google Scholar] [CrossRef]
- Régnière, J.; Cooke, B.; Béchard, A.; Dupont, A.; Therrien, P. Dynamics and management of rising outbreak spruce budworm populations. Forests 2019, 10, 748. [Google Scholar] [CrossRef]
- Leonhardt, B.A.; Mastro, V.C.; Leonard, D.S.; McLan, W.; Reardon, R.C.; Thorpe, K.W. Control of low-density gypsy moth (Lepidoptera: Lymantriidae) populations by mating disruption with pheromone. J. Chem. Ecol. 1996, 22, 1255–1272. [Google Scholar] [CrossRef]
- Stellinski, L.L.; Miller, J.R.; Ledebuhr, R.; Siegert, P.; Gut, L.J. Season-long mating disruption of Grapholita molesta (Lepidoptera: Tortricidae) by one machine application of pheromone in wax drops (SPLT-OFM). J. Pest Sci. 2007, 80, 109–117. [Google Scholar] [CrossRef]
- Kipp, L.R.; Lonergan, G.C.; Bell, W.J. Male periodicity and the timing of mating in the spruce budworm (Lepidoptera; Tortricidae): Influences of population density and temperature. Env. Entomol. 1995, 24, 1150–1159. [Google Scholar] [CrossRef]
- Greenbank, D.O.; Schaefer, G.W.; Rainey, R.C. Spruce budworm (Lepidoptera: Tortricidae) moth flight and dispersal: New understanding from canopy observations, radar and aircraft. Mem. Entomol. Soc. Can. 1980, 110, 1–49. [Google Scholar] [CrossRef]
- Miller, C.A.; Greenbank, D.O.; Kettela, E.G. Estimated egg deposition by invading spruce budworm moths (Lepidoptera: Tortricidae). Can. Entomol. 1978, 110, 609–615. [Google Scholar] [CrossRef]
- Moreau, G.; Bauce, E. Lethal and sublethal effects of single and double applications of Bacillus thuringiensis variety kurstaki on spruce budworm (Lepidoptera: Tortricidae) larvae. J. Econ Entomol. 2003, 96, 280–286. [Google Scholar] [CrossRef]
- Dallaire, R.; Labrecque, A.; Marcotte, M.; Bauce, É.; Delisle, J. The sublethal effects of tebufenozide on the precopulatory and copulatory activites of Choristoneura fumiferana and C. rosaceana. Ent. Exp. Appl. 2004, 112, 169–181. [Google Scholar] [CrossRef]
- MacLean, D.; Amirault, P.; Amos-Binks, L.; Cerleton, D.; Hennigar, C.; Johns, R.; Régnière, J. Positive results of an early intervention strategy to suppress a spruce budworm outbreak after five years of trials. Forests 2019, 10, 448. [Google Scholar] [CrossRef]

| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Year, Y | 2 | 12.8851 | 6.44253 | 146.48 | < 0.001 |
| Pheromone, T | 1 | 8.4613 | 8.46130 | 192.38 | < 0.001 |
| Error | 32 | 1.4074 | 0.04398 | ||
| Lack-of-Fit | 2 | 0.2451 | 0.12256 | 3.16 | 0.057 |
| Pure Error | 30 | 1.1623 | 0.03874 | ||
| Total | 35 | 24.8012 |
| Source | DF | Adj. Mean | Chi-Square | p-Value |
|---|---|---|---|---|
| Regression | 11 | 90.2094 | 992.30 | < 0.001 |
| Log(A) | 1 | 6.4617 | 6.46 | 0.011 |
| Year, Y | 2 | 28.2868 | 56.57 | < 0.001 |
| Pheromone, T | 1 | 2.0262 | 2.03 | 0.155 |
| Log(A) × Y | 2 | 7.1197 | 14.24 | 0.001 |
| Log(A) × T | 1 | 10.4317 | 10.43 | 0.001 |
| Y × T | 2 | 0.9070 | 1.81 | 0.404 |
| Log(A) × Y × T | 2 | 5.6419 | 11.28 | 0.004 |
| Error | 24 | 7.8267 | ||
| Total | 35 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Adults, A | 1 | 0.42619 | 0.426188 | 54.08 | < 0.001 |
| Year, Y | 2 | 0.12260 | 0.061300 | 7.78 | 0.002 |
| A × Y | 2 | 0.21352 | 0.106761 | 13.55 | < 0.001 |
| Error | 30 | 0.23641 | 0.007880 | ||
| Lack-of-Fit | 27 | 0.19422 | 0.007193 | 0.51 | 0.855 |
| Pure Error | 3 | 0.04219 | 0.014063 | ||
| Total | 35 | 1.26226 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Adult, Log(A) | 1 | 5.7092 | 5.70918 | 173.78 | < 0.001 |
| Year, Y | 2 | 0.4931 | 0.24654 | 7.50 | 0.002 |
| Log(A) × Y | 2 | 0.6170 | 0.30852 | 9.39 | 0.001 |
| Error | 30 | 0.9856 | 0.03285 | ||
| Lack-of-Fit | 24 | 0.7913 | 0.03297 | 1.02 | 0.541 |
| Pure Error | 6 | 0.1942 | 0.03237 | ||
| Total | 35 | 32.9479 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Adult, A | 1 | 1.19871 | 1.19871 | 80.93 | < 0.001 |
| Error | 22 | 0.32587 | 0.01481 | ||
| Lack-of-Fit | 17 | 0.25502 | 0.01500 | 1.06 | 0.522 |
| Pure Error | 5 | 0.07085 | 0.01417 | ||
| Total | 23 | 1.52458 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régnière, J.; Delisle, J.; Dupont, A.; Trudel, R. The Impact of Moth Migration on Apparent Fecundity Overwhelms Mating Disruption as a Method to Manage Spruce Budworm Populations. Forests 2019, 10, 775. https://doi.org/10.3390/f10090775
Régnière J, Delisle J, Dupont A, Trudel R. The Impact of Moth Migration on Apparent Fecundity Overwhelms Mating Disruption as a Method to Manage Spruce Budworm Populations. Forests. 2019; 10(9):775. https://doi.org/10.3390/f10090775
Chicago/Turabian StyleRégnière, Jacques, Johanne Delisle, Alain Dupont, and Richard Trudel. 2019. "The Impact of Moth Migration on Apparent Fecundity Overwhelms Mating Disruption as a Method to Manage Spruce Budworm Populations" Forests 10, no. 9: 775. https://doi.org/10.3390/f10090775
APA StyleRégnière, J., Delisle, J., Dupont, A., & Trudel, R. (2019). The Impact of Moth Migration on Apparent Fecundity Overwhelms Mating Disruption as a Method to Manage Spruce Budworm Populations. Forests, 10(9), 775. https://doi.org/10.3390/f10090775





