Prediction of the Long-Term Potential Distribution of Cryptorhynchus lapathi (L.) under Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Model and Software
2.1.1. CLIMEX Model
2.1.2. ArcMap Software
2.2. Data Collection
2.2.1. Global Distribution
2.2.2. Climate Data
2.3. Research Method
2.3.1. Fitting CLIMEX Parameters
2.3.2. Classification of EI Values
2.3.3. Parameter Sensitivity Analysis
3. Results
3.1. Parameter Sensitivity Analysis
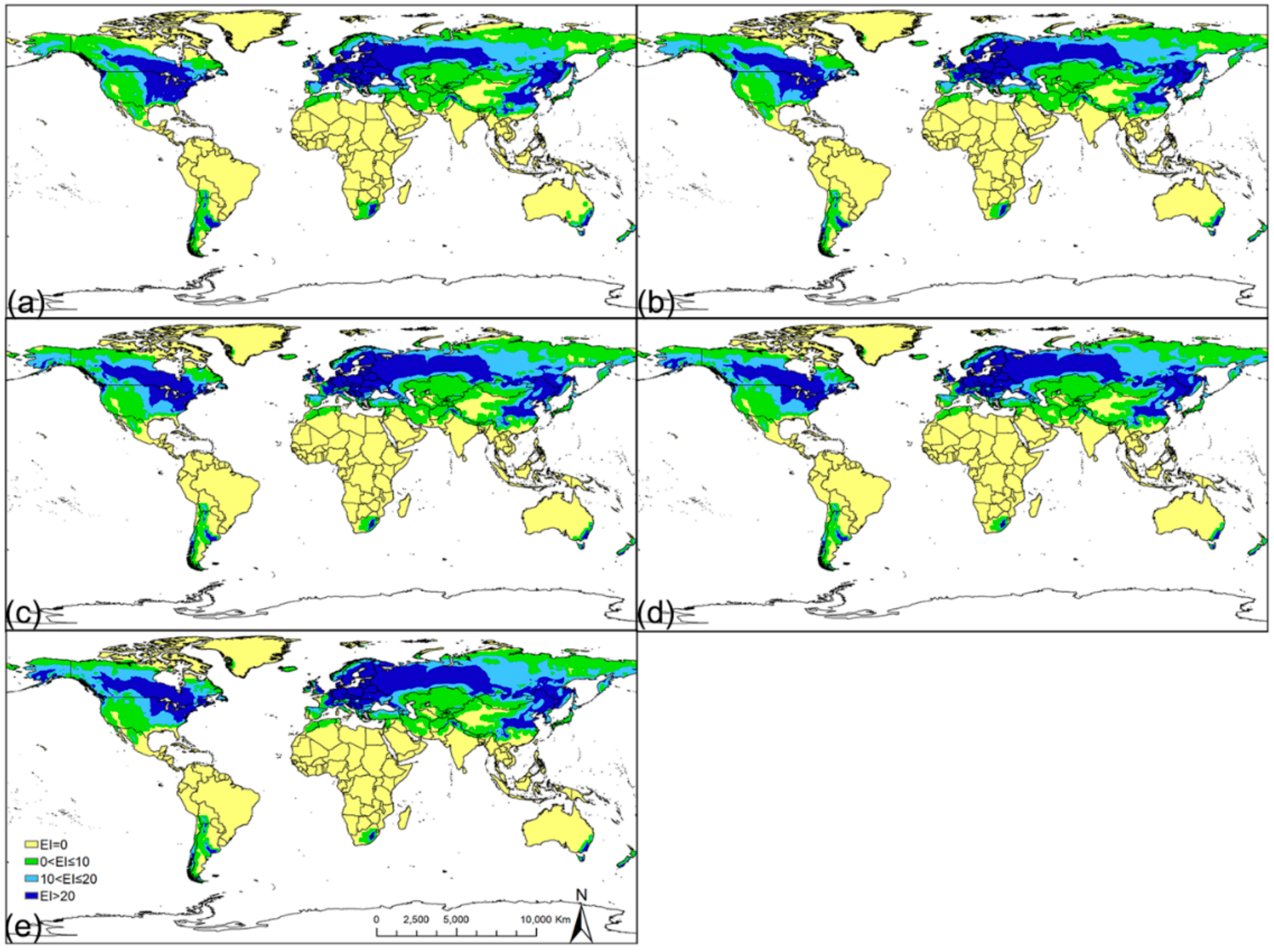
3.2. Potential Distribution under Historical Climate Conditions
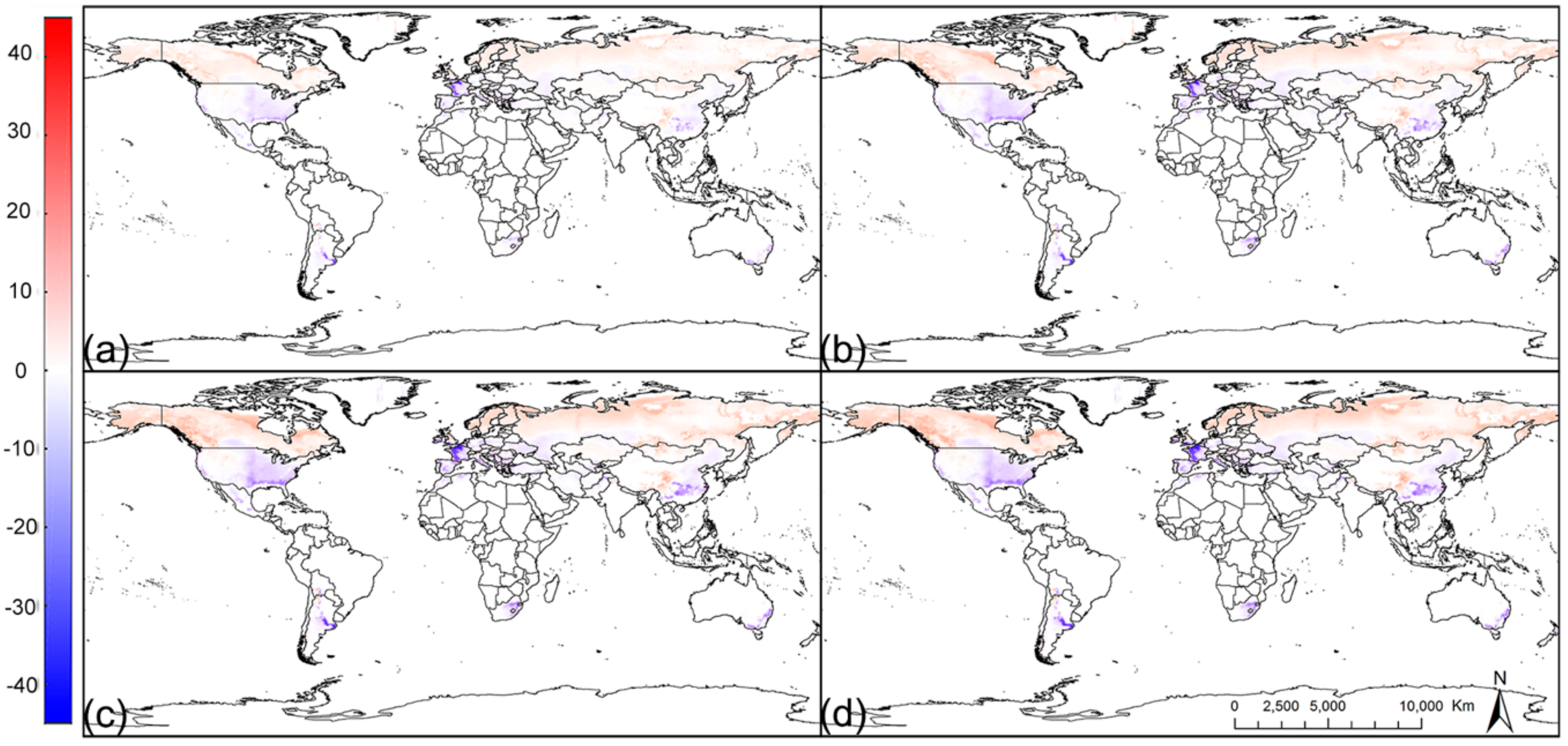
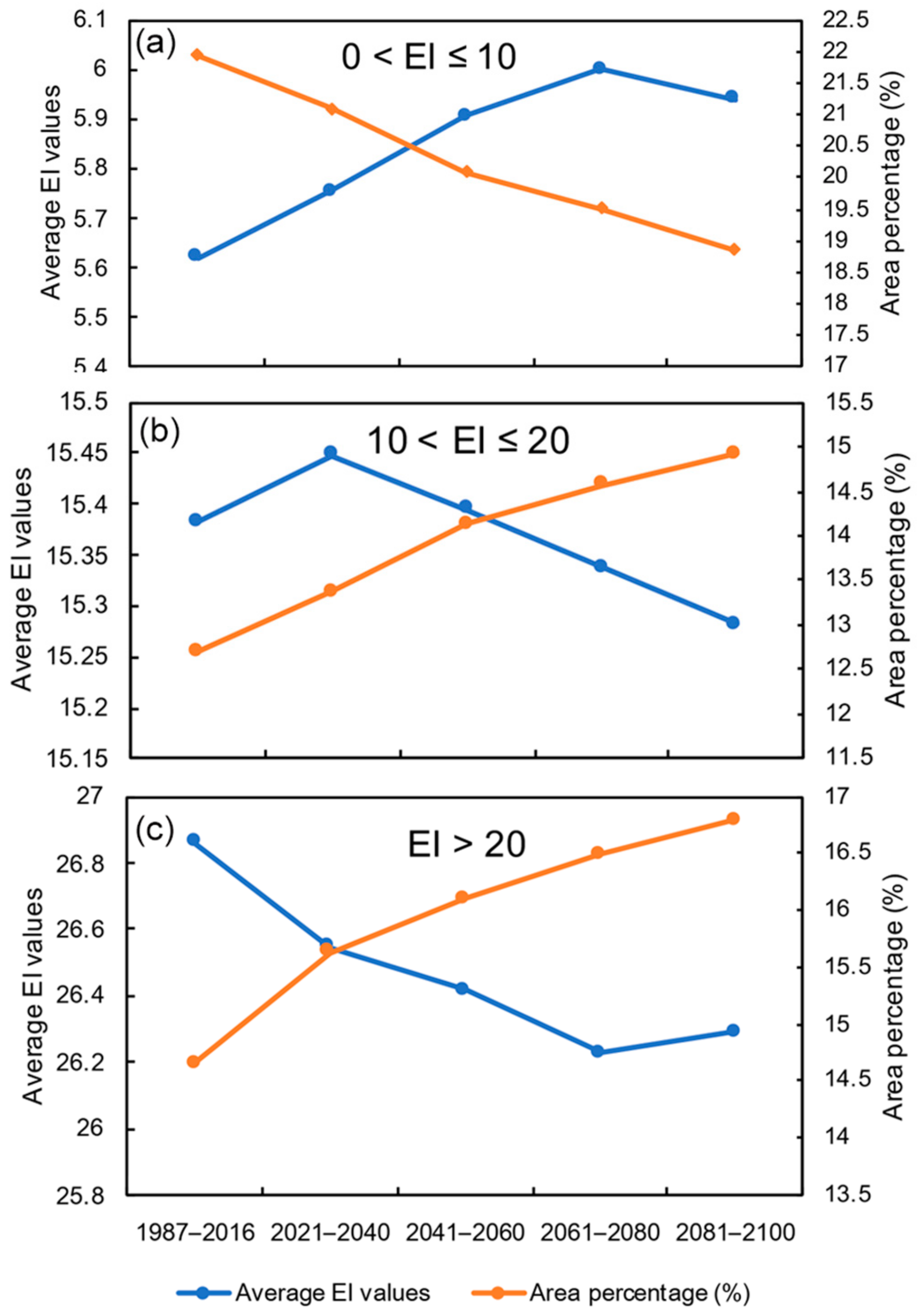
3.3. Potential Distribution under Future Climate Conditions
3.4. Indices Limiting the Potential Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Broberg, C.L.; Borden, J.H.; Humble, L.M. Distribution and abundance of Cryptorhynchus lapathi on Salix spp. Can. J. For. Res. 2002, 32, 561–568. [Google Scholar] [CrossRef]
- Schoene, W.J. The Poplar and Willow Borer (Cryptorhynchus lapathi L.); New York Agricultural Experiment Station: Geneva, NY, USA, 1907; Volume 286, pp. 83–104. [Google Scholar]
- Harris, J.W.E.; Coppel, H.C. The poplar-and-willow borer, Sternochetus (=Cryptorhynchus) lapathi (Coleoptera: Curculionidae), in British Columbia. Can. Entomol. 1967, 99, 411–418. [Google Scholar] [CrossRef]
- CABI, Cryptorhynchus lapathi (Poplar and Willow Borer). Available online: https://www.cabi.org/cpc/datasheet/16433 (accessed on 13 November 2018).
- Qin, P.C.; Yao, F.M.; Cao, X.X.; Zhang, J.H.; Cao, Q. Development process of modeling impacts of climate change on agricultural productivity based on crop models. Chin. J. Agrometeorol. 2011, 32, 240–245. [Google Scholar]
- Jia, J. Risk Aanalysis on Cryptorrhynchus lapathi in Qinghai. Sci. and Technol. of Qinghai Agriculture and For. 2015, 2, 29–31. [Google Scholar]
- Fang, S.Y. Preliminary study on poplar weevil. Sci. Silvae Sin. 1964, 10, 81–85. [Google Scholar]
- Jodal, I. A study of the susceptibility of poplar clones to the attack of poplar and willow borer (Cryptorhynchus lapathi). Radovi Institut za Topolarstvo (Yugoslavia) 1987, 1, 95–108. [Google Scholar]
- Broberg, C.L.; Borden, J.H.; Gries, R. Olfactory and feeding preferences of Cryptorhynchus lapathi L. (Coleoptera: Curculionidae) among hybrid clones and natural poplars. Environ. Entomol. 2005, 34, 1606–1613. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Zhong, Z.K.; Lin, J.H.; Qu, P. Bionomics and control of poplar weevil. Acta Entomol. Sin. 1981, 24, 390–396. [Google Scholar]
- Smith, B.D.; Stott, K.G. The life history and behaviour of the willow weevil Cryptorrhynchus lapathi L. Ann. Appl. Biol. 2010, 54, 141–151. [Google Scholar] [CrossRef]
- Gao, R.T.; Qin, X.X.; Chen, H.D.; Sun, Y.Q. Preliminary study on susceptibility of poplar varieties to Cryptorrhynchus lapathi (L.). For. Pest Dis. 1987, 3, 16–18. [Google Scholar]
- Ji, Y.C. Study on Suitable Area and Economic Loss Assessment of Ten Important Forestry Weevils in China. Master’s Thesis, Shandong Agricultural University, Shandong, China, 2015. [Google Scholar]
- Shen, G. Research of Farming Information Investigation Platform. Master’s Thesis, North China Electric Power University, Beijing, China, 2015. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Scientific Basis. Contribution of Working Group? To the Fifth Assessment Report of the Intergovernmental; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Chen, Y. Effect of global warming on insect: A literature review. Acta Ecol. Sin. 2010, 30, 2159–2172. [Google Scholar]
- Liu, E. Studies on Early-Warming Techniques and Risk Assessment for the Poplar Stem Boring Pests in Heilongjiang Province. Master’s Thesis, Northeast Forestry University, Harbin, China, 2009. [Google Scholar]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.I.; Sutherst, R. Exploring the Effects of Climate on Plants, Animals and Diseases; CLIMEX Version; CSIRO: Canberra, Australia, 2015; Volume 4, p. 184. [Google Scholar]
- Sutherst, R.W.; Maywald, G.F.; Kriticos, D.J. CLIMEX Version 3: User’s Guide; Hearne Scientific Software: Melbourne, Australia, 2007. [Google Scholar]
- Cui, Y.; Wei, F.; Zhu, Z.; Wang, X.; Peng, D.; Xie, B. Potential distribution of Diaporthe phaseolorum var. caulivora in China by using CLIMEX and GIS tools. Plant Prot. 2009, 35, 49–53. [Google Scholar]
- Harris, I.C.; Jones, P.D. CRU TS4. 01: Climatic Research Unit (CRU) Time-Series (TS) Version 4.01 of High-Resolution Gridded Data of Month-by-Month Variation in Climate (Jan. 1901-Dec. 2016); Centre for Environmental Data Analysis: Chilton, UK, 2017; Volume 4. [Google Scholar]
- Zou, Y.; Ge, X.; Guo, S.; Zhou, Y.; Wang, T.; Zong, S. Impacts of climate change and host plant availability on the global distribution of Brontispa longissima (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2019, 76, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, R.; Harris, J.W.E. Poplar and Willow Borer; Pest Leaflet—Pacific Forest Centre Forestry: Victoria, BC, Canada, 2002. [Google Scholar]
- Hannon, E.; Kittelson, N.; Eaton, J.; Brown, J. Screening hybrid poplar clones for susceptibility to Cryptorhynchus lapathi (Coleoptera: Curculionidae). J. Econ. Entomol. 2008, 101, 199–205. [Google Scholar] [CrossRef]
- Yu, X.Y.; Du, Y.D. Report and control of Cryptorrhynchus lapathi (L.). J. Heilongjiang For. 1997, 12–13. [Google Scholar]
- Doom, D. The biology, damage and control of the poplar and willow borer, Cryptorrhynchus lapathi. Neth. J. Plant Pathol. 1966, 72, 233–240. [Google Scholar] [CrossRef]
- Jin, D.; Lue, L.; Li, L. Status and Perspective of Research on Cryptorrhynchus lapathi in China. J. Northeast For. Univ. 2003, 31, 75–77. [Google Scholar] [CrossRef]
- Dong, Q.Y. Investigation of Cryptorrhynchus lapathi (L.) hazard to several poplar species. For. Invest. Des. 2004, 41–42. [Google Scholar]
- Li, X.E.; Shen, Z.A.; Zhu, H. Bionomics and Control of Cryptorrhynchus lapathi (L.); China Association for Science and Technology: Wuhan, China, 2007. [Google Scholar]
- Niu, H.X. Forest quarantine insect pest, Cryptorrhynchus lapathi L. in Hebei Province. J. Hebei. For. Sci. Technol. 1984, 28–29. [Google Scholar]
- Geier, P.W. The life history of Codling Moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae), in the Australian Capital Territory. Aust. J. Zool. 1963, 11, 323–367. [Google Scholar] [CrossRef]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.H.; Graham, C.P.; Anderson, R. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog. Phys. Geogr. 2006, 30, 751–777. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Synth. Am. Mus. Nat. Hist. 2007, 50, 54–89. [Google Scholar]
- Kearney, M.R.; Wintle, B.A.; Porter, W.P. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 2010, 3, 203–213. [Google Scholar] [CrossRef]
- Magarey, R.; Newton, L.; Hong, S.C.; Takeuchi, Y.; Christie, D.; Jarnevich, C.S.; Castro, K. Comparison of four modeling tools for the prediction of potential distribution for non-indigenous weeds in the United States. Biol. Invasions 2018, 20, 679–694. [Google Scholar] [CrossRef]
- Moilanen, A.; Wintle, B.A. Quantitative reserve network aggregation via the boundary quality penalty. Conserv. Biol. 2007, 21, 355–364. [Google Scholar] [CrossRef]
- Lantschner, M.V.; de la Vega, G.; Corley, J.C. Predicting the distribution of harmful species and their natural enemies in agricultural, livestock and forestry systems: An overview. Int. J. Pest Manage. 2019, 65, 190–206. [Google Scholar] [CrossRef]
- Hawkins, E.; Sutton, R. The potential to narrow uncertainty in regional climate predictions. Bull. Am. Meteorol. Soc. 2009, 90, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; He, S.; Zhu, C.; Wang, T.; Xu, Z.; Zong, S. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljaryian, R.; Kumar, L. Changing global risk of invading greenbug Schizaphis graminum under climate change. Crop Prot. 2016, 88, 137–148. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.U. Effect analysis of drilling injection against Cryptorrhynchus lapathi. Biol. Disaster Sci. 2017, 40, 181–184. [Google Scholar]
- Allegro, G. Mechanical control of the principal xylophagous insects of poplar using trunk barriers (In Italian). Inform. Agr 1990, 46, 91–95. [Google Scholar]
- Cao, Q.J.; Chi, D.F.; Yu, J.; Ran, Y.L. SEM and TEM observations of Beauveria brongniartii (Sacc.) Petch infecting body wall of Cryptorhynchus lapathi L. (Coleoptera: Curculionidae) larvae. J. Beijing For. Univ. 2015, 37, 96–101. [Google Scholar]
- Li, Y.X. The life habit and characteristics of Scleroderma guani Xiao et Wu. Gansu. Agric. 2014, 14, 72–73. [Google Scholar] [CrossRef]
- Broberg, C.; Borden, J.; Gries, R. Antennae of Cryptorhynchus lapathi (Coleoptera: Curculionidae) detect two pheromone components of coniferophagous bark beetles in the stems of Salix sitchensis and Salix scouleriana (Salicaceae). Can. Entomol. 2005, 137, 716–718. [Google Scholar] [CrossRef]
- Xing, Y.; Chi, D.; Yu, J.; Yan, J.; Ran, Y. EAG and behavioral responses of Cryptorrhynchus lapathi (Coleoptera: Curculionidae) to Twelve Plant Volatiles. Sci. Silvae Sin. 2017, 53, 159–167. [Google Scholar]
- Pureswaran, D.S.; Neau, M.; Marchand, M.; Grandpré, L.D.; Kneeshaw, D. Phenological synchrony between eastern spruce budworm and its host trees increases with warmer temperatures in the boreal forest. Ecol. Evol. 2018, 9, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; MožNý, M.; Žalud, Z.; Pokorný, R.; Lebeda, A. Impact of climate change on the occurrence and activity of harmful organisms. Plant Prot. Sci. 2009, 45, S48–S52. [Google Scholar] [CrossRef] [Green Version]
- Forrest, J.R. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]


| CLIMEX Parameter | Semi-Arid Template | Facultative Diapause Template | Temperate Template | Final Parameter | Units |
|---|---|---|---|---|---|
| DV0-Lower Temperature Threshold | 10 | 8 | 8 | 8 | °C |
| DV1-Lower Optimum Temperature | 20 | 18 | 18 | 16 | °C |
| DV2-Upper Optimum Temperature | 32 | 26 | 24 | 20 | °C |
| DV3-Upper Temperature Threshold | 38 | 31 | 28 | 36 | °C |
| PDD-Length of growing season | 0 | 0 | 600 | / | DD |
| DPD0-Diapause Induction Daylength | / | 11 | / | 11 | DD |
| DPT0-Diapause Induction Temperature | / | 5 | / | 10 | °C |
| DPT1-Diapause Termination Temperature | / | 0 | / | 4 | °C |
| DPD-Diapause Development Days | / | 90 | / | 90 | DD |
| DPSW-Diapause Summer or Winter indicator | / | 0 | / | 0 | |
| TTCS-Cold Stress Temperature Threshold | 0 | / | 0 | 0 | °C |
| THCS-Cold Stress Temperature Rate | 0 | / | 0 | 0 | week−1 |
| TTHS-Heat Stress Temperature Threshold | 39 | / | 30 | 36 | °C |
| THHS-Heat Stress Temperature Rate | 0.002 | / | 0.005 | 0.005 | week−1 |
| SM0-Lower Soil Moisture Threshold | 0.1 | 0.3 | 0.25 | 0.1 | |
| SM1-Lower Optimal Soil Moisture | 0.2 | 0.7 | 0.8 | 0.3 | |
| SM2-Upper Optimal Soil Moisture | 0.25 | 1 | 1.5 | 1 | |
| SM3-Upper Soil Moisture Threshold | 0.3 | 2 | 2.5 | 1.5 | |
| SMDS-Dry Stress Threshold | 0.05 | / | 0.2 | 0.01 | |
| HDS-Dry Stress Rate | −0.005 | / | −0.005 | −0.005 | week−1 |
| SMWS-Wet Stress Threshold | 0.4 | / | 2.5 | 1.5 | |
| HWS-Wet Stress Rate | 0.01 | / | 0.002 | 0.002 | week−1 |
| Parameter | Low | Default | High | Range Change | ||||
|---|---|---|---|---|---|---|---|---|
| 1987–2016 | 2021–2040 | 2041–2060 | 2061–2080 | 2081–2100 | ||||
| SM0 | 0 | 0.1 | 0.2 | 3.8 | 3.4 | 3.2 | 2.9 | 2.8 |
| SM1 | 0.2 | 0.3 | 0.4 | 0.6 | 0.5 | 0.4 | 0.4 | 0.4 |
| SM2 | 0.9 | 1 | 1.1 | 0 | 0 | 0 | 0 | 0 |
| SM3 | 1.4 | 1.5 | 1.6 | 0 | 0 | 0 | 0 | 0 |
| DV0 | 7 | 8 | 9 | 0 | 0 | 0 | 0 | 0 |
| DV1 | 15 | 16 | 17 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| DV2 | 19 | 20 | 21 | 0 | 0 | 0 | 0 | 0 |
| DV3 | 35 | 36 | 37 | 0 | 0 | 0 | 0 | 0 |
| DPD0 | 10.5 | 11 | 11.5 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 |
| DPT0 | 9 | 10 | 11 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| DPT1 | 3 | 4 | 5 | 4.2 | 3.9 | 3.6 | 3.4 | 3.3 |
| DPD | 72 | 90 | 108 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 |
| TTCS | −24 | −23 | −22 | 0 | 0 | 0 | 0 | 0 |
| THCS | −0.012 | −0.01 | −0.008 | 0 | 0 | 0 | 0 | 0 |
| TTHS | 35 | 36 | 37 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 |
| THHS | 0.004 | 0.005 | 0.006 | 0 | 0.1 | 0.1 | 0.1 | 0.1 |
| SMDS | 0 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 |
| HDS | −0.006 | −0.005 | -0.004 | 0 | 0 | 0 | 0 | 0 |
| SMWS | 1.4 | 1.5 | 1.6 | 0 | 0 | 0 | 0 | 0 |
| HWS | 0.0016 | 0.002 | 0.0024 | 0 | 0 | 0 | 0 | 0 |
| Index | Legend | Regions Limited by Indices | Change of Range |
|---|---|---|---|
| MI (Moisture Index) | MI = 0 | Northwest China, the Western United States, Chile, Argentina, South Africa, and New Zealand | The limited regions in Western United States will gradually decrease |
| GI′ (combination of Growth Index, Temperature Index, and Moisture Index) | GI′ = 0 (GI = 0, TI ≠ 0, MI ≠ 0) | Western United States, Central Asia, and scattered areas of Northwest China | The limited regions in central Asia will gradually decrease |
| DI (Diapause Index) | DI = 0 | 30°S and 30°N at low latitudes | Change is not clear |
| HS (Heat Stress) | HS ≥ 100 | Central Asia and the scattered areas of the Southern United States | The limited regions in central Asian will gradually expand northward to 40°N |
| DI+HS (Diapause Index + Heat Stress) | DI = 0, HS ≥ 100 | 5°N-15°N in Africa, India, Mexico, Brazil, sporadic regions of Venezuela | The limited regions in India will gradually increase |
| DI+GI′ (Diapause Index + combination of Growth Index, Temperature Index and Moisture Index) | DI = 0, GI′ = 0 (DI = 0, GI = 0, TI ≠ 0, MI ≠ 0) | 20°S-45°S, 20°N -40°N, Western Europe, Canada, and Northern Russia | Overall decrease |
| DI+TI (Diapause Index + Temperature Index) | DI = 0, TI = 0 | Arctic Circle and Western China | The limited regions in China will gradually increase |
| DI+MI (Diapause Index + Moisture Index) | DI = 0, TI = 0 | North and South Africa, Arabia, and South Australia | The limited regions in China and Africa will gradually decrease |
| HS+GI′ (Heat Stress + combination of Growth Index, Temperature Index and Moisture Index) | HS ≥ 100, GI′ = 0 | US-Mexico border, Iran’s sporadic regions | Change is not clear |
| HS + MI (Heat Stress + Moisture Index) | HS ≥ 100, GI′ = 0 | Roughly the same as HS+GI′ | Change is not clear |
| DI + WS (Diapause Index + Wet Stress) | DI = 0, WS ≥ 100 | Sporadic regions of Colombia and India | Change is not clear |
| DI + HS + TI (Diapause Index + Heat Stress + Temperature Index) | DI = 0, HS ≥ 100, TI = 0 | Africa, Southern Mexico, and sporadic regions of Iran after 2021 | The limited regions will gradually expand |
| DI + TI + MI (Diapause Index + Temperature Index + Moisture Index) | DI = 0, TI = 0, MI = 0 | 80°N and its north of Greenland | The limited regions will gradually decrease, and it will no longer be a limiting factor after 2061 |
| DI + WS + GI′ (Diapause Index + Wet Stress + combination of Growth Index, Temperature Index and Moisture Index) | DI = 0, WS ≥ 100, GI′ = 0 | The junction of Panama and Colombia | No change |
| DI + WS + MI (Diapause Index + Wet Stress + Moisture Index) | DI = 0, WS ≥ 100, MI = 0 | North Western South America and 10°S-5°N area in Southeast Asia | Change is not clear |
| DI + HS + GI′ (Diapause Index + Heat Stress + combination of Growth Index, Temperature Index and Moisture Index) | DI = 0, HS ≥ 100, GI′ = 0 | 10°N-15°N in Africa, Western Mexico, Northern Australia, and sporadic regions along the Arabian Sea coast | The limited regions in Australia will gradually expand southward |
| DI + HS + MI (Diapause Index + Heat Stress + Moisture Index) | DI = 0, HS ≥ 100, MI = 0 | 15°N-20°N in Africa, Pakistan, the eastern part of the Arabian Peninsula, and sporadic regions in central Australia | The limited regions in central Asia and Australia will gradually increase |
| DI + DS + MI (Diapause Index + Dry Stress + Moisture Index) | DI = 0, DS ≥ 100, MI = 0 | Northern Egypt, Southern Arabia, and coastal areas of Peru | Change is not clear |
| DI + HS + DS + MI (Diapause Index + Heat Stress + Dry Stress + Moisture Index) | DI = 0, HS ≥ 100, DS ≥ 100, MI = 0 | 20°N -30°N in Africa and Saudi Arabia | The limited regions will gradually expand to high latitudes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Zhang, L.; Ge, X.; Guo, S.; Li, X.; Chen, L.; Wang, T.; Zong, S. Prediction of the Long-Term Potential Distribution of Cryptorhynchus lapathi (L.) under Climate Change. Forests 2020, 11, 5. https://doi.org/10.3390/f11010005
Zou Y, Zhang L, Ge X, Guo S, Li X, Chen L, Wang T, Zong S. Prediction of the Long-Term Potential Distribution of Cryptorhynchus lapathi (L.) under Climate Change. Forests. 2020; 11(1):5. https://doi.org/10.3390/f11010005
Chicago/Turabian StyleZou, Ya, Linjing Zhang, Xuezhen Ge, Siwei Guo, Xue Li, Linghong Chen, Tao Wang, and Shixiang Zong. 2020. "Prediction of the Long-Term Potential Distribution of Cryptorhynchus lapathi (L.) under Climate Change" Forests 11, no. 1: 5. https://doi.org/10.3390/f11010005





