Artificial Top Soil Drought Hardly Affects Water Use of Picea abies and Larix decidua Saplings at the Treeline in the Austrian Alps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Environmental Sap Flow Density Measurements and Stem Radial Increment
2.3. Data Analysis
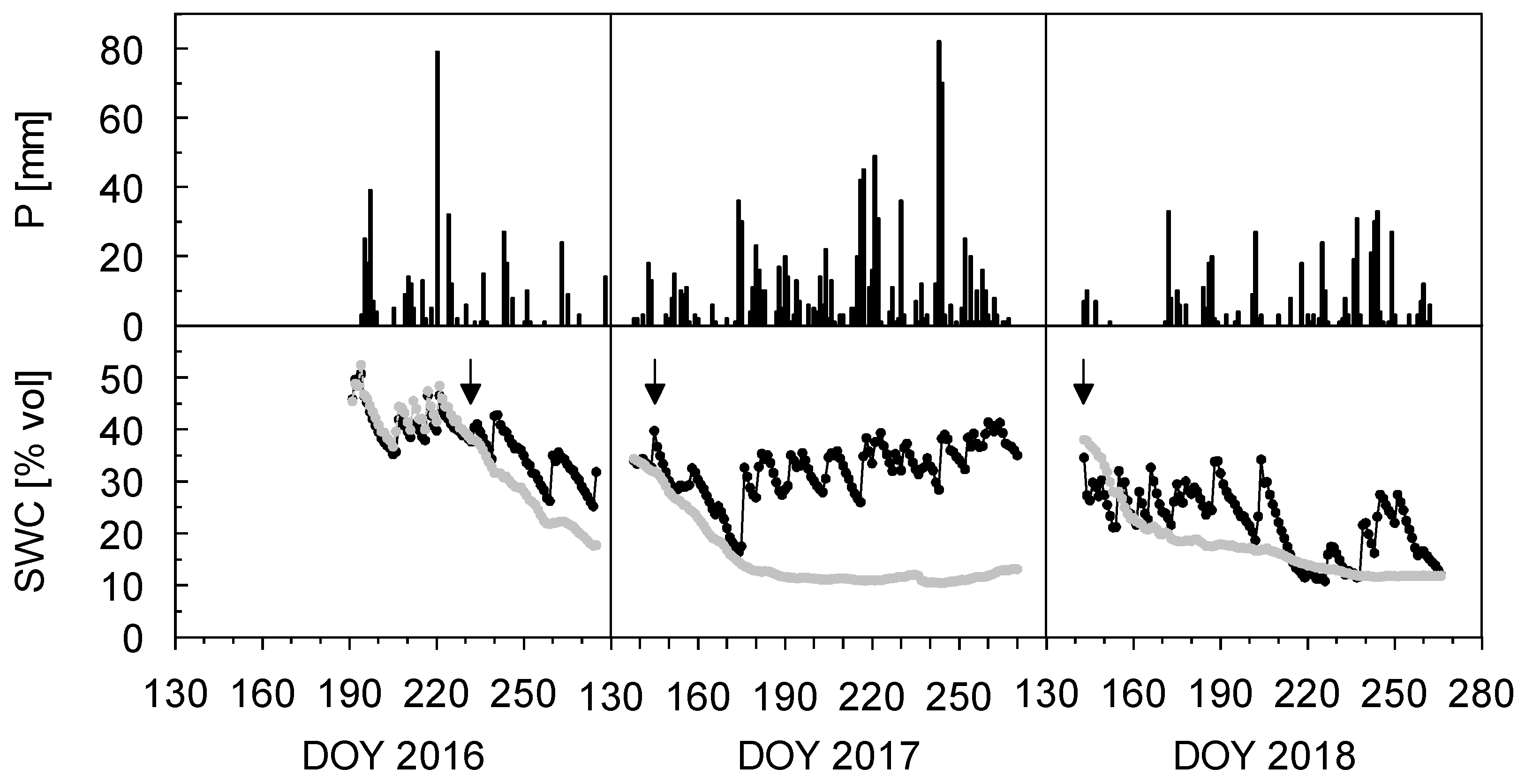
2.4. Environmental Conditions
3. Results
3.1. Diameter Growth
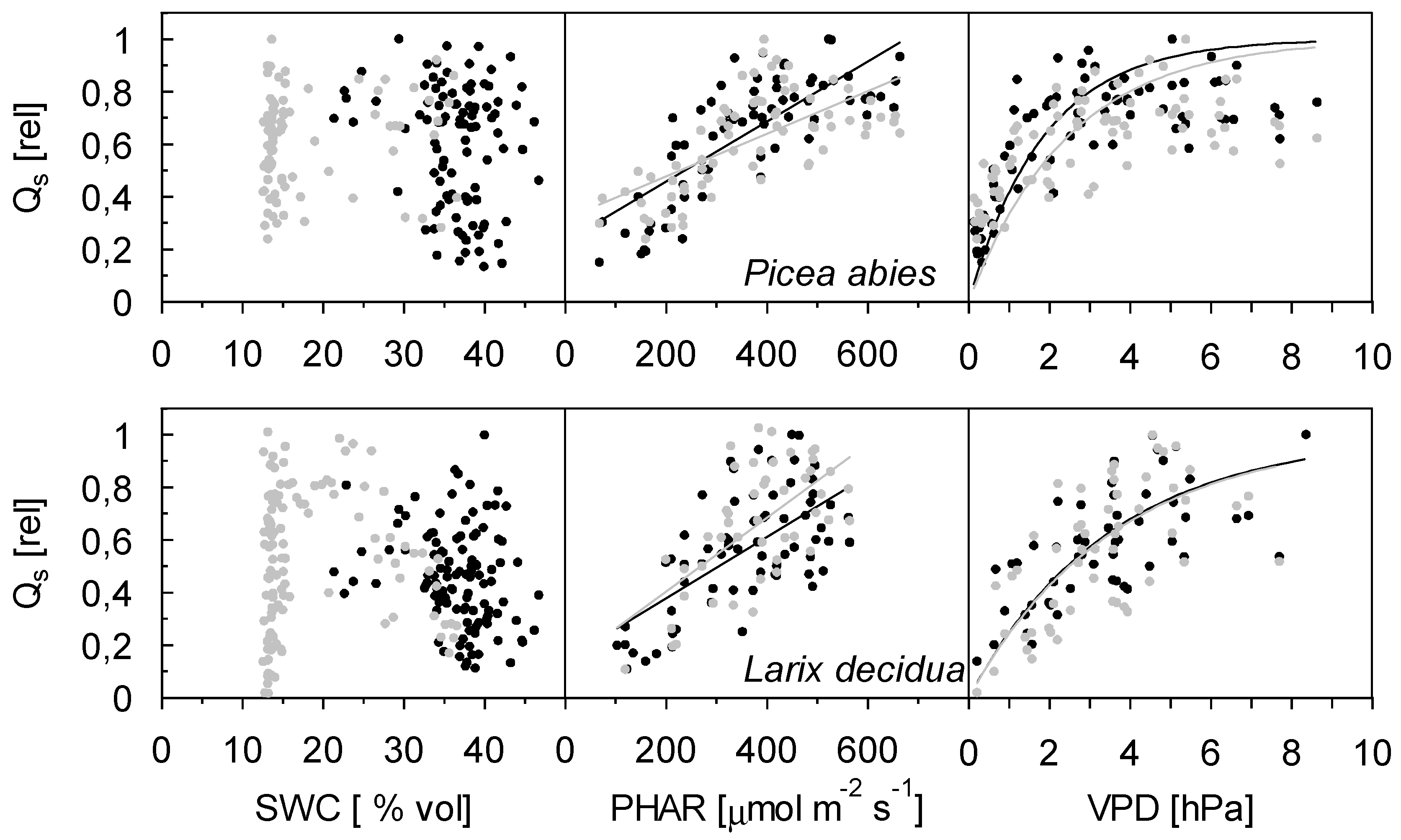
3.2. Sap Flow Density and Influencing Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holtmeier, F.-K.; Broll, G.E. Treeline advance - driving processes and adverse factors. Landsc. Online 2007, 1, 1–33. [Google Scholar] [CrossRef]
- Wieser, G.; Matyssek, R.; Luzian, R.; Zwerger, P.; Pindur, P.; Oberhuber, W.; Gruber, A. Effects of atmospheric and climate change at the timberline of the Central European Alps. Ann. For. Sci. 2009, 66, 402. [Google Scholar] [CrossRef] [PubMed]
- Böhm, R.; Auer, I.; Brunetti, M.; Maugeri, M.; Nanni, T.; Schöner, W. Regional temperature variability in the European Alps: 1760–1998 from homogenized instrumental time series. Int. J. Clim. 2001, 21, 1779–1801. [Google Scholar] [CrossRef]
- Rebetez, M.; Reinhard, M. Monthly air temperature trends in Switzerland 1901–2000 and 1975–2004. Theor. Appl. Clim. 2007, 91, 27–34. [Google Scholar] [CrossRef]
- Ciccarelli, N.; Von Hardenberg, J.; Provenzale, A.; Ronchi, C.; Vargiu, A.; Pelosini, R. Climate variability in north-western Italy during the second half of the 20th century. Glob. Planet. Chang. 2008, 63, 185–195. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change. In The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Wieser, G.; Grams, T.E.; Matysssek, R.; Oberhuber, W.; Gruber, A. Soil warming increased whole-tree water use of Pinus cembra at the treeline in the Central Tyrolean Alps. Tree Physiol. 2015, 35, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieser, G.; Oberhuber, W.; Waldboth, B.; Gruber, A.; Siegwolf, R.T.; Grams, T.E.; Matyssek, R. Long-term trends in leaf level gas exchange mirror tree-ring derived intrinsic water-use efficiency of Pinus cembra at treeline during the last century. Agric. For. Meteorol. 2018, 248, 251–258. [Google Scholar] [CrossRef]
- Obojes, N.; Meurer, A.; Newesely, C.; Tasser, E.; Oberhuber, W.; Mayr, S.; Tappeiner, U. Water stress limits transpiration and growth of European larch up to the lower subalpine belt in an inner-alpine dry valley. New Phytol. 2018, 220, 460–475. [Google Scholar] [CrossRef] [Green Version]
- Hoover, D.L.; Wilcox, K.R.; Young, K.E. Experimental droughts with rainout shelters: A methodological review. Ecosphere 2018, 9, e02088. [Google Scholar] [CrossRef]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.-M.; Rambal, S.; Miglietta, F.; Peressotti, A.; Pecchiari, M.; Tirone, G.; Valentini, R.; et al. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: Revision of current hypotheses? Glob. Chang. Biol. 2002, 8, 999–1017. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Granier, A.; Reichstein, M.; Breda, N.; Janssens, I.; Falge, E.; Ciais, P.; Grunwald, T.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; et al. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agric. For. Meteorol. 2007, 143, 123–145. [Google Scholar] [CrossRef]
- Aulitzky, H. Grundlagen und Anwendungen des vorläufigen Wind-Schnee-Ökogramms. Mitt. Forstl. Bundesversuchsanstalt Mariabrunn 1963, 60, 763–834. [Google Scholar]
- Leo, M.; Oberhuber, W.; Schuster, R.; Grams, T.E.E.; Matyssek, R.; Wieser, G. Evaluating the effect of plant water availability on inner alpine coniferous trees based on sap flow measurements. Eur. J. For. Res. 2014, 133, 691–698. [Google Scholar] [CrossRef]
- Schuster, R.; Oberhuber, W.; Gruber, A.; Wieser, G. Soil drought decreases water-use of pine and spruce but not of larch in a dry inner alpine valley. Austrian J. For. Res. 2016, 133, 1–17. [Google Scholar]
- Schmidt, O. Fichtenwälder im Klimawandel; Bayerische Landesanstalt für Wald und Forstwirtschaft: Freising, Germany, 2009. [Google Scholar]
- Wolfslehner, G.; Koeck, R.; Hochbichler, E.; Steiner, H.; Frank, G.; Formayer, H.; Arbeiter, F. Ökologische und waldbauliche Eigenschaften der Lärche (Larix decidua MILL.)—Folgerungen für die Waldbewirtschaftung in Österreich unter Berücksichtigung des Klimawandels; Endbericht Start-Clim. 2010: Anpassung an den Klimawandel: Weitere Beiträge zur Erstellung einer Anpassungsstrategie für Österreich Auftraggeber: BMLFUW, BMWF, BMWFJ, ÖBF; Austrian Academy of Science: Vienna, Austria, 2011. [Google Scholar]
- Hartl-Meier, C.; Dittmar, C.; Zang, C.; Rothe, A. Mountain forest growth response to climate change in the Northern Limestone Alps. Trees 2014, 28, 819–829. [Google Scholar] [CrossRef]
- Anfodillo, T.; Rento, S.; Carraro, V.; Furlanetto, L.; Urbinati, C.; Carrer, M. Tree water relations and climatic variations at the alpine timberline: Seasonal changes of sap flux and xylem water potential in Larix decidua Miller, Picea abies (L.) Karst. and Pinus cembra L. Ann. For. Sci. 1998, 55, 159–172. [Google Scholar] [CrossRef]
- Wieser, G. Lessons from the timberline ecotone in the Central Tyrolean Alps: A review. Plant Ecol. Divers. 2012, 5, 127–139. [Google Scholar] [CrossRef]
- Granier, A. Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann. For. Sci. 1985, 42, 193–200. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef]
- Bortz, J.; Lienert, G.A.; Boenke, K. Verteilungsfreie Methoden in der Biostatistik; Springer: Berlin, Germany, 2008. [Google Scholar]
- Köstner, B.M.M.; Schulze, E.-D.; Kelliher, F.M.; Hollinger, D.Y.; Byers, J.N.; Hunt, J.E.; McSeveny, T.M.; Meserth, R.; Weir, P.L. Transpiration and canopy conductance in a pristine broad-leaved forest of Nothofagus: An analysis of xylem sap flow and eddy correlation measurements. Oecologia 1992, 91, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Vygodskaya, N.N.; Ziegler, W.; Schulze, E.D.; Wirth, C.; McDonald, K.C. Canopy transpiration in a chronosequence of Central Siberian pine forests. Glob. Chang. Biol. 2000, 6, 25–37. [Google Scholar] [CrossRef]
- Matyssek, R.; Wieser, G.; Patzner, K.; Blaschke, H.; Häberle, K.-H. Transpiration of forest trees and stands at different altitude: Consistencies rather than contrasts? Eur. J. For. Res. 2009, 128, 579–596. [Google Scholar] [CrossRef]
- Badalotti, A.; Anfodillo, T.; Grace, J. Evidence of osmoregulation in Larix decidua at Alpine treeline and comparative responses to water availability of two co-occurring evergreen species. Ann. For. Sci. 2000, 57, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, A. Mountain climates from a perspective of forest growth. In Mountain Environments and Subalpine Forest Growth; Benecke, U., Davis, M.R., Eds.; New Zealand Forest Service: Wellington, New Zealand, 1980; pp. 27–39. [Google Scholar]
- Fliri, F. Das Klima der Alpen im Raume von Tirol; Monographien zur Landeskunde Tirols I Universitätsverlag Wagner: Innsbruck, Austria; Munchen, Germany, 1975. [Google Scholar]
- Veit, H. Die Alpen—Geoökologie und Landschaftsentwicklung; Ulmer: Stuttgart, Germany, 2002. [Google Scholar]
- Wieser, G.; Tausz, M. Trees at Their Upper Limit: Treelife Limitation at the Alpine Timberline; Springer: Berlin, Germany, 2007; Volume 5. [Google Scholar]
- Tranquillini, W. Physiological ecology of the alpine timberline. In Tree Existence at High Altitudes with Special Reference to the European Alps; Springer: Berlin, Germany, 1979; Volume 31. [Google Scholar]
- Wieser, G.; Gruber, A.; Oberhuber, W. Sap flow characteristics and whole-tree water use of Pinus cembra across the treeline ecotone of the central Tyrolean Alps. Eur. J. For. Res. 2012, 133, 287–295. [Google Scholar] [CrossRef]
- Gruber, A.; Zimmenmann, J.; Wieser, G.; Oberhuber, W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the timberline ecotone. Ann. For. Sci. 2009, 66, 503. [Google Scholar] [CrossRef]
- Dawson, D.E. Determining water use by trees and forests from isotopic, energy balance, and transpirational analyses: The role of tree site and hydraulic lift. Tree Physiol. 1994, 18, 177–184. [Google Scholar]
- Villar-Salvador, P.; Castro-Dıez, P.; Perez-Rontome, C.; Montserrat-Martí, G. Stem xylem features in three Quercus (Fagaceae) species along a climatic gradient in NE Spain. Trees 1997, 12, 90–96. [Google Scholar] [CrossRef]
- Sarris, D.; Siegwolf, R.; Körner, C. Inter- and intra-annual stable carbon and oxygen isotope signals in response to drought in Mediterranean pines. Agric. For. Meteorol. 2013, 168, 59–68. [Google Scholar] [CrossRef]
- Vincke, C.; Thiry, Y. Water table is a relevant source for water uptake by a Scots pine (Pinus sylvestris L.) stand: Evidences from continuous evapotranspiration and water table monitoring. Agric. For. Meteorol. 2008, 148, 1419–1432. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Morales, D.; Wieser, G.; Jiménez, M.S.; González-Rodríguez, Á.M. Canopy transpiration of a semi arid Pinus canariensis forest at a treeline ecotone in two hydrologically contrasting years. Agric. For. Meteorol. 2015, 201, 120–127. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen in Okologischer, Dynamischer und Historischer Sicht; Verlag Eugen Ulmer: Stuttgart, Germany, 1996. [Google Scholar]
- Holtmeier, F.-K. Mountain Timberlines. Ecology, Patchiness, and Dynamics; KLuwer Academic Publishers: Dortrecht, The Netherlands; Boston, MA, USA; London, UK, 2000; Volume 14. [Google Scholar]
- Boisvert-Marsh, L.; Perie, C.; de Blois, S. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 2014, 5, 1–33. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Sykes, M.; Prentice, I. Climate change, tree species distributions and forest dynamics: A case study in the mixed conifer/northern hardwoods zone of northern Europe. Clim. Chang. 1996, 34, 161–177. [Google Scholar] [CrossRef]
- Tognetti, R.; Alados, C.; Bebi, P.; Grunewald, K.; Zhiyanski, M.; Andonowski, V.; Hofgaard, A.; La Porta, N.; Bratanova-Doncheva, S.; Edwards-Jonášová, M.; et al. Drivers of treeline shift in different European mountains. Clim. Res. 2017, 73, 135–150. [Google Scholar]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forests worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef]
- He, J.-S.; Zhang, Q.-B.; Bazzaz, F.A. Differential drought responses between saplings and adult trees in four co-occurring species of New England. Trees 2005, 19, 442–450. [Google Scholar] [CrossRef]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between saplings and adult trees: An integration of field results by meta-analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef]
- Andivia, E.; Madrigal-González, J.; Villar-Salvador, P.; Zavala, M.A. Do adult trees increase conspecific juvenile resilience to recurrent droughts? Implications for forest regeneration. Ecosphere 2018, 9, e02282. [Google Scholar] [CrossRef]


| Treatment | Picea abies | Larix decidua | ||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| control | 3.8 ± 1.7 | 7.3 ± 1.0 | 7.5 ± 5.9 | 11.2 ± 4.8 | 9.5 ± 1.0 | 9.0 ± 1.3 |
| rain exclusion | 6.2 ± 1.6 | 10.6 ± 0.7 | 12.8 ± 1.9 | 11.8 ± 3.8 | 10.0 ± 1.9 | 9.3 ± 2.4 |
| Year | PHAR (μmol m−2 s−1) | Tair (°C) | VPD (hPa) | P (mm) | Rainout Period | P Excluded (mm) | % P Excluded |
|---|---|---|---|---|---|---|---|
| 2016 | 247 | 9.4 | 2.4 | 830 | 16 August–3 October | 137 | 16 |
| 2017 | 331 | 9.4 | 2.7 | 1171 | 18 May–13 September | 962 | 82 |
| 2018 | 349 | 10.9 | 2.8 | 563 | 23 May–23 September | 512 | 91 |
| Treatment | Soil Water Content | Soil Temperature | ||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| control | 34.6 ± 2.8 | 32.3 ± 4.0 | 29.9 ± 3.8 | 11.9 ± 0.7 | 11.6 ± 1.0 | 12.0 ± 0.9 |
| rain exclusion | 27.9 ± 4.2 | 15.3 ± 3.0 | 15.7 ± 3.2 | 11.9 ± 0.7 | 11.5 ± 1.0 | 12.0 ± 0.8 |
| Tree | Picea abies | Larix decidua | ||||||
|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2015 | 2016 | 2017 | 2018 | |
| control | 3.9 ± 1.0 | 3.9 ± 1.2 | 3.8 ± 1.1 | 5.3 ± 1.5 | 2.1 ± 0.2 | 1.8 ± 0.1 | 2.4 ± 0.2 | 2.1 ± 1.6 |
| rain exclusion | 3.3 ± 0.5 | 3.0 ± 0.1 | 2.6 ± 0.4 | 3.2 ± 0.4 | 2.8 ± 0.1 | 2.4 ± 1.1 | 2.9 ± 1.4 | 4.4 ± 18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieser, G.; Oberhuber, W.; Gruber, A.; Oberleitner, F.; Hasibeder, R.; Bahn, M. Artificial Top Soil Drought Hardly Affects Water Use of Picea abies and Larix decidua Saplings at the Treeline in the Austrian Alps. Forests 2019, 10, 777. https://doi.org/10.3390/f10090777
Wieser G, Oberhuber W, Gruber A, Oberleitner F, Hasibeder R, Bahn M. Artificial Top Soil Drought Hardly Affects Water Use of Picea abies and Larix decidua Saplings at the Treeline in the Austrian Alps. Forests. 2019; 10(9):777. https://doi.org/10.3390/f10090777
Chicago/Turabian StyleWieser, Gerhard, Walter Oberhuber, Andreas Gruber, Florian Oberleitner, Roland Hasibeder, and Michael Bahn. 2019. "Artificial Top Soil Drought Hardly Affects Water Use of Picea abies and Larix decidua Saplings at the Treeline in the Austrian Alps" Forests 10, no. 9: 777. https://doi.org/10.3390/f10090777





