Diversity, Abundance, and Distribution of Wood-Decay Fungi in Major Parks of Hong Kong
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Sampling
2.3. Culturing and Isolation
2.4. PCR Amplification
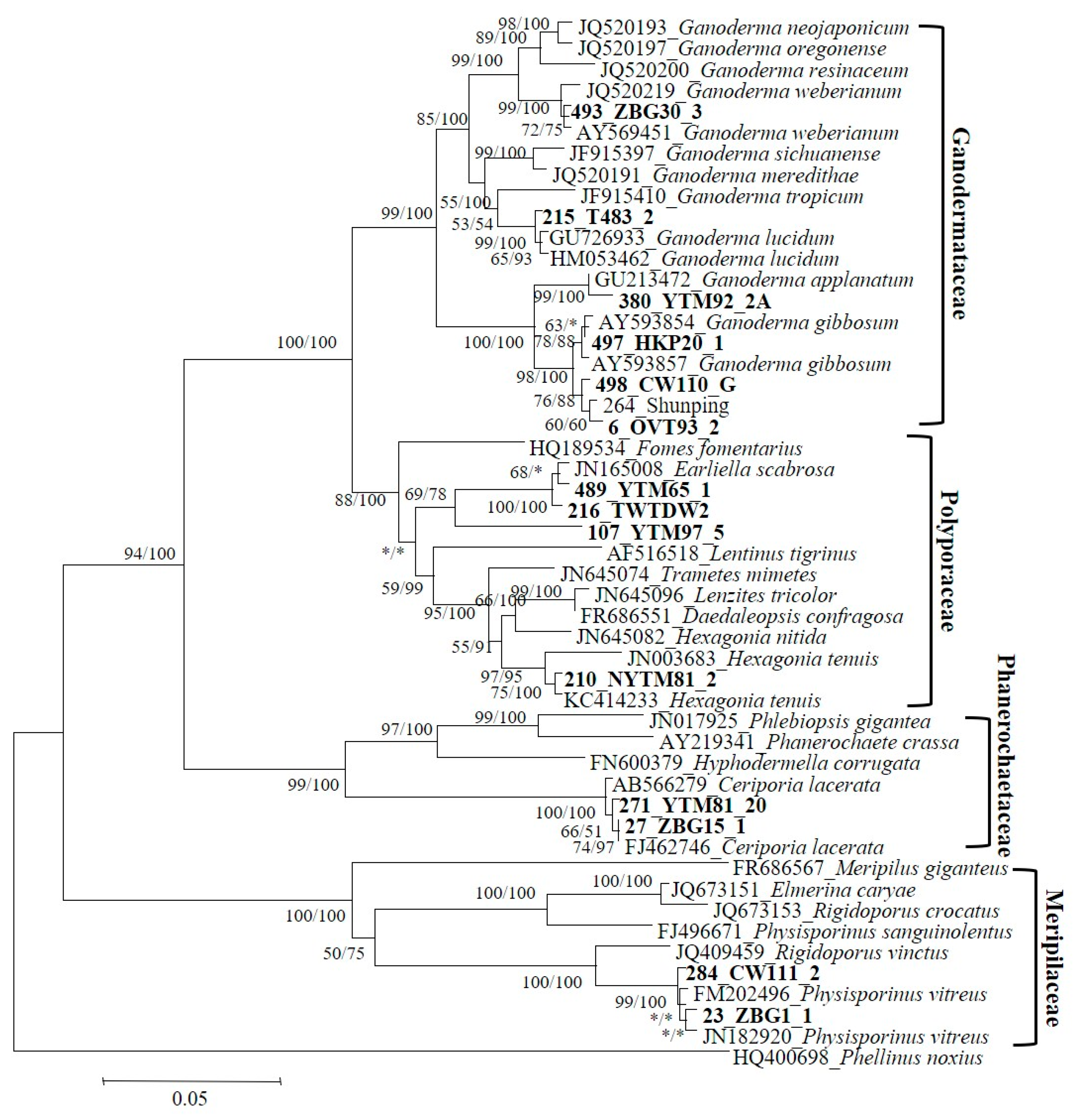
2.5. Phylogenetic and Statistical Analysis
2.6. Morphological Characters
3. Results
3.1. Diversity of Wood-Decay Fungi in Parks
3.2. Abundance and Distribution of Wood-Decay Fungi in Different Parks
3.3. The Colonization of Wood-Decay Fungi on Old Trees and Their Significance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, X.Z.; Gu, J.D. Uptake, metabolism, and toxicity of methyl tert-butyl ether (MTBE) in weeping willows. J. Hazard Mater. 2006, 137, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.I.; McPherson, E.G.; Siimpson, J.R. Air pollutant uptake by Sacrametos urban forest. J. Arboric. 1998, 24, 224–234. [Google Scholar]
- Yu, X.Z.; Gu, J.D. Metabolic responses of weeping willows to selenate and selenite. Environ. Sci. Pollut. Res. 2007, 14, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Alvey, A.A. Promoting and preserving biodiversity in the urban forest. Urban Urban Gree 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Zhang, H.; Jim, C.Y. Contributions of landscape trees in public housing estates to urban biodiversity in Hong Kong. Urban Urban Gree 2014, 13, 272–284. [Google Scholar] [CrossRef]
- McPherson, E.G.; Nowak, D.; Heisler, G.; Grimmond, S.; Souch, C.; Grant, R.; Rowntree, R. Quantifying urban forest structure, function, and value: The Chicago urban forest climate project. Urban Ecosyst. 1997, 1, 49–61. [Google Scholar] [CrossRef]
- Tyrvainen, L. Economic valuation of urban forest benefits in Finland. J. Environ. Manag. 2001, 62, 75–92. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hoehn, R.E.; Bodine, A.R.; Greenfield, E.J.; O’Neil-Dunne, J. Urban forest structure, ecosystem services and change in Syracuse, NY. Urban Ecosyst. 2016, 19, 1455–1477. [Google Scholar] [CrossRef]
- Blanchette, R.A. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 1991, 29, 381–398. [Google Scholar] [CrossRef]
- Newbound, M.; Mccarthy, M.A.; Lebel, T. Fungi and the urban environment: A review. Landsc. Urban Plan. 2010, 96, 138–145. [Google Scholar] [CrossRef]
- Tuor, U.; Winterhalter, K.; Fiechter, A. Enzymes of white-rot fungi involved in lignin degradation and ecological determinants for wood decay. J. Biotechnol. 1995, 41, 1–17. [Google Scholar] [CrossRef]
- Dai, Y.C. Illustrations of Pathogenic Wood-Decaying Fungi in China; Science Press: Beijing, China, 2005. [Google Scholar]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer: Berlin/Heidelberg, Germany; London, UK, 2000. [Google Scholar]
- Ding, S.; Hu, H.; Gu, J.D. Fungi colonizing wood sticks of Chinese fir incubated in subtropical urban soil growing with Ficus microcarpa trees. Int. J. Environ. Sci. Te 2015, 12, 3781–3790. [Google Scholar] [CrossRef] [Green Version]
- Ann, P.J.; Chang, T.T.; Ko, W.H. Phellinus noxius brown root rot of fruit and ornamental trees in Taiwan. Plant Dis. 2002, 86, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Terho, M. An assessment of decay among urban Tilia, Betula, and Acer trees felled as hazardous. Urban For. Urban Green. 2009, 8, 77–85. [Google Scholar] [CrossRef]
- Guglielmo, F.; Bergemann, S.E.; Gonthier, P.; Nicolotti, G.; Garbelotto, M. A multiplex PCR-based method for the detection and early identification of wood rotting fungi in standing trees. J. Appl. Microbiol. 2007, 103, 1490–1507. [Google Scholar] [CrossRef]
- Fukui, Y.; Miyamoto, T.; Tamai, Y.; Koizumi, A.; Yajima, T. Use of DNA sequence data to identify wood-decay fungi likely associated with stem failure caused by windthrow in urban trees during a typhoon. Trees Struct. Funct. 2018, 32, 1147–1156. [Google Scholar] [CrossRef]
- Hodges, C.S.; Tenorio, J.A. Root disease of Delonix regia and associated tree species in the Mariana Islands caused by Phellinus noxius. Plant Dis. 1984, 68, 334–336. [Google Scholar] [CrossRef]
- Griffiths, D.A. Fungi of Hong Kong; Government Printer: Hong Kong, China, 1977.
- Ho, W.H.; Hyde, K.D.; Hodgkiss, I.J. Seasonality and sequential occurrence of fungi on wood submerged in Tai Po Kau Forest Stream, Hong Kong. Fungal Divers. 2002, 10, 21–43. [Google Scholar]
- Zhou, D.Q.; Hyde, K.D. Fungal succession on bamboo in Hong Kong. Fungal Divers. 2002, 10, 213–227. [Google Scholar]
- Jim, C.Y.; Zhang, H. Species diversity and spatial differentiation of old-valuable trees in urban Hong Kong. Urban Urban Gree 2013, 12, 171–182. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.L.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Shinsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Window 95/98/NT. Nucleic Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal-W-Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004; Available online: http://www.ebc.uu.se/systzoo/staff/nylander.html (accessed on 1 September 2020).
- Bi, Z.; Zheng, G.; Li, T. The Macrofungus Flora of China’s Guangdong Province; Chinese University Press: Hong Kong, China, 1993. [Google Scholar]
- Dai, Y.C. Illustrations of Wood-Decaying fungi on Stored Wood or Structural Timber in China; Science Press: Beijing, China, 2009. [Google Scholar]
- Stancheva, Y. Atlas of Wood Decaying Fungi; Pensoft: Sofia, Bulgaria, 2009. [Google Scholar]
- Gadgil, P.D.; Dick, M.A.; Hood, I.A.; Pennycook, S.R. Fungi on trees and shrubs in New Zealand; Fungal Diversity Press: Hong Kong, China, 2005. [Google Scholar]
- Pong, V.M.; Zainal Abidin, M.A.; Almaliky, B.S.A.; Kadir, J.; Wong, M.Y. Isolation, fruiting and pathogenicity of Marasmiellus palmivorus (Sharples) Desjardin (comb. prov.) in oil palm plantations in West Malaysia. Trop. Agric. Sci. 2012, 35, 37–48. [Google Scholar]
- Fu, K.; Fu, S.Y.; Zhan, H.Y.; Zhou, P.D.; Liu, M.R.; Liu, H. A newly isolated wood-rot fungus for laccase production in submerged cultures. Bioresources 2013, 8, 1385–1397. [Google Scholar] [CrossRef]
- Dai, Y.C.; Cui, B.K.; Yuan, H.S.; Li, B.D. Pathogenic wood-decaying fungi in China. Forest Pathol. 2007, 37, 105–120. [Google Scholar] [CrossRef]
- Sahashi, N.; Akiba, M.; Ishihara, M.; Abe, Y.; Morita, S. First report of the brown root rot disease caused by Phellinus noxius, its distribution and newly recorded host plants in the Amami Islands, southern Japan. Forest Pathol. 2007, 37, 167–173. [Google Scholar] [CrossRef]
- Terho, M.; Hantula, J.; Hallaksela, A.M. Occurrence and decay patterns of common wood-decay fungi in hazardous trees felled in the Helsinki City. Forest Pathol. 2007, 37, 420–432. [Google Scholar] [CrossRef]
- Tomsovsky, M.; Vampola, P.; Sedlak, P.; Byrtusova, Z.; Jankovsky, L. Delimitation of central and northern European species of the Phellinus igniarius group (Basidiomycota, Hymenochaetales) based on analysis of ITS and translation elongation factor 1 alpha DNA sequences. Mycol. Prog. 2010, 9, 431–445. [Google Scholar] [CrossRef]
- Dai, Y.C. Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 2010, 45, 131–343. [Google Scholar] [CrossRef]
- Berrin, J.G.; Navarro, D.; Couturier, M.; Olive, C.; Grisel, S.; Haon, M.; Taussac, S.; Lechat, C.; Courtecuisse, R.; Favel, A.; et al. Exploring the natural fungal biodiversity of tropical and temperate forests toward improvement of biomass conversion. Appl. Environ. Microb. 2012, 78, 6483–6490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbett, D.S. A phylogenetic overview of the Agaricomycotina. Mycologia 2006, 98, 917–925. [Google Scholar] [CrossRef]
- Bolhassan, M.H.; Abdullah, N.; Sabaratnam, V.; Tsutomu, H.; Abdullah, S.; Rashid, N.M.N.; Musa, M.Y. Diversity and Distribution of Polyporales in Peninsular Malaysia. Sains Malays 2012, 41, 155–161. [Google Scholar]
- Sanchez-Lopez, M.I.; Vanhulle, S.F.; Mertens, V.; Guerra, G.; Figueroa, S.H.; Decock, C.; Corbisier, A.M.; Penninckx, M.J. Autochthonous white rot fungi from the tropical forest: Potential of Cuban strains for dyes and textile industrial effluents decolourisation. Afr. J. Biotechnol. 2008, 7, 1983–1990. [Google Scholar] [CrossRef] [Green Version]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. Forest Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Guglielmo, F.; Gonthier, P.; Garbelotto, M.; Nicolotti, G. Optimization of sampling procedures for DNA-based diagnosis of wood decay fungi in standing trees. Lett. Appl. Microbiol. 2010, 51, 90–97. [Google Scholar] [CrossRef]
- Park, J.H.; Pavlov, I.N.; Kim, M.J.; Park, M.S.; Oh, S.Y.; Park, K.H.; Fong, J.J.; Lim, Y.W. Investigating wood decaying fungi diversity in Central Siberia, Russia using ITS sequence analysis and interaction with host trees. Sustainability 2020, 12, 2532. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Meinholz, K.; Cleveland, K.; Jordan, S.A.; Gevens, A.J. Diversity and virulence of Alternaria spp. causing potato early blight and brown spot in Wisconsin. Phytopathology 2019, 109, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.L.; Huang, S.Y.; Huang, Y.C.; Tzean, S.S.; Ann, P.J.; Tsai, J.N.; Yang, C.C.; Lee, H.H.; Huang, T.W.; Huang, H.Y.; et al. The genetic structure of Phellinus noxius and dissemination pattern of brown root rot disease in Taiwan. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ann, P.J.; Lee, H.L.; Huang, T.C. Brown root rot of 10 species of fruit trees caused by Phellinus noxius in Taiwan. Plant Dis. 1999, 83, 746–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.T. Survival of Phellinus noxius in soil and in the roots of dead host plants. Phytopathology 1996, 86, 272–276. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Meng, H.; Gu, V.W.; Gu, J.-D. Molecular diagnosis of the brown root rot disease agent Phellinus noxius on trees and in soil by rDNA ITS analysis. Appl. Environ. Biotechnol. 2016, 1, 81–91. [Google Scholar] [CrossRef]
- Lodge, D.J. Factors related to diversity of decomposer fungi in tropical forests. Biodivers. Conserv. 1997, 6, 681–688. [Google Scholar] [CrossRef]
- Wardlaw, T.; Grove, S.; Hopkins, A.; Yee, M.; Harrison, K.; Mohammed, C. The uniqueness of habitats in old eucalypts: Contrasting wood-decay fungi and saproxylic beetles of young and old eucalypts. Tasforests 2009, 18, 17–32. [Google Scholar]
- Jang, Y.; Lee, S.W.; Jang, S.; Lim, J.W.; Lee, J.S.; Kim, J.J. Four unrecorded wood decay fungi from Seoul in Korea. Mycobiology 2012, 40, 195–201. [Google Scholar] [CrossRef] [Green Version]
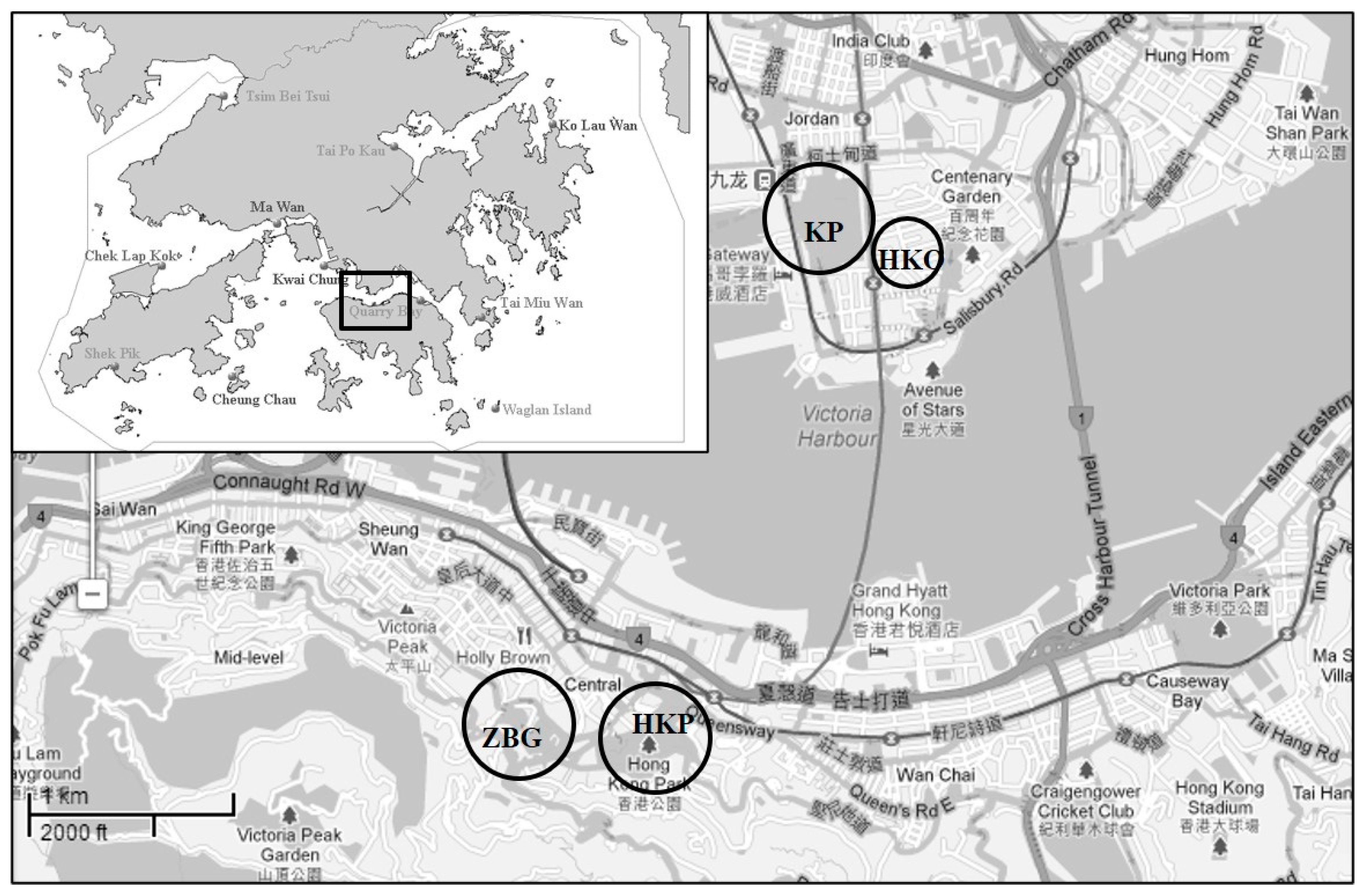




| Parks a | Number of OVTs | Area (Hectares) | Opening Year (Since) | Tree Species (Partial) | Location |
|---|---|---|---|---|---|
| HKP | 14 | 8 | 1991 | F. microcarpa, M. indica, B. ceiba, Ficus virens W.T. Aiton, Ficus elastic Roxb. ex Hornem, ect. | Central, north of Garden Road |
| ZBG | 25 | 5.6 | 1864 | F. microcarpa, Araucaria bidwillii Hook, Nauclea orientalis (L.) L., Sophora japónica (L.) Schott, Podocarpus neriifolius D. Don, ect. | Central, south of Garden Road |
| KP | 49 | 13.3 | 1970 | F. microcarpa, C. camphora, A. lebbeck, Cassia fistula L., C. sinensis, ect. | Tsim Sha Tsui, west of Nathan Road |
| HKO | 0 | 2.5 | Non-public | F. microcarpa, L. confertus, A. moluccana, A. lebbeck, C. camphora, etc. | Tsim Sha Tsui, east of Nathan Road |
| Fungal Species | Host Trees (Codes If Applicable and Location) | Notes |
|---|---|---|
| Auricularia polytricha (Mont.) Sacc. | F. microcarpa (HKP CW/110) wood stumps (ZBG, KP) | white rot fungus, saprotrophic; occur on the barks |
| Botryobasidium conspersum J. Erikss. | P. neriifolius (ZBG CW/69) | fruiting body occurs inside the hollow on the branch |
| Ceriporia lacerata N. Maek., Suhara & R. Kondo | Ficus variegata (Blume) (ZBG) F. microcarpa (KP YTM/81) | white rot fungus, causing stem and butt decay to broad-leaved trees |
| Cryptococcus heveanensis (Groen.) Baptist & Kurtzman | A. lebbeck (KP YTM/94) | occur on the wounded part |
| Earliella scabrosa(Pers.) Gilb. & Ryvarden | F. microcarpa (KP YTM/65) fallen branch (HKO) | white rot fungus, fruiting body occur on branch intersection; also saprophytically live on fallen branch |
| Fuscoporia senex (Nees & Mont.) Ghob.-Nejh | Syzygium samarangense (Blume) Merr. & L.M. Perry (ZBG) Sophora japonica (L.) Schott (ZBG CW/62) | white rot fungus, cause stem decay, discovered in the cavities on the tree trunk |
| Fomes sp. | F. microcarpa (KP YTM/97) | white rot fungus, cause heart wood-decay; fruiting body discovered on fallen branches, |
| Ganoderma applanatum (Pers.) Pat. | F. microcarpa (KP YTM/92) | white rot fungus, cause stem, root, and butt rot; fruiting body occur on wounded part, causing cavity on branch intersection |
| Ganoderma gibbosum (Nees) Pat. | F. microcarpa (HKP CW/110, CW/107; KP YTM/93, YTM/74; HKO) wood stump (HKP) | white rot fungus, fruiting body discovered both on standing trees and wood stumps, usually observed on wounded part of the trees, and cavities occur in most cases |
| Ganoderma lucidum (Curtis) P. Karst. | dead A. lebbeck (HKO) | white rot fungus, cause root rot, fruiting bodies occur around the dead tree |
| Ganoderma webrianum Bres. & Henn. | wood stump (ZBG) | white rot fungus, cause butt rot; fruiting bodies were detected when the tree was declined and the wood stump after the tree was cut down |
| Gymnopus gibbosus (Corner) A.W.Wilson, Desjardin & E.Horak | F. microcarpa (KP YTM/91) | the fruiting bodies were occurred in small clusters on the branch, and there was a noticeable hollow on the branch |
| Helicobasidium mompa Nobuj. Tanaka | F. microcarpa (KP) | on root |
| Hypoxylon fendleri Berk. ex Cooke | F. microcarpa (HKP CW/107) | around the tree |
| Hypoxylon sp. | F. microcarpa (HKO) | detected from culturing |
| Hypoxylon vinosopulvinatum Y.M. Ju, J.D. Rogers &H.M. Hsieh | F. microcarpa (KP YTM/81) | detected from culturing |
| Hexagonia tenuis (Hook.) Fr. | F. microcarpa (KP YTM/67 ect.) | white rot fungus, live on fallen branches, or dead part of the tree |
| Kretzschmaria deusta (Hoffm.) P.M.D. Martin | C. sinensis (KP) | White rot fungus, cause root decay |
| Kretzschmaria sp. | F. microcarpa (KP YTM/74) | white rot fungus, cause stem and root rot; patches of fruiting body occur on the trunk |
| Lopharia sp. | F. microcarpa (KP) | saprotroph, fruiting body occur on dead part of the tree |
| Marasmiellus palmivorus (Sharples) Desjardin (comb. prov.) | Asplenium nidus L. (ZBG) | can cause bunch rot on palm [35], discovered in rhizosphere soil of a palm |
| Phellinus noxius | F. microcarpa (HKP CW/110; KP YTM/65; HKO) L. confertus (HKO) A. moluccana (HKO) wood stumps (HKO) | white rot fungus, cause brown root rot disease |
| Physisporinus vitreus (Pers.) P. Karst. | M. indica (HKP CW/111) Lysidice rhodostegia Hance (ZBG CW/58) | white rot fungus, cause white rot and root decay |
| Psathyrella candolleana (Fr.) Maire | C. sinensis (KP YTM/69) | white rot fungus [36], fruiting bodies on the butt |
| Rigidoporus vinctus (Berk.) Ryvarden | Drypetes roxburghii (Wall.) Hurus (ZBG CW/67) | white rot fungus, inside and on the brim of the cavity on the tree trunk |
| Trametes hirsute (Wulfen) Lloyd | dead Paulownia fortunei (Seem.) Hemsl. (ZBG) dead wood (HKO) | white rot fungus, saprophyte |
| Xylaria escharoidea (Berk.) Fr. | F. microcarpa (HKP CW/107) | around the root |
| Xylogone sphaerospora Arx & T. Nilsson | A. lebbeck (KP YTM/94) | detected from culturing |
| Tree Species or Types | Wood-Decay Fungal Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. polytricha | C. lacerata | E. scabrosa | G. gibbosum | P. noxius | P. senex | P. vitreus | T. hirsuta | H. tenuis | G. lucidum | G. webrianum | K. deusta | |
| Ficus microcarpa | 1 | 1 | 1 | 5 | 3 | 2 | ||||||
| dead tree/wood stump | 2 | 1 | 3 | 2 | 1 | |||||||
| Aleurites moluccana | 4 | |||||||||||
| Celtis sinensis | 1 | |||||||||||
| Albizia lebbeck | 1 | |||||||||||
| Ficus variegata | 1 | |||||||||||
| fallen branch | 1 | |||||||||||
| Lophostemon confertus | 1 | |||||||||||
| shrub | 1 | |||||||||||
| Syzygium samarangense | 1 | |||||||||||
| Sophora japonica | 1 | |||||||||||
| Mangifera indica | 1 | |||||||||||
| Lysidice rhodostegia | 1 | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Hu, H.; Gu, J.-D. Diversity, Abundance, and Distribution of Wood-Decay Fungi in Major Parks of Hong Kong. Forests 2020, 11, 1030. https://doi.org/10.3390/f11101030
Ding S, Hu H, Gu J-D. Diversity, Abundance, and Distribution of Wood-Decay Fungi in Major Parks of Hong Kong. Forests. 2020; 11(10):1030. https://doi.org/10.3390/f11101030
Chicago/Turabian StyleDing, Shunping, Hongli Hu, and Ji-Dong Gu. 2020. "Diversity, Abundance, and Distribution of Wood-Decay Fungi in Major Parks of Hong Kong" Forests 11, no. 10: 1030. https://doi.org/10.3390/f11101030
APA StyleDing, S., Hu, H., & Gu, J.-D. (2020). Diversity, Abundance, and Distribution of Wood-Decay Fungi in Major Parks of Hong Kong. Forests, 11(10), 1030. https://doi.org/10.3390/f11101030





