A Spatial Relationship between Canopy and Understory Leaf Area Index in an Old-Growth Cool-Temperate Deciduous Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Leaf Area Index
3. Results
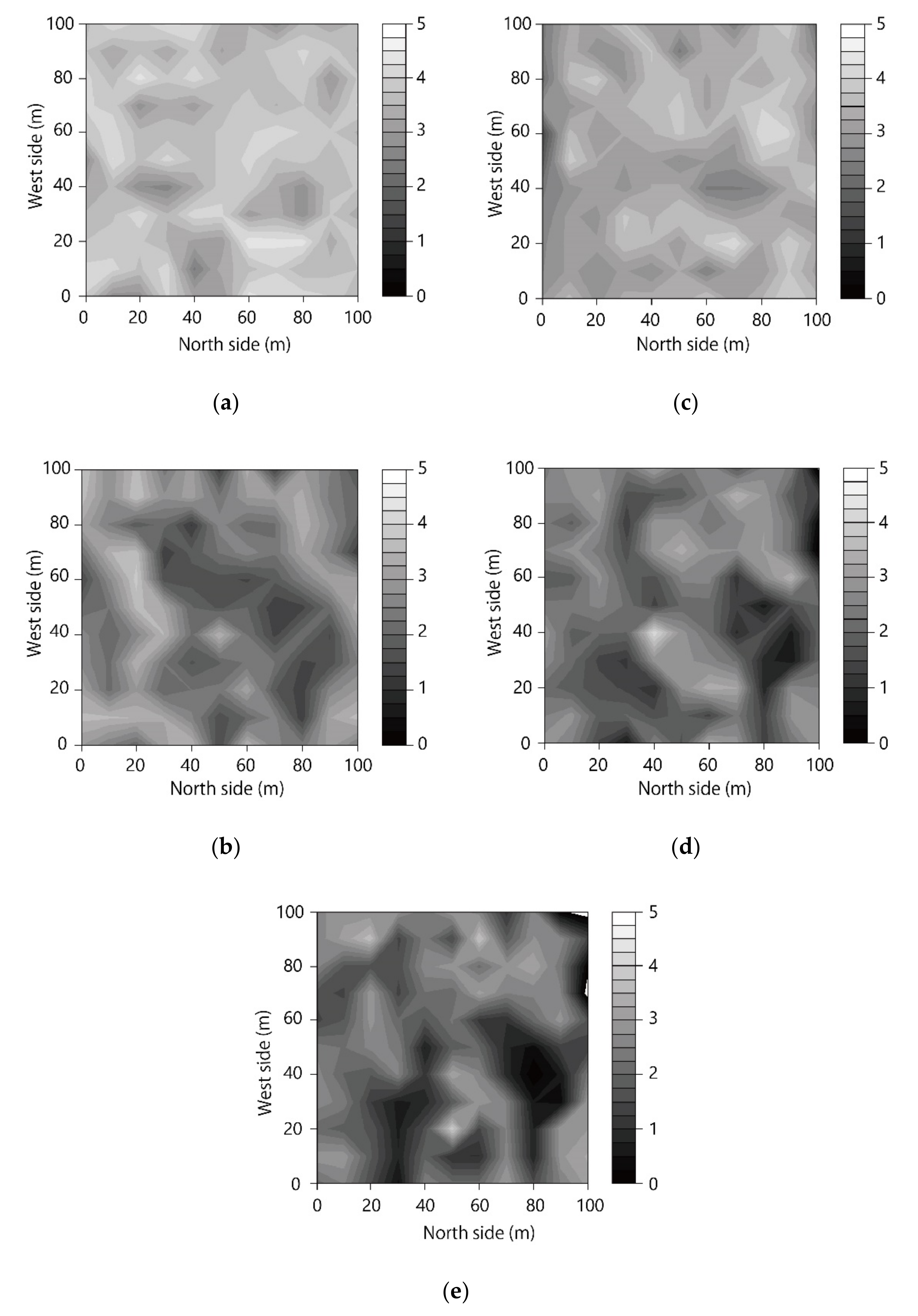
3.1. Spatial Distribution of LAI
3.2. The Relationship among Layers
4. Discussion
4.1. Spatial Distribution of LAI and Relationship among Layers
4.2. Rapid Changes in LAI Estimated from 2 Years of Measurements
4.3. Future Task
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.; Asner, J.M.O.S.; Hicke, J.A. Global synthesis of leaf area index observations: Implications for ecological and remote sensing studies. Glob. Ecol. Biogeogr. 2003, 12, 191–205. [Google Scholar]
- Nasahara, K.N.; Muraoka, H.; Nagai, S.; Mikami, H. Vertical integration of leaf area index in a Japanese deciduous broad-leaved forest. Agric. For. Meteorol. 2008, 148, 1136–1146. [Google Scholar] [CrossRef] [Green Version]
- Gough, C.M.; Hardiman, B.S.; Nave, L.E.; Bohrer, G.; Maurer, K.D.; Vogel, C.S.; Nadelhoffer, K.J.; Curtis, P.S. Sustained carbon uptake and storage following moderate disturbance in a Great Lakes forest. Ecol. Appl. 2013, 23, 1202–1215. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Bi, J.; Pan, Y.; Ganguly, S.; Anav, A.; Xu, L.; Samanta, A.; Piao, S.; Nemani, R.R.; Myneni, R.B. Global data sets of vegetation leaf area index (LAI)3g and fraction of photosynthetically active radiation (FPAR)3g derived from global inventory modeling and mapping studies (GIMMS) normalized difference vegetation index (NDVI3G) for the period 1981 to 2. Remote Sens. 2013, 5, 927–948. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Hu, R.; Luo, J.; Weiss, M.; Jiang, H.; Mu, X.; Xie, D.; Zhang, W. Review of indirect optical measurements of leaf area index: Recent advances, challenges, and perspectives. Agric. For. Meteorol. 2019, 265, 390–411. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Smith, G.J.; Jonckheere, I.; Coppin, P. Review of methods for in situ leaf area index (LAI) determination. Agric. For. Meteorol. 2004, 121, 37–53. [Google Scholar] [CrossRef]
- Bequet, R.; Campioli, M.; Kint, V.; Muys, B.; Bogaert, J.; Ceulemans, R. Spatial variability of leaf area index in homogeneous forests relates to local variation in tree characteristics. For. Sci. 2012, 58, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Naithani, K.J.; Baldwin, D.C.; Gaines, K.P.; Lin, H.; Eissenstat, D.M. Spatial Distribution of Tree Species Governs the Spatio-Temporal Interaction of Leaf Area Index and Soil Moisture across a Forested Landscape. PLoS ONE 2013, 8, e58704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xiang, W.; Pan, Q.; Zeng, Y.; Ouyang, S.; Lei, P.; Deng, X.; Fang, X.; Peng, C. Spatial and seasonal variations of leaf area index (LAI) in subtropical secondary forests related to floristic composition and stand characters. Biogeosciences 2016, 13, 3819–3831. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiang, F.; Zhu, Y.; Li, F.; Jin, G. Spatial heterogeneity of leaf area index in a temperate old-growth forest: Spatial autocorrelation dominates over biotic and abiotic factors. Sci. Total Environ. 2018, 634, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kume, A.; Nasahara, K.N.; Nagai, S.; Muraoka, H. The ratio of transmitted near-infrared radiation to photosynthetically active radiation (PAR) increases in proportion to the adsorbed PAR in the canopy. J. Plant Res. 2011, 124, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Moskal, L.M.; Kim, S.-H. Modeling approaches to estimate effective leaf area index from aerial discrete-return LIDAR. Agric. For. Meteorol. 2009, 149, 1152–1160. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Bohrer, G.; Gough, C.M.; Vogel, C.S.; Curtis, P.S. The role of canopy structural complexity in wood net primary production of a maturing northern deciduous forest. Ecology 2011, 92, 1818–1827. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Li, F.; Liu, Z.; Jin, G. Impact of understorey on overstorey leaf area index estimation from optical remote sensing in five forest types in northeastern China. Agric. For. Meteorol. 2014, 198–199, 72–80. [Google Scholar] [CrossRef]
- IDA, H. Snow depth in a cool-temperate deciduous forest. Unpublished work.
- Ishihara, M.I.; Suzuki, S.N.; Nakamura, M.; Enoki, T.; Fujiwara, A.; Hiura, T.; Hoshino, D.; Homma, K.; Hoshizaki, K.; Ida, H.; et al. Forest stand structure, composition, and dynamics in 34 sites over Japan. Ecol. Res. 2011, 26, 1007–1008. [Google Scholar] [CrossRef]
- IDA, H. Forest structure in a beech (Fagus crenata Blume) stand on a 1-ha permanent plot for the Monitoring Sites 1000 Project in Kayanodaira, central Japanese snowbelt. Bull. Inst. Nat. Educ. Shiga Height. Shinshu Univ. 2013, 50, 33–40. [Google Scholar]
- Melnikova, I.; Awaya, Y.; Saitoh, T.; Muraoka, H.; Sasai, T. Estimation of Leaf Area Index in a Mountain Forest of Central Japan with a 30-m Spatial Resolution Based on Landsat Operational Land Imager Imagery: An Application of a Simple Model for Seasonal Monitoring. Remote Sens. 2018, 10, 179. [Google Scholar] [CrossRef] [Green Version]
- Granier, A.; Bréda, N.; Longdoz, B.; Gross, P.; Ngao, J. Ten years of fluxes and stand growth in a young beech forest at Hesse, North-eastern France. Ann. For. Sci. 2008, 65, 704. [Google Scholar] [CrossRef]
- Ngao, J.; Epron, D.; Delpierre, N.; Bréda, N.; Granier, A.; Longdoz, B. Spatial variability of soil CO2 efflux linked to soil parameters and ecosystem characteristics in a temperate beech forest. Agric. For. Meteorol. 2012, 154–155, 136–146. [Google Scholar] [CrossRef]
- Černý, J.; Haninec, P.; Pokorný, R. Leaf area index estimated by direct, semi-direct, and indirect methods in European beech and sycamore maple stands. J. For. Res. 2020, 31, 827–836. [Google Scholar] [CrossRef]
- Glatthorn, J.; Pichler, V.; Hauck, M.; Leuschner, C. Effects of forest management on stand leaf area: Comparing beech production and primeval forests in Slovakia. For. Ecol. Manag. 2017, 389, 76–85. [Google Scholar] [CrossRef]
- Fischer, A.; Marshall, P.; Camp, A. Disturbances in deciduous temperate forest ecosystems of the northern hemisphere: Their effects on both recent and future forest development. Biodivers. Conserv. 2013, 22, 1863–1893. [Google Scholar] [CrossRef]
- Majasalmi, T.; Rautiainen, M. The impact of tree canopy structure on understory variation in a boreal forest. For. Ecol. Manag. 2020, 466, 118100. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Wirth, C.; Messier, C.; Bergeron, Y.; Frank, D.; Fankhänel, A. Old-Growth Forest Definitions: A Pragmatic View. In Old-Growth Forests; Springer: Berlin/Heidelberg, Germany, 2009; pp. 11–33. [Google Scholar]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415–416, 38–46. [Google Scholar] [CrossRef]

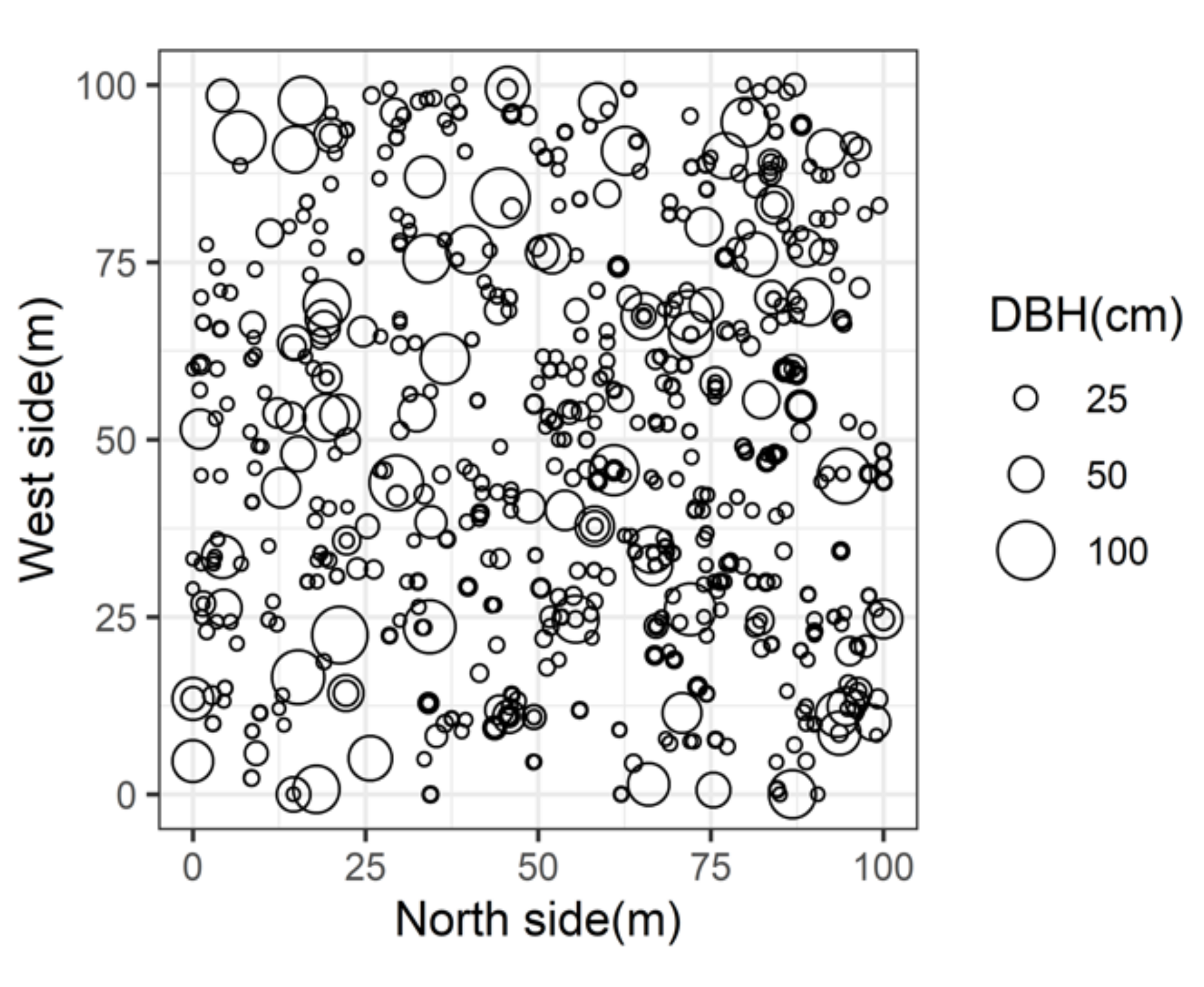


| Species | Trees (No/ha) | Basal Area 1 (m2/ha) | Relative Trees (%) | Relative Basal Area (%) | Average DBH 2 (cm) |
|---|---|---|---|---|---|
| Fagus crenata | 220 | 27.250 | 23.305 | 82.510 | 29.797 |
| Betula ermanii | 5 | 1.168 | 0.530 | 3.535 | 48.020 |
| Aesculus turbinata | 17 | 0.924 | 1.801 | 2.799 | 22.237 |
| Acer nipponicum | 183 | 0.914 | 19.386 | 2.767 | 7.612 |
| Hydrangea paniculata | 132 | 0.521 | 13.983 | 1.577 | 6.945 |
| Chengiopanax sciadophylloides | 22 | 0.502 | 2.331 | 1.519 | 15.066 |
| Acer japonicum | 74 | 0.435 | 7.839 | 1.316 | 8.182 |
| Viburnum furcatum | 87 | 0.266 | 9.216 | 0.804 | 6.166 |
| Phellodendron amurense | 10 | 0.189 | 1.059 | 0.573 | 13.920 |
| Sorbus commixta | 34 | 0.173 | 3.602 | 0.523 | 7.712 |
| Cornus controversa | 54 | 0.166 | 5.720 | 0.504 | 6.205 |
| Padus grayana | 21 | 0.100 | 2.225 | 0.303 | 7.618 |
| Acer pictum | 1 | 0.095 | 0.106 | 0.287 | 34.728 |
| Euonymus macropterus | 18 | 0.074 | 1.907 | 0.223 | 7.075 |
| Acer rufinerve | 11 | 0.069 | 1.165 | 0.210 | 8.826 |
| Corylus sieboldiana | 23 | 0.057 | 2.436 | 0.173 | 5.591 |
| Tilia japonica | 7 | 0.057 | 0.742 | 0.171 | 9.572 |
| Symplocos sawafutagi | 20 | 0.052 | 2.119 | 0.158 | 5.723 |
| Acer tschonoskii | 2 | 0.008 | 0.212 | 0.025 | 7.257 |
| Toxicodendron trichocarpum | 3 | 0.008 | 0.318 | 0.025 | 5.931 |
| Total | 944 | 33.027 |
| Year | Layer | Average (LAI) | SE 1 (LAI) | CV (LAI) |
|---|---|---|---|---|
| 2018 | LAI0 | 3.66 | 0.0386 | 11.6 |
| LAI2.5 | 2.41 | 0.0659 | 31.1 | |
| 2019 | LAI0 | 3.01 | 0.0428 | 15.7 |
| LAI2.5 | 2.17 | 0.0711 | 36.0 | |
| LAI5 | 2.00 | 0.0828 | 45.6 |
| Study | Site (Region) | Method or Tool | Time | LAI (±SE) | Dominant Species |
|---|---|---|---|---|---|
| This study | Kayanodaira (Nagano, Japan) | NIR/PAR ratio | August 2018 | 2.5 (±0.039) | Fagus crenata |
| This study | Kayanodaira (Nagano, Japan) | NIR/PAR ratio | August 2019 | 2.2 (±0.082) | Fagus crenata |
| Kume et al., 2011 [15] | Takayama (Gifu, Japan) | NIR/PAR ratio | June 2006 | 5.1 | Betula ermanii, Quercus crispula |
| Nasahara et al., 2008 [3] | Takayama (Gifu, Japan) | PAR transmittance | 2005~2006 | 5.1~5.9 | Betula ermanii, Quercus crispula |
| Nasahara et al., 2008 [3] | Takayama (Gifu, Japan) | Litter fall | 2005~2006 | 5.0 | Betula ermanii, Quercus crispula |
| Nasahara et al., 2008 [3] | Takayama (Gifu, Japan) | LAI-2000 1 | 2005~2006 | 3.0 | Betula ermanii, Quercus crispula |
| Melnikova et al., 2018 [22] | Takayama (Gifu, Japan) | PAR transmittance | May~August 2013 | 5.9 | Betula ermanii, Quercus crispula |
| Melnikova et al., 2018 [22] | Takayama (Gifu, Japan) | Litter fall | May~August 2013 | 5.0 | Betula ermanii, Quercus crispula |
| Melnikova et al., 2018 [22] | Takayama (Gifu, Japan) | remote sensing by satellite | May~August 2013 | 5.5 | Betula ermanii, Quercus crispula |
| Bequet et al., 2012 [11] | Flanders (Belgium) | Hemispherical photographs | August 2008 | 2.5~3.3 | Fagus sylvatica Quercus robur |
| Granier et al., 2008 [23] | Hesse forest (north-eastern France) | Litter fall | 1996~2005 | 4.6~7.2 | Fagus sylvatica |
| Ngao et al., 2011 [24] | Hesse forest (north-eastern France) | LAI-2000 1 | 2004 | 4~8.1 | Fagus sylvatica |
| Cerny et al., 2020 [25] | Training Forest Enterprise Masaryk Forest (Křtiny, Czech) | Litter fall | 2013 | 5.2~5.6 | Fagus sylvatica |
| Cerny et al., 2020 [25] | Training Forest Enterprise Masaryk Forest (Křtiny, Czech) | Needle Technique | 2013 | 3.4~6.0 | Fagus sylvatica |
| Cerny et al., 2020 [25] | Training Forest Enterprise Masaryk Forest (Křtiny, Czech) | LAI-2000 1 | 2013 | 4.5~5.1 | Fagus sylvatica |
| Glatthorn et al., 2018 [26] | eastern, Slovakia | LAI-2000 1 | 2013 | 6.2 (±0.39) | Fagus sylvatica |
| Glatthorn et al., 2018 [26] | eastern, Slovakia | Litter fall | 2013 | 8.5 (±0.54) | Fagus sylvatica |
| Asner et al., 2003 [2] | Various | Various | Various | 5.1 (±0.13) | Various |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanioka, Y.; Cai, Y.; Ida, H.; Hirota, M. A Spatial Relationship between Canopy and Understory Leaf Area Index in an Old-Growth Cool-Temperate Deciduous Forest. Forests 2020, 11, 1037. https://doi.org/10.3390/f11101037
Tanioka Y, Cai Y, Ida H, Hirota M. A Spatial Relationship between Canopy and Understory Leaf Area Index in an Old-Growth Cool-Temperate Deciduous Forest. Forests. 2020; 11(10):1037. https://doi.org/10.3390/f11101037
Chicago/Turabian StyleTanioka, Yosuke, Yihan Cai, Hideyuki Ida, and Mitsuru Hirota. 2020. "A Spatial Relationship between Canopy and Understory Leaf Area Index in an Old-Growth Cool-Temperate Deciduous Forest" Forests 11, no. 10: 1037. https://doi.org/10.3390/f11101037
APA StyleTanioka, Y., Cai, Y., Ida, H., & Hirota, M. (2020). A Spatial Relationship between Canopy and Understory Leaf Area Index in an Old-Growth Cool-Temperate Deciduous Forest. Forests, 11(10), 1037. https://doi.org/10.3390/f11101037






