Do Coffee Agroforestry Systems Always Improve Soil Carbon Stocks Deeper in the Soil?—A Case Study from Turrialba, Costa Rica
Abstract
:1. Introduction
- (1)
- To compare the differences in whole soil and aggregate-associated carbon stocks among coffee AFS with the above-mentioned management variables, a coffee monoculture (sun coffee), and an adjacent forest across varying depth classes up to 1 m.
- (2)
- To assess whether differences in management practices within coffee AFS helped in improving soil aggregation and SOC storage relative to monoculture sun coffee.
- (3)
- To evaluate if AFS promote aggregate associated C within the smallest aggregate fraction (<53 µm) across varying depth classes.
2. Materials and Methods
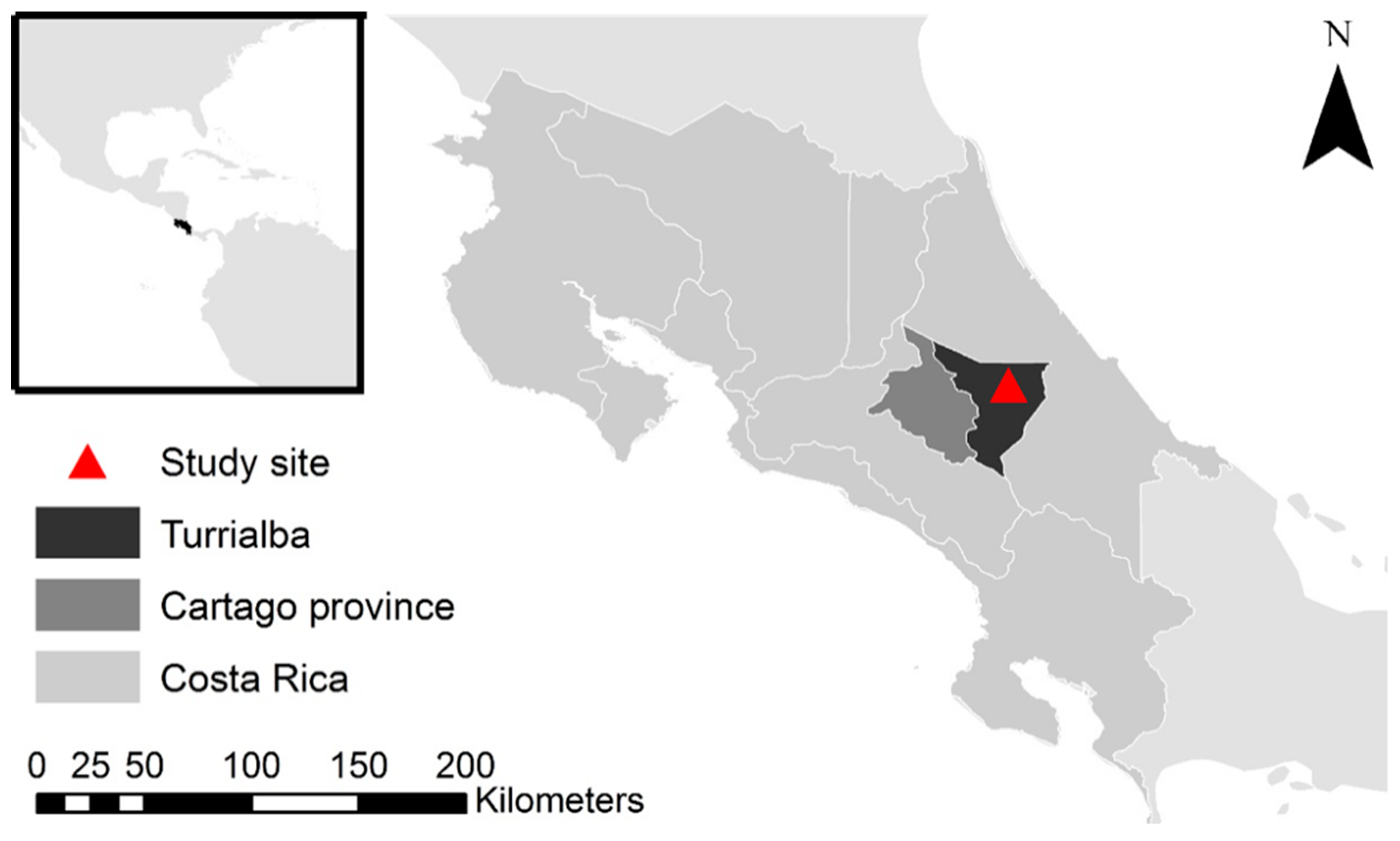
2.1. Study Location
2.2. Management Practices under Coffee AFS
- Conventional intensively managed coffee + N2 fixing species (Erythrina poeppigiana (Walp.) O.F. Cook.) as shade tree (CE)
- Conventional intensively managed coffee + timber species (Terminalia amazonia J.F.Gmel.) as shade tree (CT)
- Organic intensively managed coffee + N2 species (Erythrina poeppigiana (Walp.) O.F. Cook.) as shade tree (OE)
- Organic intensively managed coffee + timber species (Terminalia amazonia J.F.Gmel.) as shade tree (OT)
- Full sun grown coffee (Coffea arabica L.) (SC) (monoculture)
- Forest: Native, Talamancan montane forest (Bosque Florencia) from a nearby site (FO)
2.3. Soil Sampling
2.4. Soil Preparation and Analysis
2.5. Statistical Analyses
- .
- = overall effect,
- = effect due to the th treatments
- = effect due to the th depth level,
- = effect of the th fraction size.
- = interaction effect of the th treatments and the th depth level
- = interaction effect of the th depth level and the th fraction size
- = interaction effect of the th treatments and the th fraction size
- = normal random error
3. Results
3.1. Various Interaction Effects and the Analysis of Variance (ANOVA)
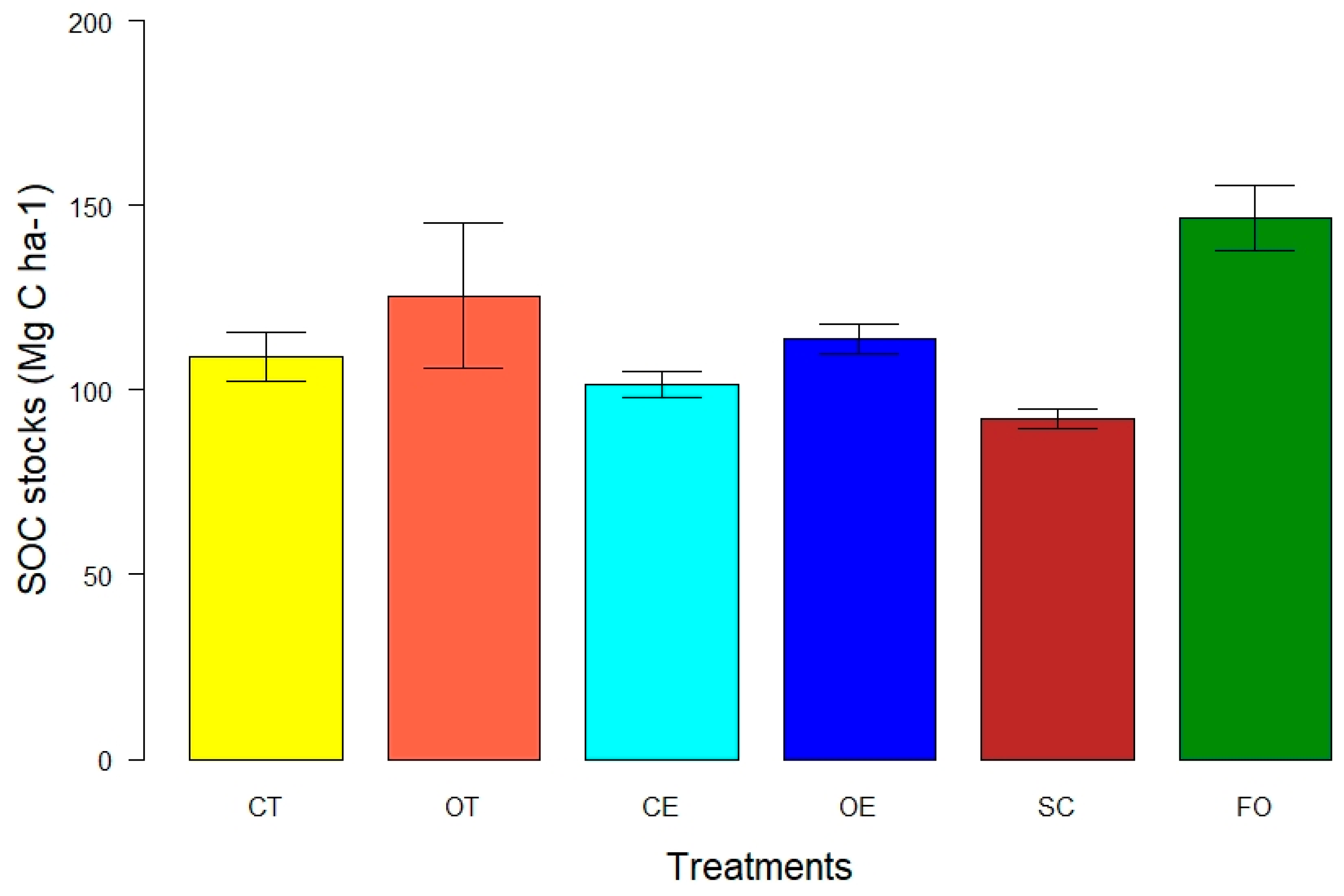
3.2. Soil Organic Carbon Stock in Whole Soil up to 1 m
3.3. Soil Organic Carbon Stocks in Macroaggregates (>250 μm)
3.4. Soil Organic Carbon Stock in Microaggregates (250–53 μm)
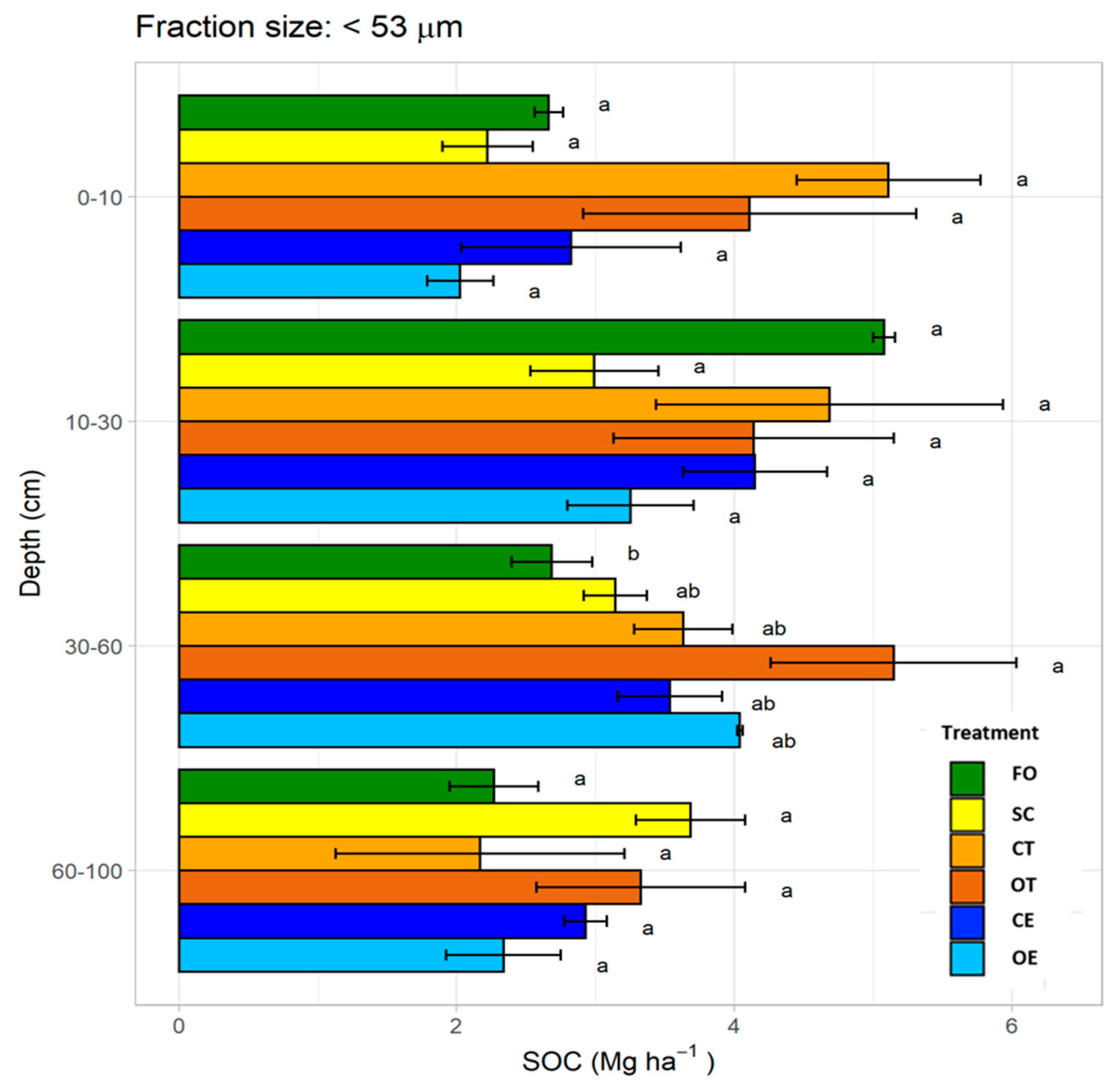
3.5. Soil Organic Carbon Stock in Silt and Clay Fraction (<53 μm)
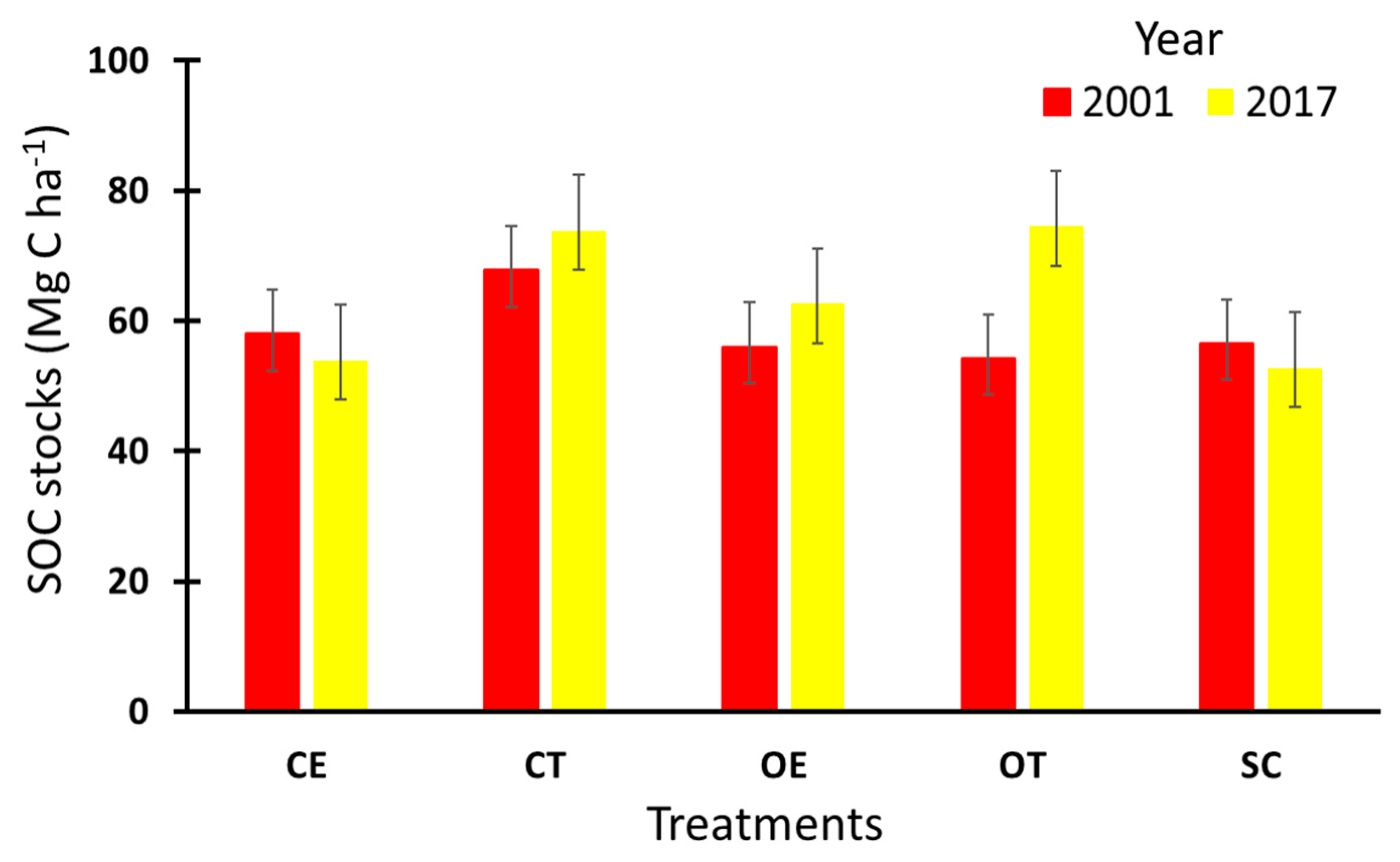
3.6. Carbon Sequestration Potential (CSP)
3.7. Effect of Management and Type of Shade on SOC Stocks
3.8. Modeling the Effect of Textural Variability on SOC Stocks in Whole Soil
4. Discussion
4.1. Land-Use System—Soil Depth Class—Aggregate Size Interactions in SOC Storage under Shaded AFS
4.2. SOC Stocks in Whole Soil
4.3. Soil Organic Carbon in Various Aggregate-Size Fractions
4.3.1. Macroaggregates (>250 µm)
4.3.2. Microaggregates (250–53 µm)
4.3.3. Silt and Clay Fraction (<53 μm)
4.4. Rhizodeposition and Management under Shaded Perennial Systems
4.4.1. Timber Species versus N2 Fixing Species
4.4.2. Organic Versus Conventional Management
4.4.3. Do Trees in AFS Always Improve and Sequester Carbon Stocks Deeper in the Soil?
4.5. Are Textural Variations Influencers of SOC Stocks in Soil?
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.K.R.; McBratney, A.B.; de Moraes Sá, J.C.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A–152A. [Google Scholar] [CrossRef] [Green Version]
- Stout, B.; Lal, R.; Monger, C. Carbon capture and sequestration: The roles of agriculture and soils. Integr. J. Agric. Biol. Eng. 2016, 9, 1–8. [Google Scholar]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- Powlson, D.S.; Gregory, P.J.; Whalley, W.R.; Quinton, J.N.; Hopkins, D.W.; Whitmore, A.P.; Hirsch, P.R.; Goulding, K.W.T. Soil management in relation to sustainable agriculture and ecosystem services. Food Policy 2011, 36, S72–S87. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Blanchart, E.; Skiba, U.; Hénault, C.; Harmand, J.M. Changes in carbon stock and greenhouse gas balance in a coffee (Coffea arabica) monoculture versus an agroforestry system with Inga densiflora, in Costa Rica. Agric. Ecosyst. Environ. 2012, 148, 102–110. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef] [Green Version]
- Soto-Pinto, L.; Anzueto, M.; Mendoza, J.; Ferrer, G.J.; de Jong, B. Carbon sequestration through agroforestry in indigenous communities of Chiapas, Mexico. Agrofor. Syst. 2010, 78, 39–51. [Google Scholar] [CrossRef]
- Noponen, M.R.A.; Edwards-Jones, G.; Haggar, J.P.; Soto, G.; Attarzadeh, N.; Healey, J.R. Greenhouse gas emissions in coffee grown with differing input levels under conventional and organic management. Agric. Ecosyst. Environ. 2012, 151, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.R.; Nair, V.D. Solid-fluid-gas: The state of knowledge on carbon-sequestration potential of agroforestry systems in africa. Curr. Opin. Environ. Sustain. 2014, 6, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Montagnini, F.; Nair, P.K.R. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2004, 61, 281–295. [Google Scholar]
- Noponen, M.R.A.; Healey, J.R.; Soto, G.; Haggar, J.P. Sink or source-The potential of coffee agroforestry systems to sequester atmospheric CO2 into soil organic carbon. Agric. Ecosyst. Environ. 2013, 175, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Sanderman, J.; Baldock, J.A. Accounting for soil carbon sequestration in national inventories: A soil scientist’s perspective. Environ. Res. Lett. 2010, 5, 34–45. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Bhagwat, S.A.; Buchori, D.; Faust, H.; Hertel, D.; Hölscher, D.; Juhrbandt, J.; Kessler, M.; Perfecto, I.; et al. Multifunctional shade-tree management in tropical agroforestry landscapes—A review. J. Appl. Ecol. 2011, 48, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Monroe, P.H.M.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Marques, J.R.B. Soil carbon stocks and origin under different cacao agroforestry systems in Southern Bahia, Brazil. Agric. Ecosyst. Environ. 2016, 221, 99–108. [Google Scholar] [CrossRef]
- Gama-Rodrigues, E.F.; Nair, P.K.R.; Nair, V.D.; Gama-Rodrigues, A.C.; Baligar, V.C.; Machado, R.C.R. Carbon storage in soil size fractions under Two cacao agroforestry systems in Bahia, Brazil. Environ. Manag. 2010, 45, 274–283. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Nair, V.D.; Viswanath, S.; Bhattacharjee, A. Depth-wise distribution of soil-carbon stock in aggregate-sized fractions under shaded-perennial agroforestry systems in the Western Ghats of Karnataka, India. Agrofor. Syst. 2019. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Chakraborty, S.; Nair, V.D. Agriculture, Ecosystems and Environment Changes in soil carbon stocks across the Forest-Agroforest-Agriculture/Pasture continuum in various agroecological regions: A meta-analysis. Agric. Ecosyst. Environ. 2018, 266, 55–67. [Google Scholar] [CrossRef]
- Beer, J. Litter production and nutrient cycling in coffee (Coffea arabica) or cacao (Theobroma cacao) plantations with shade trees. Agrofor. Syst. 1988, 7, 103–114. [Google Scholar] [CrossRef]
- Oelbermann, M.; Voroney, R.P. Carbon and nitrogen in a temperate agroforestry system: Using stable isotopes as a tool to understand soil dynamics. Ecol. Eng. 2007, 29, 342–349. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Chevallier, T.; Blanchart, E.; Albrecht, A.; Feller, C. The physical protection of soil organic carbon in aggregates: A mechanism of carbon storage in a Vertisol under pasture and market gardening (Martinique, West Indies). Agric. Ecosyst. Environ. 2004, 103, 375–387. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Chang. 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with silt and clay particles. Soil Sci. Soc. Am. J. 1997, 61, 131–139. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Haile, S.G.; Nair, P.K.R.; Nair, V.D. Carbon Storage of Different Soil-Size Fractions in Florida Silvopastoral Systems All rights reserved. J. Environ. Qual. 2008, 37, 1789–1797. [Google Scholar] [CrossRef]
- Howlett, D.S.; Mosquera-Losada, M.R.; Nair, P.K.R.; Nair, V.D.; Rigueiro-Rodríguez, A. Soil carbon storage in silvopastoral systems and a treeless pasture in northwestern Spain. J. Environ. Qual. 2011, 40, 825–832. [Google Scholar] [CrossRef]
- Tonucci, R.G.; Nair, V.D.; Ramachandran Nair, P.K.; Garcia, R. Grass vs. tree origin of soil organic carbon under different land-use systems in the Brazilian Cerrado. Plant Soil 2017, 419, 281–292. [Google Scholar] [CrossRef]
- Tonucci, R.G.; Nair, P.K.R.; Nair, V.D.; Garcia, R.; Bernardino, F.S. Soil Carbon Storage in Silvopasture and Related Land-Use Systems in the Brazilian Cerrado. J. Environ. Qual. 2011, 40, 833–841. [Google Scholar] [CrossRef]
- Takimoto, A.; Nair, V.D.; Nair, P.K.R. Contribution of trees to soil carbon sequestration under agroforestry systems in the West African Sahel. Agrofor. Syst. 2009, 76, 11–25. [Google Scholar] [CrossRef]
- RStudio Team. RStudio Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019. [Google Scholar]
- The Food and Agriculture Organization. Agriculture in Costa Rica. Available online: http://www.fao.org/3/y4632e/y4632e0a.htm (accessed on 29 November 2019).
- Haggar, J.; Barrios, M.; Bolaños, M.; Merlo, M.; Moraga, P.; Munguia, R.; Ponce, A.; Romero, S.; Soto, G.; Staver, C.; et al. Coffee agroecosystem performance under full sun, shade, conventional and organic management regimes in Central America. Agrofor. Syst. 2011, 82, 285–301. [Google Scholar] [CrossRef]
- Muschler, R.G. Shade improves coffee quality in a sub-optimal coffee-zone of Costa Rica. Agrofor. Syst. 2001, 51, 131–139. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Haile, S.G.; Nair, V.D.; Nair, P.K.R. Contribution of trees to carbon storage in soils of silvopastoral systems in Florida, USA. Glob. Chang. Biol. 2010, 16, 427–438. [Google Scholar] [CrossRef]
- Saha, S.K.; Nair, P.K.R.; Nair, V.D.; Kumar, B.M. Carbon storage in relation to soil size-fractions under tropical tree-based land-use systems. Plant Soil 2010, 328, 433–446. [Google Scholar] [CrossRef]
- Vargas, M.; Glaz, B.; Alvarado, G.; Pietragalla, J.; Morgounov, A.; Zelenskiy, Y.; Crossa, J. Analysis and interpretation of interactions in agricultural research. Agron. J. 2015, 107, 748–762. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, A.; Nair, P.K.R.; Nair, V.D. Carbon stock and sequestration potential of traditional and improved agroforestry systems in the West African Sahel. Agric. Ecosyst. Environ. 2008, 125, 159–166. [Google Scholar] [CrossRef]
- Hombegowda, H.C.; van Straaten, O.; Köhler, M.; Hölscher, D. On the rebound: Soil organic carbon stocks can bounce back to near forest levels when agroforests replace agriculture in southern India. SOIL Discuss. 2015, 2, 871–902. [Google Scholar] [CrossRef]
- Häger, A. The effects of management and plant diversity on carbon storage in coffee agroforestry systems in Costa Rica. Agrofor. Syst. 2012, 86, 159–174. [Google Scholar] [CrossRef]
- Soto-Pinto, L.; Aguirre-Dávila, C.M. Carbon stocks in organic coffee systems in Chiapas, Mexico. J. Agric. Sci. 2014, 7, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Abou Rajab, Y.; Leuschner, C.; Barus, H.; Tjoa, A.; Hertel, D. Cacao Cultivation under Diverse Shade Tree Cover Allows High Carbon Storage and Sequestration without Yield Losses. PLoS ONE 2016, 11, e0149949. [Google Scholar]
- Tumwebaze, S.B.; Byakagaba, P. Soil organic carbon stocks under coffee agroforestry systems and coffee monoculture in Uganda. Agric. Ecosyst. Environ. 2016, 216, 188–193. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Bevilacqua, E.; Briggs, R.; Volk, T. Soil organic carbon under a linear simultaneous agroforestry system in Uganda. Agrofor. Syst. 2012, 84, 11–23. [Google Scholar] [CrossRef]
- Dossa, E.L.; Fernandes, E.C.M.; Reid, W.S.; Ezui, K. Above-and belowground biomass, nutrient and carbon stocks contrasting an open-grown and a shaded coffee plantation. Agrofor. Syst. 2008, 72, 103–115. [Google Scholar] [CrossRef]
- Ehrenbergerová, L.; Cienciala, E.; Kučera, A.; Guy, L.; Habrová, H. Carbon stock in agroforestry coffee plantations with different shade trees in Villa Rica, Peru. Agrofor. Syst. 2016, 90, 433–445. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Jiang, X.; Wu, J. Effects of rubber-based agroforestry systems on soil aggregation and associated soil organic carbon: Implications for land use. Geoderma 2017, 299, 13–24. [Google Scholar] [CrossRef]
- Jastrow, D. Soil aggregate formation and the accrual of particulate and mineral-associated organic carbon. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Chenu, C.; Plante, A.T. Clay-sized organo-mineral complexes in a cultivation chronosequence: Revisiting the concept of the “primary organo-mineral complex”. Eur. J. Soil Sci. 2006, 57, 596–607. [Google Scholar] [CrossRef]
- Angers, D.A.; Recous, S.; Aita, C. Fate of carbon and nitrogen in water-stable aggregates during decomposition of 13C15N-labelled wheat straw in situ. Eur. J. Soil Sci. 1997, 48, 295–300. [Google Scholar] [CrossRef]
- Gale, W.J.; Cambardella, C.A.; Bailey, T.B. Root-derived carbon and the formation and stabilization of aggregates. Soil Sci. Soc. Am. J. 2000, 64, 201–207. [Google Scholar] [CrossRef]
- Garces, A.M. Characterization of the Spatial Variability of Soil Properties and Coffee Fine Roots in Shade Tree-Coffee Associations under Organic and Conventional Management Practices. Ph.D. Thesis, Tropical Agriculture Research and Higher education Centre, Turrialba, Costa Rica, 2011. [Google Scholar]
- Defrenet, E.; Roupsard, O.; van den Meersche, K.; Charbonnier, F.; Stokes, A.; Roumet, C.; Rapidel, B.; De Melo, E.; Filho, V.; Vargas, V.J.; et al. Root biomass, turnover and net primary productivity of a coffee agroforestry system in Costa Rica: Effects of soil depth, shade trees, distance to row and coffee age. Ann. Bot. 2016, 118, 833–851. [Google Scholar] [CrossRef] [Green Version]
- Nair, P. Managed Multi-strata Tree + Crop Systems: An Agroecological Marvel. Front. Environ. Sci. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Cuenca, G.; Aranguren, J.; Herrera, R. Root growth and litter decomposition in a coffee plantation under shade trees. Plant Soil 1983, 71, 477–486. [Google Scholar] [CrossRef]
- Pierret, A.; Maeght, J.-L.; Clément, C.; Montoroi, J.-P.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef] [Green Version]
- Montagnini, F. Accumulation in above-ground biomass and soil storage of mineral nutrients in pure and mixed plantations in a humid tropical lowland. For. Ecol. Manag. 2000, 134, 257–270. [Google Scholar] [CrossRef]
- Redondo-Brenes, A.; Montagnini, F. Growth, productivity, aboveground biomass, and carbon sequestration of pure and mixed native tree plantations in the Caribbean lowlands of Costa Rica. For. Ecol. Manag. 2006, 232, 168–178. [Google Scholar] [CrossRef]
- Richter, D.D.; Markewitz, D.; Trumbore, S.E.; Wells, C.G. Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 1999, 400, 56–58. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.D.B.; Hofmockel, M.; Callaham, M.A.; Powlson, D.S.; Smith, P. Long-term soil experiments: Keys to managing Earth’s rapidly changing ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 266–279. [Google Scholar] [CrossRef]
- Meylan, L.; Gary, C.; Allinne, C.; Ortiz, J.; Jackson, L.; Rica, C. Agriculture, Ecosystems and Environment Evaluating the effect of shade trees on provision of ecosystem services in intensively managed coffee plantations. Agric. Ecosyst. Environ. 2017, 245, 32–42. [Google Scholar] [CrossRef]
- Cerda, R.; Allinne, C.; Gary, C.; Tixier, P.; Harvey, C.A.; Krolczyk, L.; Mathiot, C.; Clément, E.; Aubertot, J.; Avelino, J. Effects of shade, altitude and management on multiple ecosystem services in coffee agroecosystems. Eur. J. Agron. 2017, 82, 308–319. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C. Is soil carbon mostly root carbon? Mechanisms for specific stabilization. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Sinacore, K.; Hall, J.S.; Potvin, C.; Royo, A.A.; Ducey, M.J.; Ashton, M.S. Unearthing the hidden world of roots: Root biomass and architecture differ among species within the same guild. PLoS ONE 2017, 12, e0185934. [Google Scholar] [CrossRef] [PubMed]
- Freibauer, A.; Rounsevell, M.D.A.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Scialabba, N.E.; Muller-Lindenlauf, M. Organic agriculture and climate change. Renew. Agric. Food Syst. 2010, 25, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Powlson, D.S.; Stirling, C.M.; Thierfelder, C.; White, R.P.; Jat, M.L. Does conservation agriculture deliver climate change mitigation through soil carbon sequestration in tropical agro-ecosystems? Agric. Ecosyst. Environ. 2016, 220, 164–174. [Google Scholar] [CrossRef]
- Powlson, D.S.; Whitmore, A.P.; Goulding, K.W.T. Soil carbon sequestration to mitigate climate change: A critical re-examination to identify the true and the false. Eur. J. Soil Sci. 2011, 62, 42–55. [Google Scholar] [CrossRef]
- Smith, P.; Powlson, D.; Glendining, M.; Smith, J. Potential for carbon sequestration in European soils: Preliminary estimates for five scenarios using results from long-term experiments. Glob. Chang. Biol. 1997, 3, 67–79. [Google Scholar] [CrossRef]
- Parton, W.J.; Stewart, J.W.B.; Cole, C.V. Dynamics of C, N, P and S in grassland soils: A model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Carbon and nitrogen storage by deep-rooted tall fescue (Lolium arundinaceum) in the surface and subsurface soil of a fine sandy loam in eastern Canada. Agric. Ecosyst. Environ. 2010, 136, 125–132. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Percival, H.J.; Parfitt, R.L.; Scott, N.A. Factors controlling soil carbon levels in New Zealand grasslands is clay content important? Soil Biol. Biochem. 2000, 64, 1623–1630. [Google Scholar]
- Zhong, Z.; Chen, Z.; Xu, Y.; Ren, C.; Yang, G.; Han, X.; Ren, G.; Feng, Y. Relationship between soil organic carbon stocks and clay content under different climatic conditions in Central China. Forests 2018, 9, 598. [Google Scholar] [CrossRef] [Green Version]



| Name of Treatment | Conventional Intensive (CI) | Organic Intensive (OI) |
|---|---|---|
| Soil Amendments | 300 kg N ha−1 20 kg P ha−1 150 kg K ha−1 | 287 kg N ha−1 205 kg P ha−1 326 kg K ha−1 Chicken manure 9 Mg ha−1 Coffee Pulp 5 Mg ha−1 |
| Shade Regulation | Drastic pruning | Regulated pruning |
| Weed Control | Herbicide | Selective mechanical removal |
| Disease Control | Fungicide | As required |
| Treatment | Herb | Litterfall | Coffee Pruning | Tree Pruning | Total |
|---|---|---|---|---|---|
| CE | 225 | 4104 | 4104 | 9997 | 17,357 |
| CT | 123 | 3832 | 1659 | 4513 | 10,126 |
| OE | 164 | 3077 | 7837 | 6352 | 17,428 |
| OT | 1338 | 2199 | 955 | 4203 | 8696 |
| Shade Tree | Shade before Pruning (%) | Shade after Pruning (%) | Height (m) | DBH (cm) | Basal Area per Tree (cm2) |
|---|---|---|---|---|---|
| Erythrina | 77.4 | 51.3 | 6.08 | 22.9 | 43.9 |
| Terminalia | 45.8 | 36.1 | 9.14 | 11 | 10.9 |
| Land-Use Types/Treatments | Depth (cm) | Bulk Density (Mg m−3) | pH | Particle Size Distribution (g 100 g−1 Soil) | ||
|---|---|---|---|---|---|---|
| Sand ‡ | Clay | Silt | ||||
| CE | 0–10 | 0.73 (0.02) | 6.1 | 37.6 (1.21) | 40.8 (1.2) | 21.6 (0.58) |
| 10–30 | 0.62 (0.08) | 5.5 | 45.6 (0.51) | 36.8 (0.41) | 17.6 (0.24) | |
| 30–60 | 0.99 (0.05) | 5.5 | 43.6 (0.96) | 36.4 (1.11) | 20 (0.88) | |
| 60–100 | 1.03 (0.06) | 5.3 | 43.6 (1.03) | 32.4 (1.11) | 24 (0.99) | |
| CT | 0–10 | 1.13 (0.03) | 6.0 | 36.8 (0.87) | 42 (0.81) | 21.2 (1.01) |
| 10–30 | 0.90 (0.03) | 5.1 | 32.4 (0.84) | 44.4 (1.16) | 23.2 (1.08) | |
| 30–60 | 0.83 (0.07) | 5.4 | 40.4 (0.21) | 36.4 (0.77) | 23.2 (0.36) | |
| 60–100 | 0.83 (0.02) | 5.4 | 38 (1.81) | 34.4 (1.22) | 27.6 (0.76) | |
| FO | 0–10 | 0.64 (0.05) | 4.5 | 29.2 (0.98) | 58.4 (1.25) | 12.4 (0.42) |
| 10–30 | 0.69 (0.02) | 5.1 | 24.8 (1.01) | 66.4 (1.08) | 8.8 (0.96) | |
| 30–60 | 0.73 (0.04) | 5.3 | 35.2 (0.87) | 58.4 (0.55) | 6.4 (0.34) | |
| 60–100 | 0.76 (0.01) | 5.1 | 33.2 (1.11) | 56.4 (1.08) | 10.4 (1.23) | |
| OE | 0–10 | 0.86 (0.04) | 6.3 | 37.6 (0.95) | 40.4 (1.21) | 22 (1.23) |
| 10–30 | 0.92 (0.04) | 6.3 | 39.6 (1.47) | 40.4 (1.16) | 20 (1.02) | |
| 30–60 | 1.12 (0.01) | 5.8 | 41.6 (1.15) | 36.4 (1.01) | 22 (0.55) | |
| 60–100 | 0.98 (0.03) | 5.9 | 37.2 (0.76) | 34.4 (1.51) | 28.4 (0.98) | |
| OT | 0–10 | 0.89 (0.03) | 6.5 | 47.6 (1.31) | 30.4 (1.13) | 22 (0.56) |
| 10–30 | 0.77 (0.02) | 6.1 | 37.6 (0.94) | 38.4 (1.82) | 24 (1.02) | |
| 30–60 | 1.15 (0.06) | 5.7 | 41.6 (1.54) | 38.8 (0.87) | 19.6 (1.11) | |
| 60–100 | 0.91 (0.08) | 5.9 | 41.6 (0.34) | 32.8 (1.21) | 25.6 (0.45) | |
| SC | 0–10 | 0.81 (0.02) | 6.4 | 33.2 (1.67) | 42.4 (1.43) | 24.4 (1.12) |
| 10–30 | 0.95 (0.02) | 6.6 | 45.2 (0.77) | 36.8 (0.76) | 18 (0.88) | |
| 30–60 | 0.99 (0.05) | 5.8 | 45.2 (1.21) | 32.4 (1.8) | 22.4 (1.2) | |
| 60–100 | 1.04 (0.19) | 5.3 | 43.2 (1.01) | 34.4 (0.96) | 22.4 (0.74) | |
| Average Percentage Weight (%) Distribution of Size Fraction at Various Depth | |||||||
|---|---|---|---|---|---|---|---|
| Soil Depth (cm) | Size Fraction (µm) | CE | CT | FO | OE | OT | SC |
| 0–10 | >250 | 81 (5.1) | 75.8 (1.11) | 90.5 (2.8) | 81.7(3.6) | 75.9 (2.1) | 81.5 (2.8) |
| 250 < x < 53 | 13.9 (4.3) | 15.7 (2.3) | 10.3 (5.1) | 15.6 (2.2) | 18.0 (2.4) | 15.5 (1.8) | |
| <53 | 11.5 (2.3) | 19.6 (1.5) | 8.9 (3.6) | 6.8 (2.2) | 13.6 (1.3) | 8.8 (0.6) | |
| 10–30 | >250 | 75.9 (6.7) | 71.5 (3.2) | 74.1 (6.2) | 74.5 (3.1) | 84.1 (2.2) | 73.9 (3.8) |
| 250 < x < 53 | 20.5 (4.8) | 22.2 (3.8) | 15.6 (4.2) | 16.1 (4.2) | 15.1 (4.5) | 17.1 (4.1) | |
| <53 | 12.9 (3.1) | 16.5 (1.1) | 15.3 (1.7) | 11.8 (2.8) | 11.9 (0.9) | 15.3 (0.7) | |
| 30–60 | >250 | 66.1 (5.2) | 65.0 (4.5) | 83.7 (5.2) | 62.2 (4.6) | 58.4 (5.4) | 51.9 (6.6) |
| 250 < x < 53 | 25.8 (6.6) | 28.5 (2.7) | 20.0 (3.8) | 25.0 (2.2) | 29.7 (1.7) | 38.3 (3.2) | |
| <53 | 19.9 (1.4) | 20.4 (2.1) | 17.8 (2.1) | 22.1 (1.9) | 20.7 (1.4) | 19.8 (1.4) | |
| 60–100 | >250 | 68.7 (4.1) | 53.5 (6.2) | 70.6 (5.4) | 55.2 (6.3) | 53.0 (5.9) | 38.5 (2.5) |
| 250 < x < 53 | 23.1 (3.2) | 35.0 (3.7) | 29.6 (3.7) | 33.8 (4.5) | 37.2 (4.1) | 38.5 (4.1) | |
| <53 | 22.9 (3.6) | 22.1 (1.1) | 28.2 (1.8) | 18.6 (3.1) | 18.8 (1.2) | 25.5 (0.6) | |
| Category | Df | Sum Sq. | Mean Sq. | F Value | Pr (>F) |
|---|---|---|---|---|---|
| Treatment | 5 | 492 | 98 | 5.03 | <0.0001 |
| Depth | 3 | 1109 | 370 | 18.8 | <0.0001 |
| Fraction Size | 2 | 11,115 | 5557 | 283.9 | <0.0001 |
| Treatment × Depth | 15 | 324 | 22 | 1.1 | 0.054 |
| Treatment × Fraction Size | 10 | 628 | 63 | 3.2 | <0.0001 |
| Depth × Fraction Size | 6 | 2158 | 360 | 18.3 | <0.0001 |
| Treatment × Depth × Fraction Size | 30 | 555 | 19 | 0.94 | 0.0299 |
| Residuals | 144 | 2818 | 20 |
| Category | Df | Sum Sq. | Mean Sq. | F Value | Pr (>F) |
|---|---|---|---|---|---|
| Treatment | 5 | 492 | 98 | 5.34 | <0.0001 |
| Depth | 3 | 1109 | 370 | 18.8 | <0.0001 |
| Fraction Size | 2 | 11,115 | 5557 | 284.1 | <0.0001 |
| Treatment × Fraction Size | 10 | 628 | 63 | 3.2 | <0.0001 |
| Depth × Fraction Size | 6 | 2158 | 360 | 18.4 | <0.0001 |
| Residuals | 189 | 3697 | 20 |
| Depth (cm) | Treatment | SOC Stocks in 2001 (Mg C ha−1) | SOC Stocks in 2017 (Mg C ha−1) | Soil CSP (Mg C ha−1 yr−1) (2001–2017) |
|---|---|---|---|---|
| 0–10 | CE | 26.4 | 25.3 | −0.1 |
| 10–40 | CE | 30.5 | 28.5 | −0.1 |
| 0–40 | CE | 58 | 53.9 | −0.2 |
| 0–10 | CT | 28.4 | 32.9 | 0.3 |
| 10–40 | CT | 39.4 | 41.0 | 0.1 |
| 0–40 | CT | 67.8 | 73.9 | 0.4 |
| 0–10 | OE | 24.3 | 29.5 | 0.3 |
| 10–40 | OE | 31.8 | 33.1 | 0.1 |
| 0–40 | OE | 56 | 62.6 | 0.4 |
| 0–10 | OT | 26.4 | 35.5 | 0.6 |
| 10–40 | OT | 27.8 | 39 | 0.7 |
| 0–40 | OT | 54.2 | 74.5 | 1.3 |
| 0–10 | SC | 24.2 | 27.7 | 0.2 |
| 10–40 | SC | 32.3 | 25.1 | −0.5 |
| 0–40 | SC | 56.5 | 52.8 | −0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, N.; Nair, P.K.R.; Nair, V.D.; Bhattacharjee, A.; Filho, E.d.M.V.; Muschler, R.G.; Noponen, M.R.A. Do Coffee Agroforestry Systems Always Improve Soil Carbon Stocks Deeper in the Soil?—A Case Study from Turrialba, Costa Rica. Forests 2020, 11, 49. https://doi.org/10.3390/f11010049
Chatterjee N, Nair PKR, Nair VD, Bhattacharjee A, Filho EdMV, Muschler RG, Noponen MRA. Do Coffee Agroforestry Systems Always Improve Soil Carbon Stocks Deeper in the Soil?—A Case Study from Turrialba, Costa Rica. Forests. 2020; 11(1):49. https://doi.org/10.3390/f11010049
Chicago/Turabian StyleChatterjee, Nilovna, P. K. Ramachandran Nair, Vimala D. Nair, Abhishek Bhattacharjee, Elias de Melo Virginio Filho, Rheinhold G. Muschler, and Martin R. A. Noponen. 2020. "Do Coffee Agroforestry Systems Always Improve Soil Carbon Stocks Deeper in the Soil?—A Case Study from Turrialba, Costa Rica" Forests 11, no. 1: 49. https://doi.org/10.3390/f11010049
APA StyleChatterjee, N., Nair, P. K. R., Nair, V. D., Bhattacharjee, A., Filho, E. d. M. V., Muschler, R. G., & Noponen, M. R. A. (2020). Do Coffee Agroforestry Systems Always Improve Soil Carbon Stocks Deeper in the Soil?—A Case Study from Turrialba, Costa Rica. Forests, 11(1), 49. https://doi.org/10.3390/f11010049






