Lateral Export of Dissolved Inorganic and Organic Carbon from a Small Mangrove Estuary with Tidal Fluctuation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Water Sampling and Analysis
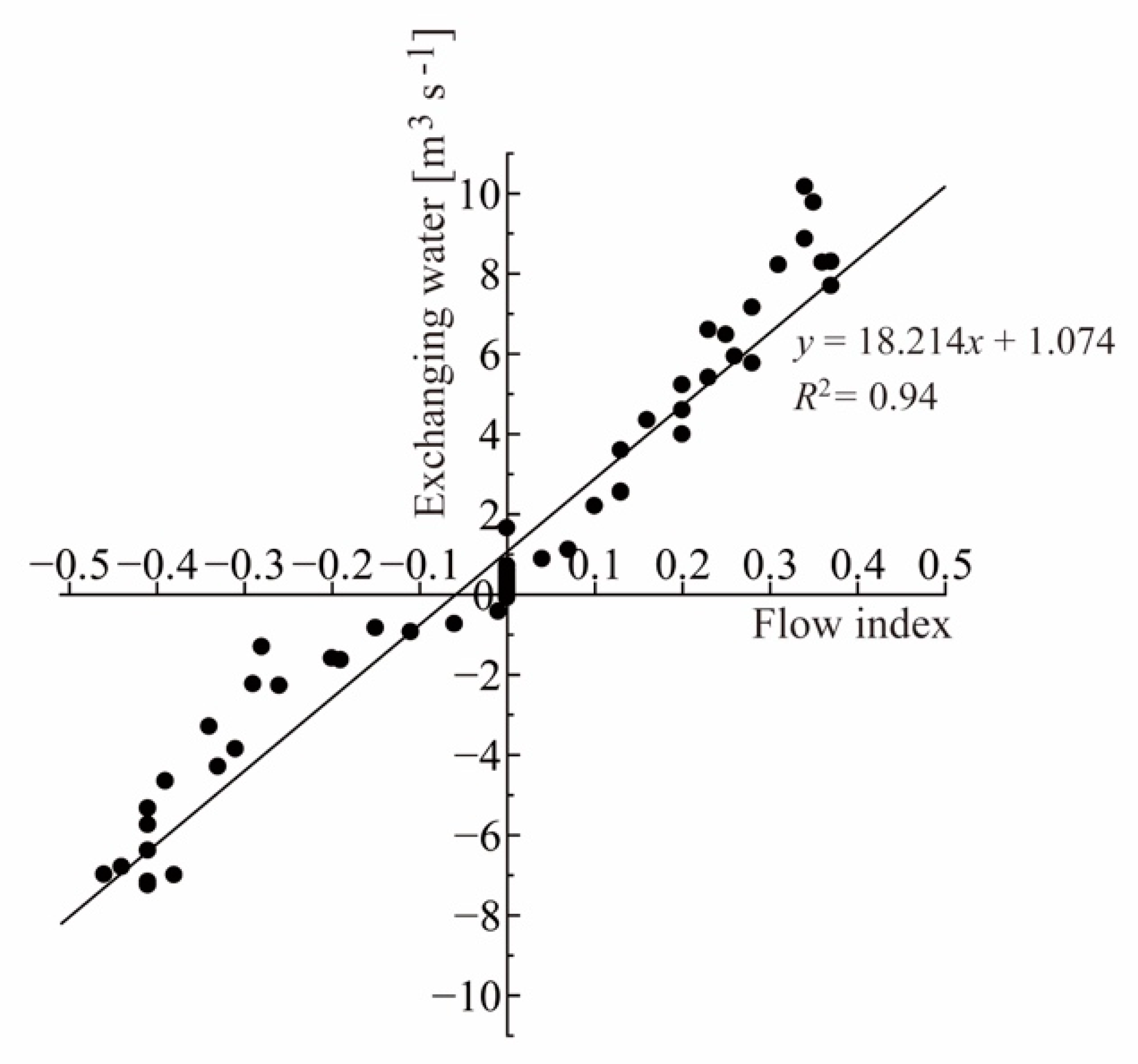
2.3. Estimation of the Water Flux at the River Mouth
2.4. Estimation of DIC and DOC Flux from the Mangrove Estuary
3. Results
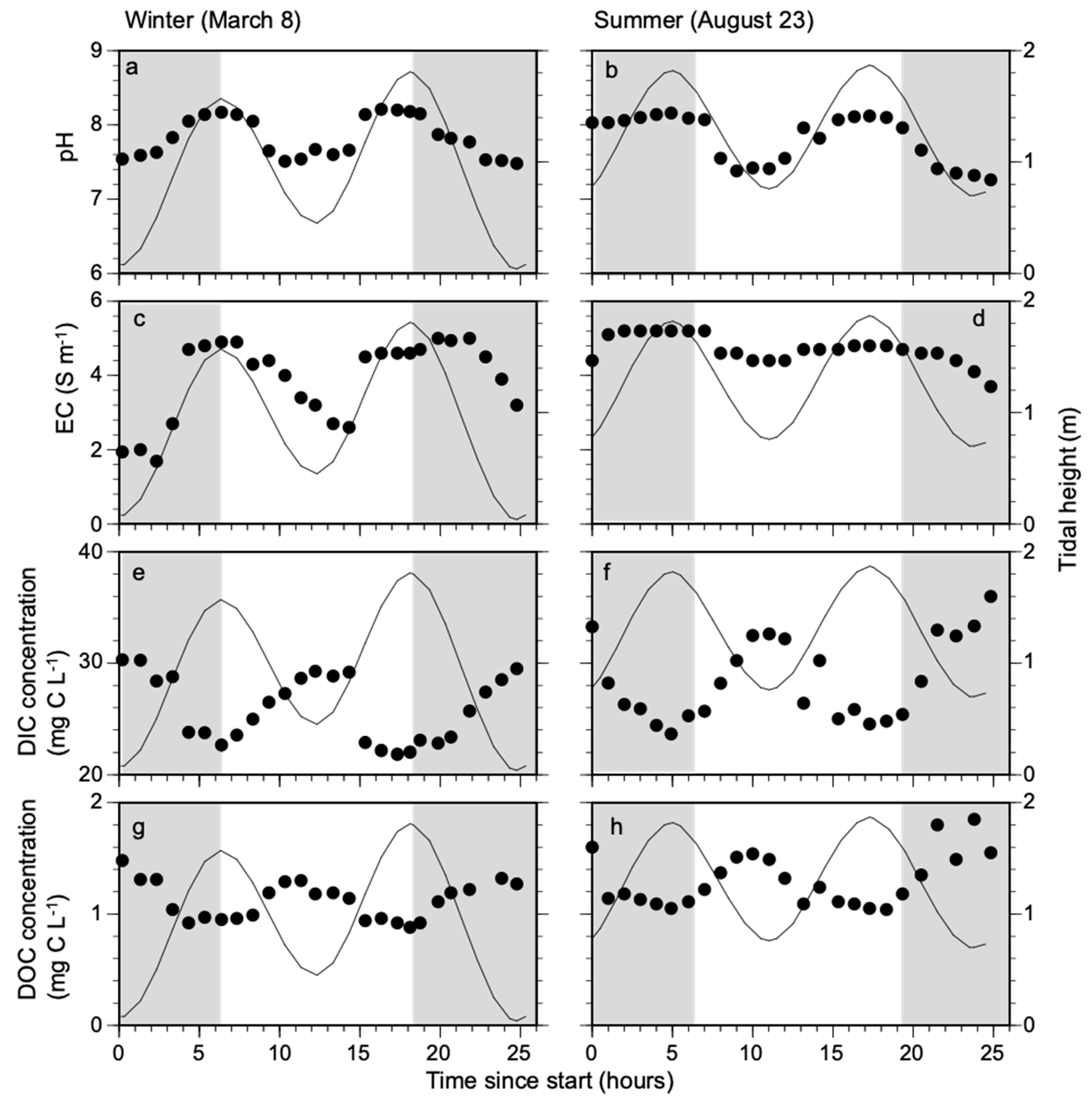
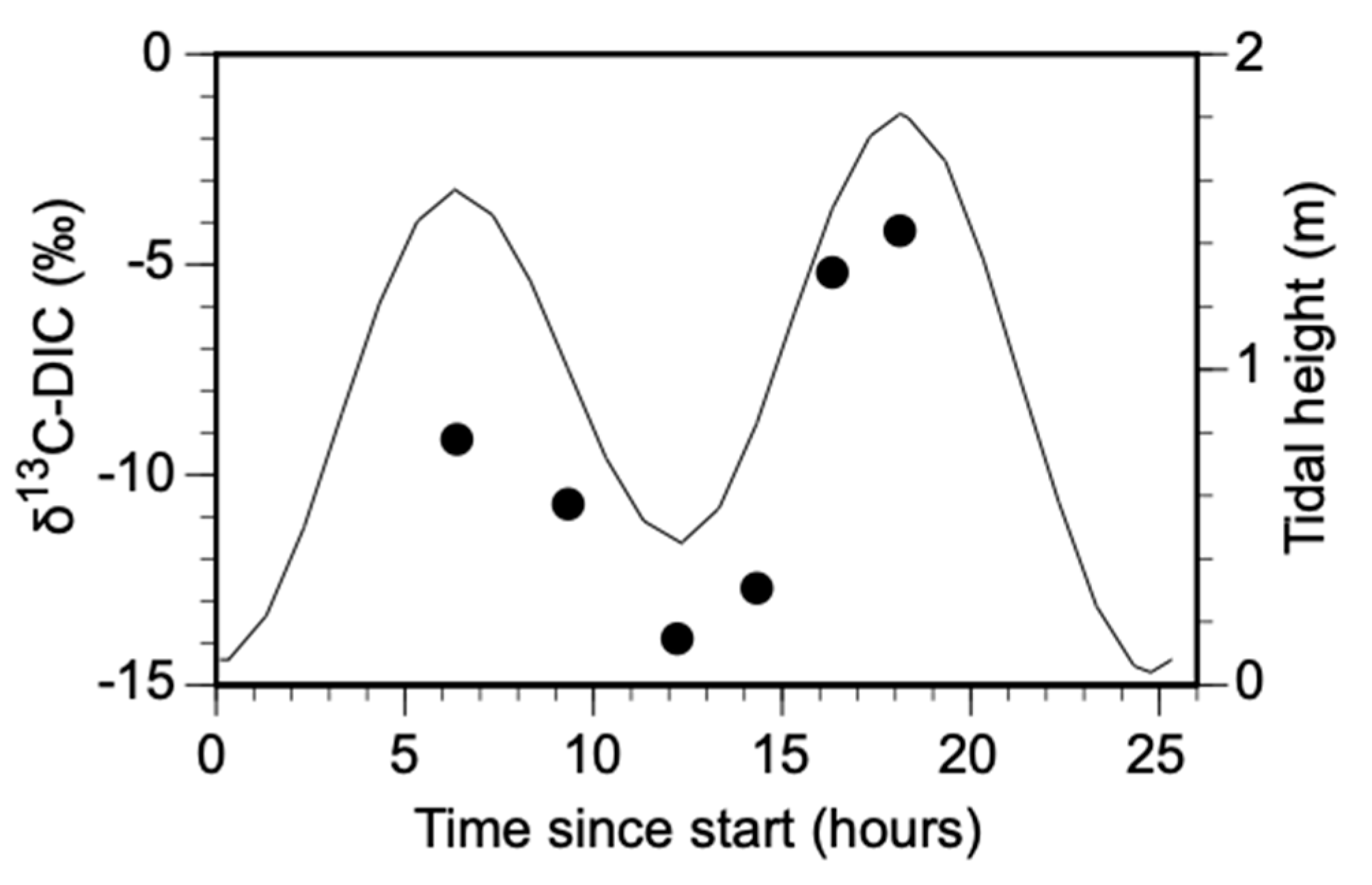
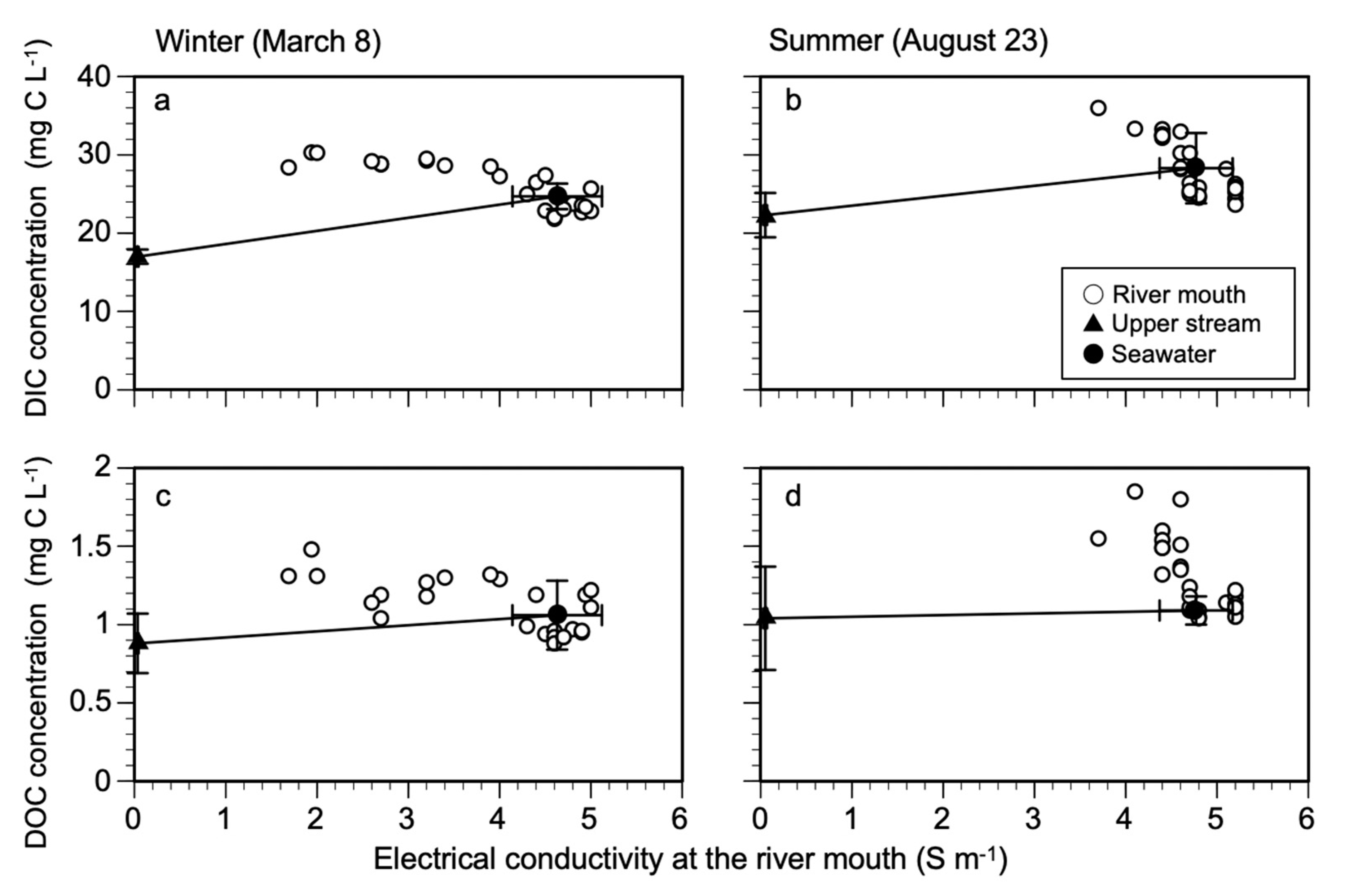
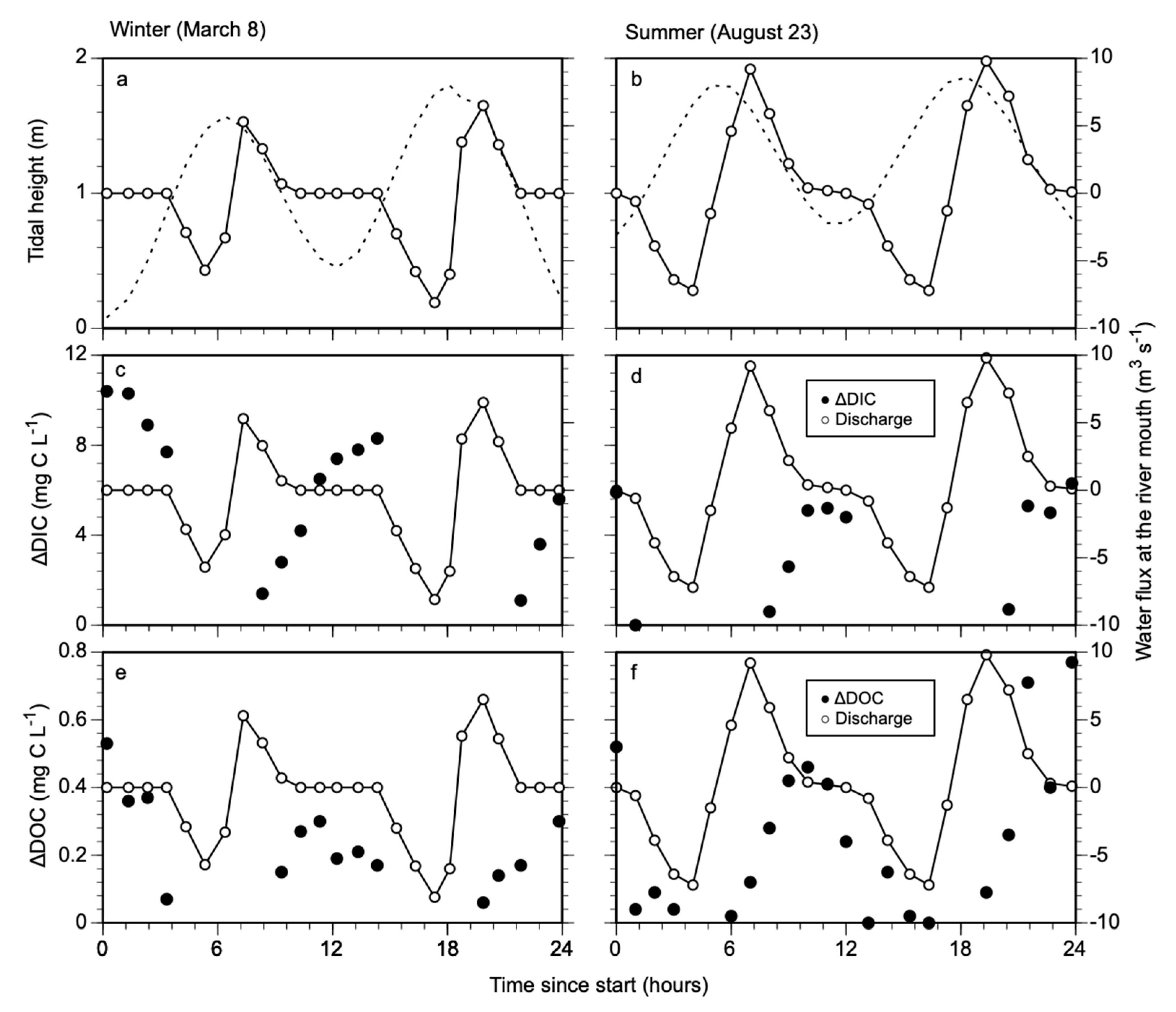
3.1. Changes in DIC and DOC Concentration with Tidal Amplitude
3.2. Daily Flux of Dissolved Carbon (DIC and DOC) from Mangrove
4. Discussion
4.1. Methods for Dissolved Carbon Export Estimates
4.2. Dissolved Carbon Flux with Tidal Amplitude
4.3. Mangrove Carbon Budget and Lateral C Fluxes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Ann. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Ewers Lewis, C.J.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Alongi, D.M. The Energetics of Mangrove Forests; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Barr, J.G.; Engel, V.; Fuentes, J.D.; Zieman, J.C.; O’Halloran, T.L.; Smith III, T.J.; Anderson, G.H. Controls on mangrove forest-atmosphere carbon dioxide exchanges in western Everglades National Park. J. Geophys. Res. 2010, 115, G02020. [Google Scholar] [CrossRef]
- Poungparn, S.; Komiyama, A.; Sangteian, T.; Maknual, C.; Patanaponpaiboon, P.; Suchewaboripont, V. High primary productivity under submerged soil raises the net ecosystem productivity of a secondary mangrove forest in eastern Thailand. J. Trop. Ecol. 2012, 28, 303–306. [Google Scholar] [CrossRef]
- Kato, T.; Tang, Y. Spatial variability and major controlling factors of CO2 sink strength in Asian terrestrial ecosystems: Evidence from eddy covariance data. Glob. Chang. Biol. 2008, 14, 2333–2348. [Google Scholar] [CrossRef]
- Odum, W.E.; Heald, E.J. The Detritus-Based Food Web of an Estuarine Mangrove Community; Cronin, L.E., Ed.; Estuarine Research; Academic Press: New York, NY, USA, 1975; pp. 265–286. [Google Scholar]
- Adame, M.F.; Lovelock, C.E. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 2007, 4, 311–322. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Oceanogr. 2013, 58, 475–488. [Google Scholar] [CrossRef]
- Taillardat, P.; Ziegler, A.D.; Friess, D.A.; Widory, D.; Van, V.T.; David, F.; Nguyen, T.N.; Marchand, C. Carbon dynamics and inconstant porewater input in a mangrove tidal creek over contrasting seasons and tidal amplitudes. Geochem. Cosmochim. Acta 2018, 237, 32–48. [Google Scholar] [CrossRef]
- Ray, R.; Michaud, E.; Aller, R.C.; Vantrepotte, V.; Gleixner, G.; Walcker, R.; Devesa, J.; le Goff, M.; Morvan, S.; Thouzeau, G. The sources and distribution of carbon (DOC, POC, DIC) in a mangrove dominated estuary (French Guiana, South America). Biogeochemistry 2018, 138, 297–321. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Yu, D.; Tam, N.F.Y.; Ye, Y.; Chen, S. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; Mckee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Cameron, C.; Hutley, L.B.; Friess, D.A. Estimating the full greenhouse gas emissions offset potential and profile between rehabilitating and established mangroves. Sci. Total Environ. 2019, 665, 419–431. [Google Scholar] [CrossRef]
- Miyajima, T.; Tsuboi, Y.; Tanaka, Y.; Koike, I. Export of inorganic carbon from two Southeast Asian Mangrove forests to adjacent estuaries as estimated by the stable isotope composition of dissolved inorganic carbon. J. Geophys. Res. Biogeosci. 2009, 114, 1–12. [Google Scholar] [CrossRef]
- Sippo, J.Z.; Maher, D.T.; Tait, D.R.; Holloway, C.; Santos, I.R. Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal transect. Glob. Biogeochem. Cycles 2016, 30, 753–766. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tomotsune, M.; Suchewaboripont, V.; Iimura, Y.; Kida, M.; Yoshitake, S.; Kondo, M.; Kinjo, K. Stand dynamics and aboveground net primary productivity of a mature subtropical mangrove forest on Ishigaki Island, South-Western Japan. Reg. Stud. Mar. Sci. 2019, 27, 100516. [Google Scholar] [CrossRef]
- Sharma, S.; Yasuoka, J.; Nakamura, T.; Watanabe, A.; Nadaoka, K. The role of hydroperiod, soil moisture and distance from the river mouth on soil organic matter in Fukido mangrove forest. In Proceedings of the International Conference on Advances in Applied Science and Environmental Engineering–ASEE, Kuala Lumpur, Malaysia, 2–3 August 2014; pp. 44–48, ISBN 978-1-63248-004-0. [Google Scholar] [CrossRef]
- Tomotsune, M.; Yoshitake, S.; Iimura, Y.; Kida, M.; Fujitake, N.; Koizumi, H.; Ohtsuka, T. Effects of soil temperature and tidal condition on variation in carbon dioxide flux from soil sediment in a Subtropical mangrove forest. J. Trop. Ecol. 2018, 34, 268–275. [Google Scholar] [CrossRef]
- Kida, M.; Tanabe, M.; Tomotsune, M.; Yoshitake, S.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. Changes in dissolved organic matter composition and dynamics in a subtropical mangrove river driven by rainfall. Estuar. Coast. Shelf Sci. 2019, 223, 6–17. [Google Scholar] [CrossRef]
- Kida, M.; Kondo, M.; Tomotsune, M.; Kinjo, K.; Ohtsuka, T.; Fujitake, N. Molecular composition and decomposition stages of organic matter in a mangrove mineral soil with time. Estuar. Coast. Shelf Sci. 2019, 231, 106478. [Google Scholar] [CrossRef]
- Iimura, Y.; Kinjo, K.; Kondo, M.; Ohtsuka, T. Soil carbon stocks and their primary origin at mature mangrove ecosystems in the estuary of Fukido River, Ishigaki Island, southwestern Japan. Soil Sci. Plant Nutr. 2019, 65, 435–443. [Google Scholar] [CrossRef]
- Ho, D.T.; Ferrón, S.; Engel, V.C.; Anderson, W.T.; Swart, P.K.; Price, R.M.; Barbero, L. Dissolved carbon biogeochemistry and export in mangrove-dominated rivers of the Florida Everglades. Biogeosciences 2017, 14, 2543–2559. [Google Scholar] [CrossRef]
- Li, S.B.; Chen, P.H.; Huang, J.S.; Hsueh, M.L.; Hsieh, L.Y.; Lee, C.L.; Lin, H.J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef] [PubMed]
- Dausse, A.; Garbutt, A.; Norman, L.; Papadimitriou, S.; Jones, L.M.; Robins, P.E.; Thomas, D.N. Biogeochemical functioning of grazed estuarine tidal marshes along a salinity gradient. Estuar. Coast. Shelf Sci. 2012, 100, 83–92. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.; Eyre, B.D. Seasonal and temporal CO2 dynamics in three tropical mangrove creeks—A revision of global mangrove CO2 emissions. Geochem. Cosmochim. Acta 2018, 222, 729–745. [Google Scholar] [CrossRef]
- Call, M.; Sanders, C.J.; Macklin, P.A.; Santos, I.R.; Maher, D.T. Carbon outwelling and emissions from two contrasting mangrove creeks during the monsoon storm season in Palau, Micronesia. Estuar. Coast. Shelf Sci. 2019, 218, 340–348. [Google Scholar] [CrossRef]
- Ray, R.; Thouzeau, G.; Walcker, R.; Vantrepotte, V.; Gleixner, G.; Morvan, S.; Devesa, J.; Michaud, E. Mangrove-derived organic and inorganic carbon exchanges between the Sinnamary estuarine system (French Guiana, South America) and Atlantic Ocean. JGR Biogeosci. 2020, 125. [Google Scholar] [CrossRef]
- Stigelitz, T.C.; Clark, J.F.; Hancock, G.J. The mangrove pump: The tidal flushing of animal burrows in a tropical mangrove forest determined from radionuclide budgets. Geochem. Cosmochim. Acta 2013, 102, 12–22. [Google Scholar] [CrossRef]
- Webb, J.; Santos, I.R.; Maher, D.T.; Finlay, K. The importance of aquatic carbon fluxed in net ecosystem carbon budgets: A catchment-scale review. Ecosystems 2019, 22, 508–527. [Google Scholar] [CrossRef]
- Poungparn, S.; Charoenphonphakdi, T.; Sangtiean, T.; Patanaponpaiboon, P. Fine root production in three zones of secondary mangrove forest in eastern Thailand. Trees 2015, 30, 467–474. [Google Scholar] [CrossRef]
- Robertson, A.I.; Alongi, D.M. Massive turnover rates of fine root detrital carbon in tropical Australian mangroves. Oecologia 2016, 180, 841–851. [Google Scholar] [CrossRef] [PubMed]

| Sample | EC (S m−1) | pH | DIC (mg C L−1) | DOC (mg C L−1) |
|---|---|---|---|---|
| 8 March 2016 | ||||
| Marine | 4.63 ± 0.49 | 8.06 ± 0.42 | 24.7 ± 1.63 | 1.06 ± 0.22 |
| Riverine | 0.036 ± 0.010 | 7.85 ± 0.21 | 17.0 ± 0.91 | 0.88 ± 0.19 |
| 23 August 2016 | ||||
| Marine | 4.77 ± 0.40 | 7.50 ± 0.77 | 28.3 ± 4.49 | 1.09 ± 0.09 |
| Riverine | 0.052 ± 0.020 | 7.24 ± 0.31 | 22.3 ± 2.83 | 1.04 ± 0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtsuka, T.; Onishi, T.; Yoshitake, S.; Tomotsune, M.; Kida, M.; Iimura, Y.; Kondo, M.; Suchewaboripont, V.; Cao, R.; Kinjo, K.; et al. Lateral Export of Dissolved Inorganic and Organic Carbon from a Small Mangrove Estuary with Tidal Fluctuation. Forests 2020, 11, 1041. https://doi.org/10.3390/f11101041
Ohtsuka T, Onishi T, Yoshitake S, Tomotsune M, Kida M, Iimura Y, Kondo M, Suchewaboripont V, Cao R, Kinjo K, et al. Lateral Export of Dissolved Inorganic and Organic Carbon from a Small Mangrove Estuary with Tidal Fluctuation. Forests. 2020; 11(10):1041. https://doi.org/10.3390/f11101041
Chicago/Turabian StyleOhtsuka, Toshiyuki, Takeo Onishi, Shinpei Yoshitake, Mitsutoshi Tomotsune, Morimaru Kida, Yasuo Iimura, Miyuki Kondo, Vilanee Suchewaboripont, Ruoming Cao, Kazutoshi Kinjo, and et al. 2020. "Lateral Export of Dissolved Inorganic and Organic Carbon from a Small Mangrove Estuary with Tidal Fluctuation" Forests 11, no. 10: 1041. https://doi.org/10.3390/f11101041
APA StyleOhtsuka, T., Onishi, T., Yoshitake, S., Tomotsune, M., Kida, M., Iimura, Y., Kondo, M., Suchewaboripont, V., Cao, R., Kinjo, K., & Fujitake, N. (2020). Lateral Export of Dissolved Inorganic and Organic Carbon from a Small Mangrove Estuary with Tidal Fluctuation. Forests, 11(10), 1041. https://doi.org/10.3390/f11101041






