Comparison of Two Sampling Methods to Estimate the Abundance of Lucanus cervus with Application of n-Mixture Models
Abstract
:1. Introduction
2. Materials and Methods
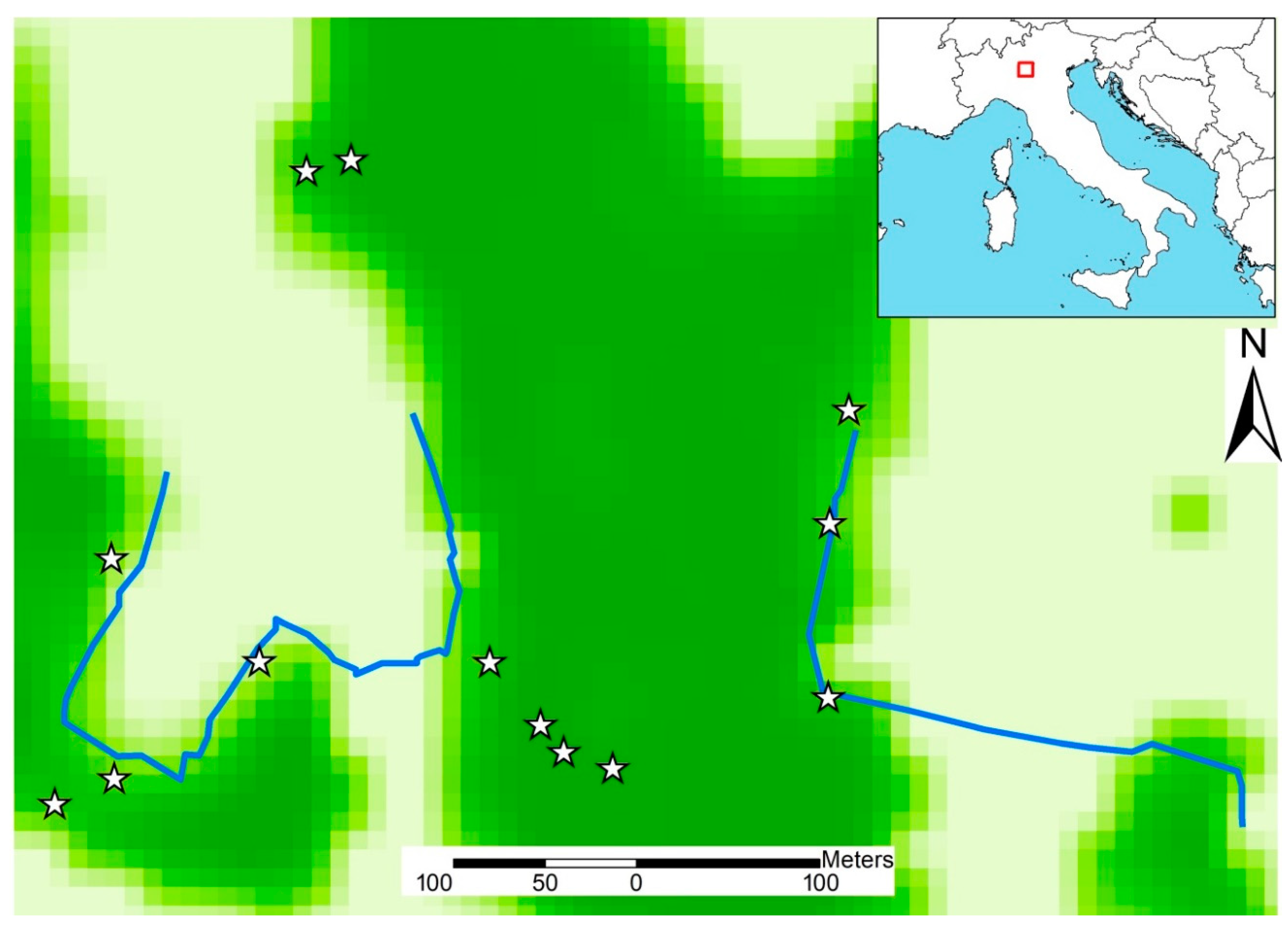
2.1. Study Area
2.2. Study Species and Data Collection
2.3. Covariates of Detection and Abundance
2.4. Modelling Procedures
3. Results
3.1. Covariates Effects on Detection and Abundance
3.2. Estimates of Detection and Abundance
4. Discussion
4.1. Effectiveness of Transect Walks and Tree Surveys for Estimating L. cervus Abundance
4.2. Pros and Cons of Implementing Transect Walks and Tree Surveys for L. cervus Abundance
4.3. N-Mixture Models to Estimate Lucanus cervus Abundance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Medeiros, M.J.; Eiben, J.A.; Haines, W.P.; Kaholoaa, R.L.; King, C.B.A.; Krushelnycky, P.D.; Magnacca, K.N.; Rubinoff, D.; Starr, F.; Starr, K. The importance of insect monitoring to conservation actions in Hawaii. Proc. Hawaii Entomol. Soc. 2013, 45, 149–166. [Google Scholar]
- Yang, L.H.; Gratton, C. Insects as drivers of ecosystem processes. Curr. Opin. Insect Sci. 2014, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speight, M.C.D. Saproxylic invertebrates and their conservation. In Nature and Environment Series; Council of Europe: Strasbourg, France, 1989; Volume 42, p. 81. [Google Scholar]
- Alexander, K. Tree biology and saproxylic coleoptera: Issues of definitions and conservation language. Rev. Ecol. Terre Vie 2008, 63, 1–7. [Google Scholar]
- Cavalli, R.; Mason, F. Techniques for re-establishment of dead wood for saproxylic fauna conservation. In LIFE Nature Project NAT/IT/99/6245 Bosco Della Fontana (Mantova, Italy); Gianluigi Arcari Editore: Mantova, Italy, 2003; Volume 112. [Google Scholar]
- Carpaneto, G.M.; Mazziotta, A.; Coletti, G.; Luiselli, L.; Audisio, P. Conflict between insect conservation and public safety: The case study of a saproxylic beetle (Osmoderma eremita) in urban parks. J. Insect Conserv. 2010, 14, 555–565. [Google Scholar] [CrossRef]
- Bütler, R.; Lachat, T.; Larrieu, L.; Paillet, Y. Habitat trees: Key elements for forest biodiversity. In Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; Kraus, D., Krumm, F., Eds.; European Forest Institute: Joensuu, Finland, 2013; pp. 84–91. [Google Scholar]
- Cálix, M.; Alexander, K.N.A.; Nieto, A.; Dodelin, B.; Soldati, F.; Telnov, D.; Vazquez-Albalate, X.; Aleksandrowicz, O.; Audisio, P.; Istrate, P.; et al. European Red List of Saproxylic Beetles; IUCN: Brussels, Belgium, 2018; p. 13. [Google Scholar]
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Müller, J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conser. Biol. 2015, 29, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, G.M.; Campanaro, A.; Hardersen, S.; Audisio, P.; Bologna, M.A.; Roversi, P.F.; Sabbatini Peverieri, G.; Mason, F. The LIFE Project “Monitoring of insects with public participation” (MIPP): Aims, methods and conclusions. Nat. Conserv. 2017, 20, 1–35. [Google Scholar] [CrossRef]
- Bardiani, M.; Chiari, S.; Maurizi, E.; Tini, M.; Toni, I.; Zauli, A.; Campanaro, A.; Carpaneto, G.M.; Audisio, P. Guidelines for the monitoring of Lucanus cervus. Nat. Conserv. 2017, 20, 37–78. [Google Scholar] [CrossRef]
- Campanaro, A.; Hardersen, S.; Redolfi De Zan, L.; Antonini, G.; Bardiani, M.; Maura, M.; Maurizi, E.; Mosconi, F.; Zauli, A.; Bologna, M.A.; et al. Analyses of occurrence data of protected insect species collected by citizens in Italy. Nat. Conserv. 2017, 20, 265–297. [Google Scholar] [CrossRef] [Green Version]
- Maurizi, E.; Campanaro, A.; Chiari, S.; Maura, M.; Mosconi, F.; Sabatelli, S.; Zauli, A.; Audisio, P.; Carpaneto, G.M. Guidelines for the monitoring of Osmoderma eremita and closely related species. Nat. Conserv. 2017, 20, 79–128. [Google Scholar] [CrossRef] [Green Version]
- Redolfi De Zan, L.; Bardiani, M.; Antonini, G.; Campanaro, A.; Chiari, S.; Mancini, E.; Maura, M.; Sabatelli, S.; Solano, E.; Zauli, A.; et al. Guidelines for the monitoring of Cerambyx cerdo. Nat. Conserv. 2017, 20, 129–164. [Google Scholar] [CrossRef] [Green Version]
- Thomaes, A.; Verschelde1, P.; Mader, D.; Sprecher-Uebersax, E.; Fremlin, M.; Onkelinx, T.; Méndez, M. Can we successfully monitor a population density decline of elusive invertebrates? A statistical power analysis on Lucanus cervus. Nat. Conserv. 2017, 19, 118. [Google Scholar] [CrossRef]
- Kull, T.; Sammul, M.; Kull, K.; Lanno, K.; Tali, K.; Gruber, B.; Schmeller, D.; Henle, K. Necessity and reality of monitoring threatened European vascular plants. Biodivers. Conserv. 2008, 17, 3383–3402. [Google Scholar] [CrossRef]
- Chiari, S.; Zauli, A.; Audisio, P.; Campanaro, A.; Donzelli, P.F.; Romiti, F.; Svensson, G.P.; Tini, M.; Carpaneto, G.M. Monitoring presence abundance and survival probability of the stag beetle Lucanus cervus using visual and odour-based capture methods: Implications for conservation. J. Insect Conserv. 2014, 1, 99–109. [Google Scholar] [CrossRef]
- Nachman, G.; Skovgård, H. A simulation study of how simple mark-recapture methods can be combined with destructive subsampling to facilitate surveys of flying insects. Environ. Entomol. 2012, 41, 141–151. [Google Scholar] [CrossRef]
- Royle, J.A.; Nichols, J.D. Estimating abundance from repeated presence-absence data or point counts. Ecology 2003, 84, 777–790. [Google Scholar] [CrossRef]
- Royle, J.A. N-mixture models for estimating population size from spatially replicated counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. UNMARKED: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.; Hines, J. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier: San Diego, CA, USA, 2006; p. 324. [Google Scholar]
- Knaus, P.; Antoniazza, S.; Wechsler, S.; Guélat, J.; Kéry, M.; Strebel, N.; Sattler, T. Distribution and population trends of birds in Switzerland and Liechtenstein. In Swiss Breeding Bird Atlas 2013–2016; Swiss Ornithological Institute: Sempach, Switzerland, 2018. [Google Scholar]
- Prandelli, A. Inquadramento Dendrometrico-Strutturale di un Bosco Planiziale in Località Capriano del Colle (BS). Master’s Thesis, University of Padua, Padua, Italy, 2005; p. 97. [Google Scholar]
- Audisio, P.; Baviera, C.; Carpaneto, G.M.; Biscaccianti, A.B.; Battistoni, A.; Teofili, C.; Rondinini, C. Lista Rossa IUCN dei Coleotteri Saproxilici Italiani; Comitato Italiano IUCN e Ministero Dell’ambiente e Della Tutela del Territorio e del Mare: Roma, Italy, 2014. [Google Scholar]
- Hawes, C.J. The Stag Beetle Lucanus cervus (L.) (Coleoptera: Lucanidae) in the County of Suffolk (England): Distribution and monitoring. In Proceedings of the 3rd Symposium and Workshop on the Conservation of Saproxylic Beetles, Riga, Latvia, 7–11 July 2004; pp. 51–67. [Google Scholar]
- Harvey, D.J.; Gange, A.C.; Hawes, C.J.; Rink, M.; Abdehalden, M.; Al-Fulaij, N.; Asp, T.; Ballerio, A.; Bartolozzi, L.; Brustel, H.; et al. Bionomics and distribution of the stag beetle Lucanus cervus across Europe. Insect Conserv. Diver. 2011, 4, 23–38. [Google Scholar] [CrossRef]
- Campanaro, A.; Bardiani, M.; Spada, L.; Carnevali, L.; Montalto, F.; Antonini, G.; Mason, F.; Audisio, P. Linee guida per il monitoraggio e la conservazione dell’entomofauna saproxilica. In Quaderni Conservazione Habitat 6; Cierre Grafica: Verona, Italy, 2011; pp. 1–8. [Google Scholar]
- Nieto, A.; Alexander, K.N.A. European Red List of Saproxylic Beetles; Publications Office of the European Union: Luxemburg, 2010; pp. 1–56. [Google Scholar]
- Thomaes, A.; Kervyn, T.; Maes, D. Applying species distribution modelling for the conservation of the threatened saproxylic Stag Beetle (Lucanus cervus). Biol. Conserv. 2008, 141, 1400–1410. [Google Scholar] [CrossRef]
- Percy, C.; Bassford, G.; Keeble, V. Findings of the 1998 National Stag Beetle Survey; People’s Trust for Endangered Species: London, UK, 2000; pp. 1–46. [Google Scholar]
- Buse, J.; Ranius, T.; Assmann, T. An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer. Conserv. Biol. 2008, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ducasse, J.J.; Brustel, H. Saproxylic beetles in the Grésigne forest management. Rev. Ecol. Terre Vie 2008, 10, 75–80. [Google Scholar]
- Roberge, J.M.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Bardiani, M.; Tini, M.; Carpaneto, G.M.; Audisio, P.; Bussola, E.; Campanaro, A.; Cini, A.; Maurizi, E.; Mason, F.; Sabbatini Peverieri, G.; et al. Effects of trap baits and height on stag beetle and flower chafer monitoring: Ecological and conservation implications. J. Insect Conserv. 2017, 21, 157–168. [Google Scholar] [CrossRef]
- Fremlin, M.; Hendriks, P. Sugaring for stag beetles—Different feeding strategies of Lucanus cervus and Dorcus parallelipipedus. Bull. Amat. Entomol. Soc. 2011, 70, 57–67. [Google Scholar]
- Jansson, N. Attraction of stag beetles with artificial sap in Sweden. Bull. Amat. Entomol. Soc. 2011, 70, 51–56. [Google Scholar]
- Campanaro, A.; Zapponi, L.; Hardersen, S.; Méndez, M.; Al Fulaij, N.; Audisio, P.; Bardiani, M.; Carpaneto, G.M.; Corezzola, S.; Della Rocca, F.; et al. A European monitoring protocol for the stag beetle; a saproxylic flagship species. Insect Conserv. Diver. 2016, 9, 574–584. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Della Rocca, F.; Bogliani, G.; Milanesi, P. Patterns of distribution and landscape connectivity of the stag beetle in a human-dominated landscape. Nat. Conserv. 2017, 19, 19–37. [Google Scholar] [CrossRef]
- Hudgins, R.M.; Norment, C.; Schlesinger, M.D. Assessing detectability for monitoring of rare species: A case study of the cobblestone tiger beetle (Cicindela marginipennis Dejean). J. Insect Conserv. 2012, 16, 447–455. [Google Scholar] [CrossRef]
- Chiari, S.; Bardiani, M.; Zauli, A.; Hardersen, S.; Mason, F.; Spada, L.; Campanaro, A. Monitoring of the saproxylic beetle Morimus asper (Sulzer, 1776) (Coleoptera: Cerambycidae) with freshly cut log piles. J. Insect Conserv. 2013, 17, 1255–1265. [Google Scholar] [CrossRef]
- Vrezec, A.; Ambrožič, Š.; Kapla, A. An overview of sampling methods tests for monitoring schemes of saproxylic beetles in the scope of Natura 2000 in Slovenia. In Saproxylic beetles in Europe: Monitoring Biology and Conservation, Ljubljana (Slovenia), June 2010; Jurc, M., Ed.; Slovenian Forestry Institute Sliva Slovenica: Ljubljana, Slovenia, 2012; pp. 73–90. [Google Scholar]
- Neumann, V.; Schmidt, V. New ecological-faunistic aspects of Cerambyx cerdo. (Col.: Cerambycidae). Hercynia NF 2001, 34, 286–288. [Google Scholar]
- Tini, M.; Bardiani, M.; Campanaro, A.; Chiari, S.; Mason, F.; Maurizi, E.; Toni, I.; Audisio, P.; Carpaneto, G.M. A stag beetle’s life: Sex-related differences in daily activity and behaviour of Lucanus cervus (Coleoptera: Lucanidae). J. Insect Conserv. 2017, 21, 897–906. [Google Scholar] [CrossRef]
- Kéry, M. Identifiability in N-mixture models: A large-scale screening test with bird data. Ecology 2018, 99, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Kéry, M.; Royle, J.A. Applied hierarchical modelling in ecology—Modeling distribution, abundance and species richness using R and BUGS. In Volume 1: Prelude and Static Models; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Kéry, M. Towards the modelling of true species distribution. J. Biogeogr. 2011, 38, 617–618. [Google Scholar] [CrossRef]
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvaňová, A.; Kalyakin, M.V.; et al. European bird census. In European Breeding Bird Atlas 2: Distribution, Abundance and Change; Council & Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
| Covariates | Unit | Mean | Range | VIF | ||
|---|---|---|---|---|---|---|
| Detection probability | Date of observation | Date | giulian day | 191.77 | 177–204 | 1.951 |
| Time of observation | Time_mean | min | 13:49 | 08:30–21:35 | 1.375 | |
| Time spent during the session | Time_spent | min | 103.41 | 30–240 | 2.392 | |
| Mean temperature | Temp_mean | °C | 27.36 | 23.02–27.36 | 2.981 | |
| Mean relative humidity | UR_Mean | % | 68.96 | 58.31–75.91 | 1.299 | |
| Duration of Leaf wetness | BF_MeanTime | min | 6.25 | 3.75–8.96 | 1.618 | |
| Daily Precipitations | Precipitations | mm | 0 | 0–0 | >3 | |
| Mean Daily insolation | Ins_Mean | mV | 4883.79 | 4733.01–5000.25 | 1.711 | |
| Abundance | Distance to water | Dist_water | m | 1667.61 | 1382.43–1838.41 | >3 |
| Distance to dense human settlements | Dist_hum_sett | m | 623.16 | 452.55–832.55 | 2.139 | |
| Distance to roads | Dist_roads | m | 2909.42 | 2738.77–3089.73 | 2.022 | |
| Distance to broadleaf forests | Dist_bl_forests | m | 71.80 | 0–218.28 | 1.924 | |
| Distance to riparian formations | Dist_rip_forests | m | 138.27 | 0–370 | 1.882 | |
| Distance to shrublands | Dist_shrubs | m | 380.41 | 227.99–571.42 | 1.868 | |
| Distance to grasslands | Dist_grass | m | 601.99 | 296.27–772.25 | 1.826 | |
| Distance to urban green parks | Dist_ug_parks | m | 1331.90 | 1080–1730 | 1.736 | |
| Distance to croplands | Dist_crops | m | 6.35 | 0–90 | 1.201 | |
| Distance to sparse human settlements | Dist_shum_sett | m | 179.52 | 84.85–366.98 | 1.124 | |
| Tree cover density | TCD | n/m2 | 36.47 | 0–85 |
| Covariates | β ± s.d. | ||
|---|---|---|---|
| Transect Walks | Tree Surveys | ||
| Detection probability | Intercept * | −11.3 ± 0.51 | −3.71 ± 0.45 |
| Date * | 1.05 ± 0.59 | 1.41 ± 0.18 | |
| Time_mean | 0.98 ± 0.05 | 0.92 ± 0.04 | |
| Time_spent ** | NA | 2.77 ± 0.14 | |
| Temp_mean | 0.29 ± 0.13 | 0.16 ± 0.07 | |
| UR_Mean | −0.73 ± 0.02 | −0.03 ± 0.04 | |
| BF_MeanTime | −0.41 ± 0.17 | −0.04 ± 0.07 | |
| Ins_Mean | 0.57 ± 0.04 | 0.08 ± 0.01 | |
| Abundance | Intercept * | −3.93 ± 0.52 | −1.38 ± 0.83 |
| Dist_bl_forests * | −5.83 ± 0.17 | −3.03 ± 0.47 | |
| Dist_crops * | 1.32 ± 0.03 | 2.56 ± 0.74 | |
| Dist_grass * | 0.02 ± 0.09 | 0.48 ± 0.22 | |
| Dist_roads | 0.48 ± 0.43 | 1.75 ± 0.94 | |
| Dist_hum_sett * | 1.41 ± 0.12 | 1.19 ± 0.93 | |
| Dist_shum_sett | −0.85 ± 0.03 | 0.96 ± 0.15 | |
| Dist_shrubs | −1.38 ± 0.06 | −0.72 ± 0.31 | |
| Dist_rip_forests * | −2.49 ± 0.05 | −3.26 ± 1.01 | |
| Dist_ug_parks | −0.74 ± 0.13 | 0.76 ± 0.99 | |
| TCD * | 1.01 ± 0.11 | 2.51 ± 0.06 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rocca, F.; Milanesi, P.; Magna, F.; Mola, L.; Bezzicheri, T.; Deiaco, C.; Bracco, F. Comparison of Two Sampling Methods to Estimate the Abundance of Lucanus cervus with Application of n-Mixture Models. Forests 2020, 11, 1085. https://doi.org/10.3390/f11101085
Della Rocca F, Milanesi P, Magna F, Mola L, Bezzicheri T, Deiaco C, Bracco F. Comparison of Two Sampling Methods to Estimate the Abundance of Lucanus cervus with Application of n-Mixture Models. Forests. 2020; 11(10):1085. https://doi.org/10.3390/f11101085
Chicago/Turabian StyleDella Rocca, Francesca, Pietro Milanesi, Francesca Magna, Livio Mola, Tea Bezzicheri, Claudio Deiaco, and Francesco Bracco. 2020. "Comparison of Two Sampling Methods to Estimate the Abundance of Lucanus cervus with Application of n-Mixture Models" Forests 11, no. 10: 1085. https://doi.org/10.3390/f11101085
APA StyleDella Rocca, F., Milanesi, P., Magna, F., Mola, L., Bezzicheri, T., Deiaco, C., & Bracco, F. (2020). Comparison of Two Sampling Methods to Estimate the Abundance of Lucanus cervus with Application of n-Mixture Models. Forests, 11(10), 1085. https://doi.org/10.3390/f11101085





