Stump Sprout Characteristics of Three Commercial Tree Species in Suriname
Abstract
:1. Introduction
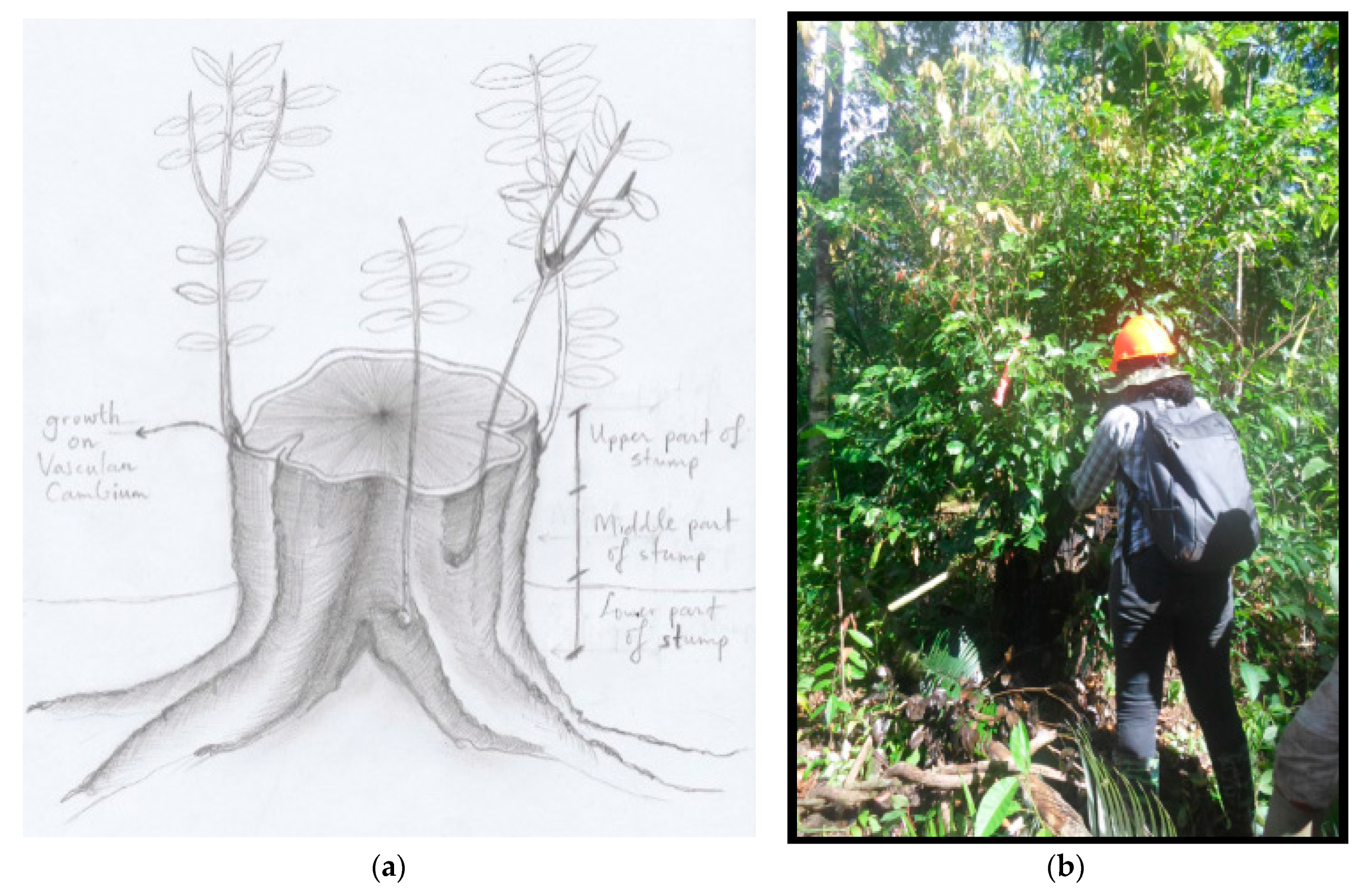
2. Materials and Methods
3. Results
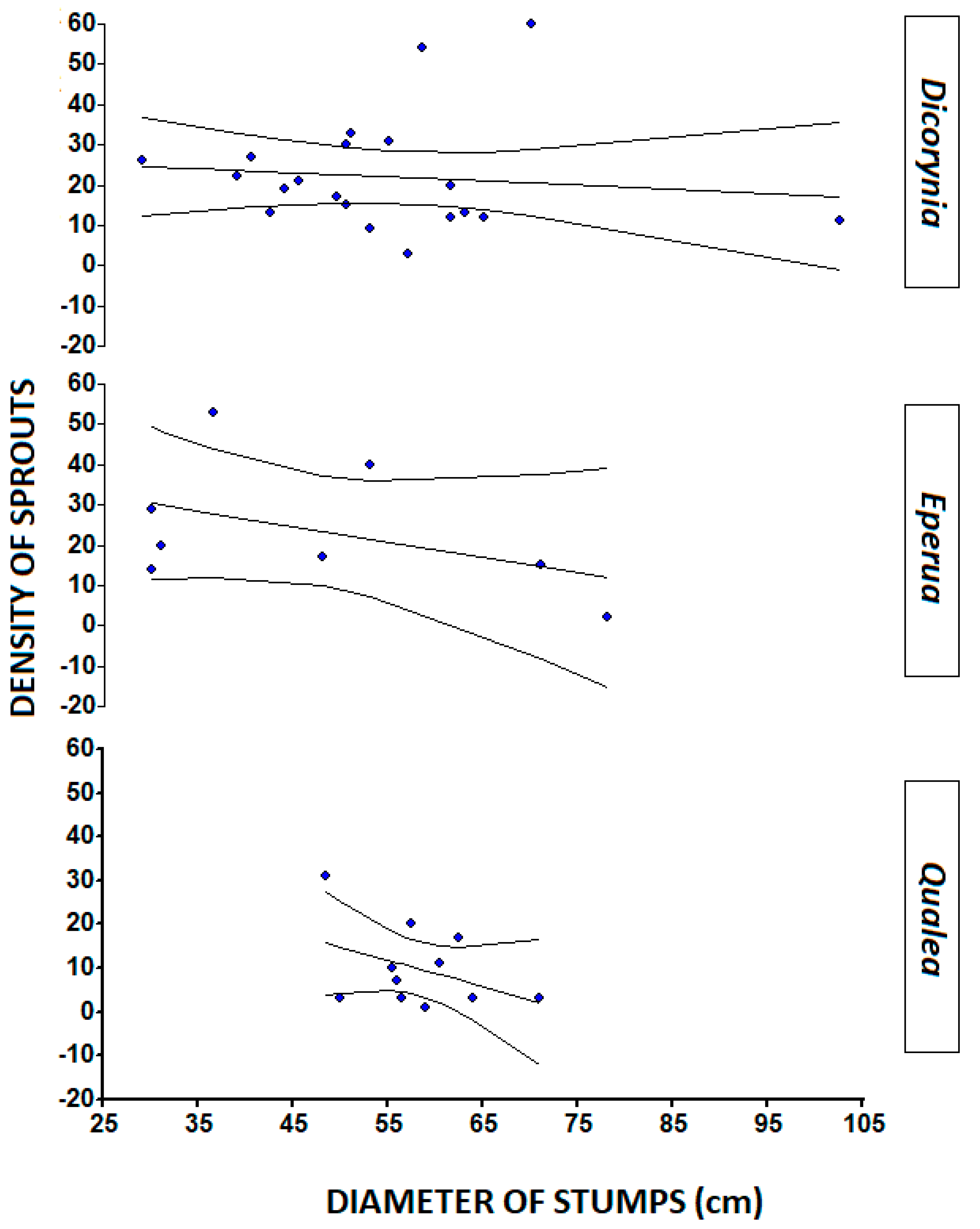
3.1. Stump Characteristics
3.2. Comparisons of Sprouts and Conspecific Saplings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Tredici, P. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Putz, F.E.; Brokaw, V.L. Sprouting of broken trees on Barro Colorado Island, Panama. Ecology 1989, 70, 508–512. [Google Scholar] [CrossRef]
- Kamo, K.; Sato, A.; Javing, A.L. Coppice growth of some tropical tree species in Mindanao Island, the Philippines. Jpn. Agric. Res. Q. 1990, 24, 235–241. [Google Scholar]
- Mushove, P.T.; Makoni, J.T. The ecology and management of indigenous forests in Southern Africa. In Silviculture, Ecology and Management, Proceedings of the International Symposium, Victoria Falls, Zimbabwe, 27–29 July 1992; Piearce, G.D., Gumbo, D.J., Shaw, P., Eds.; The Forestry Commission: Harare, Zimbabwe, 1993; pp. 226–230. [Google Scholar]
- Mostacedo, B.; Putz, F.E.; Fredericksen, T.S.; Villca, A.; Palacios, T. Contributions of root and stump sprouts to natural regeneration of a logged tropical dry forest in Bolivia. For. Ecol. Manag. 2009, 258, 978–985. [Google Scholar] [CrossRef]
- Rackham, O. Ancient woodlands: Modern threats. New Phytol. 2008, 180, 71–86. [Google Scholar] [CrossRef]
- Cañellas, I.; Del Rio, M.; Roig, S.; Montero, G. Growth response to thinning in Quercus pyrenaica Willd. coppice stands in Spanish central mountain. Ann. For. Sci. 2004, 61, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Kennard, D.K.; Gould, K.; Putz, F.E.; Fredericksen, T.S.; Morale, F. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. For. Ecol. Manag. 2002, 162, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.I.; Flores, O.; Schurr, F.M. Costs of persistence and the spread of competing seeders and sprouters. J. Ecol. 2008, 96, 679–686. [Google Scholar] [CrossRef]
- Dietze, M.; Clark, J.S. Rethinking gap dynamics: The impact of damaged trees and sprouts. Ecol. Monogr. 2008, 78, 331–347. [Google Scholar] [CrossRef]
- Mcdonald, M.A.; Mclaren, K.P.; Newton, A.C. What Are the Mechanisms of Regeneration Post-Disturbance in Tropical Dry Forest? CEE Review 07-013 (SR37). Environmental Evidence. 2010. Available online: www.environmentalevidence.org/SR37.html (accessed on 10 August 2020).
- Vickers, L.A.; Fox, T.R.; Loftis, D.L.; Boucugnani, D.A. Predicting forest regeneration in the Central Appalachians using the REGEN expert system. J. Sustain. For. 2011, 30, 790–822. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [Green Version]
- Lust, N.; Mohammady, M. Regeneration of coppice. Sylva Gandav. 1973, 39, 1–29. [Google Scholar] [CrossRef]
- Ewel, K.C. Pondcypress Swamps. In Southern Forested Wetlands: Ecology and Management; Lewis Publishers: Boca Raton, FL, USA, 1998; pp. 405–420. [Google Scholar]
- Randall, C.K.; Duryea, M.L.; Vince, S.W.; English, R.J. Factors influencing stump sprouting by pondcypress (Taxodium distichum var. nutans (Ait.) Sweet). New For. 2005, 29, 245–260. [Google Scholar] [CrossRef]
- Britez, D.D.; Romero, C.; Putz, F.E. Coppicing of two native but invasive oak species in Florida. For. Ecol. Manag. 2020, 477, 118487. [Google Scholar] [CrossRef]
- Mwavu, E.N.; Witkowski, E.T.F. Sprouting of woody species following cutting and tree-fall in a lowland semi-deciduous tropical rainforest, North-Western Uganda. For. Ecol. Manag. 2008, 255, 982–992. [Google Scholar] [CrossRef]
- Baraloto, C.; Goldberg, D.E. Microhabitat associations and seedling bank dynamics in a neotropical forest. Oecologia 2004, 141, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Norden, N.; Chave, J.; Belbenoit, P.; Riéra, B.; Caubère, A.; Châtelet, P.; Forget, P.-M.; Viers, J.; Thébaud, C. Interspecific variation in seedling responses to seed limitation and habitat conditions for 14 Neotropical woody species. J. Ecol. 2009, 97, 186–197. [Google Scholar] [CrossRef]
- Villadas, P.J.; Fernández-López, M.; Ramírez-Saad, H.; Toro, N. Rhizosphere-bacterial community in Eperua falcata (Caesalpiniaceae) a putative nitrogen-fixing tree from French Guiana rainforest. Microb. Ecol. 2007, 53, 317–327. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Putz, F.E. The influence of neighbors on tree form: Effects of lateral shade and prevention of sway on the allometry of Liquidambar styraciflua (sweet gum). Am. J. Bot. 1989, 76, 1740–1749. [Google Scholar] [CrossRef]
- Jagernath, R.; Landburg, J.S.; Paal, A.P.; Sewdien, A.V.; Wortel, V.; Putz, F.E. Likelihoods of stump sprouting by felled trees in a selectively logged forest in Suriname. J. Trop. For. Sci. 2020. in review. [Google Scholar]
- Bellingham, P.; Sparrow, A. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Weiner, J.; Thomas, S.C. Competition and allometry in three species of annual plants. Ecology 1992, 73, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Mensah, S.; Kakaï, R.G.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 49–60. [Google Scholar] [CrossRef] [Green Version]




| Species | N | Mean Stump Diameter (cm) | S.D. | Range (cm) | Mean # Sprouts Per Stump | SD | Range (#) |
|---|---|---|---|---|---|---|---|
| Dicorynia guianensis Amsh. | 19 | 61.79 b | 16.14 | 29–102.5 | 23.73 a | 14.46 | 3–60 |
| Eperua falcata Aubl. | 5 | 48.60 c | 18.56 | 31–75.5 | 23.75 a | 16.26 | 2–53 |
| Qualea rosea Aubl. | 10 | 71.55 a | 17.83 | 51.50–110 | 4.17 b | 3.49 | 1–11 |
| Species | Sprouts (s.d., N) | Saplings (s.d., N) | t | p |
|---|---|---|---|---|
| Height:Diameter | ||||
| Dicorynia | 139.8 (41.48, 18) | 126.6 (18.15, 10) | 1.2 | 0.26 |
| Eperua | 132.3 (54.71, 5) | 109.3 (27.26, 3) | 0.8 | 0.46 |
| Qualea | 116.9 (40.09, 9) | 123.2 (26.85, 21) | 0.4 | 0.67 |
| % Mass in Leaves | % Mass in Leaves | |||
| Dicorynia | 48.9 (12.34, 18) | 33.7 (13.91, 10) | 2.9 | 0.01 |
| Eperua | 43.4 (8.546, 5) | 16.3 (7.81, 3) | 4.6 | <0.01 |
| Qualea | 47.8 (8.836, 9) | 41.2 (12.63, 21) | 1.6 | 0.11 |
| % H2O in Leaves | % H2O in Leaves | |||
| Dicorynia | 64.4 (4.35, 13) | 59.07 (5.82, 6) | 2.0 | 0.08 |
| Eperua | 52.756 (3.36, 5) | 57.121 (7.24, 3) | 1.0 | 0.41 |
| Qualea | 67.397 (8.79, 5) | 67.281 (2.14, 5) | 0.1 | 0.98 |
| Leaf Area (cm2) | Leaf Area (cm2) | |||
| Dicorynia | 224.2 (73.30, 50) | 168.8 (20.39, 10) | 4.5 | <0.0001 |
| Eperua | 101.7 (45.27, 20) | 64.0 (20.73, 20) | 3.1 | <0.005 |
| Qualea | 65.1 (17.49, 25) | 70.2 (8.88, 14) | 1.2 | 0.23 |
| LMA (g m−2) | LMA (g m−2) | |||
| Dicorynia | 58.9 (11.49,50 ) | 57.7 ( 14.99, 10) | 0.3 | 0.08 |
| Eperua | 49.9 (21.09, 19) | 51.1 (30.94, 9) | 0.1 | 0.91 |
| Qualea | 73.5 (38.63, 25) | 77.4 (21.13, 14) | 0.4 | 0.68 |
| Wood Density (g cm−3) | Wood Density (g cm−3) | |||
| Dicorynia | 0.42 (0.06, 39) | 0.51 (0.08, 18) | 4.5 | <0.001 |
| Eperua | 0.50 (0.04, 15) | 0.59 (0.03, 9) | 6.3 | <0.01 |
| Qualea | 0.41 (0.10, 15) | 0.50 (0.07, 15) | 2.8 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramdial, D.; Sewdien, A.; Rasdan, J.; Critchlow, S.; Tjong-A-Hung, N.; Ospina, A.; Wortel, V.; Putz, F.E. Stump Sprout Characteristics of Three Commercial Tree Species in Suriname. Forests 2020, 11, 1130. https://doi.org/10.3390/f11111130
Ramdial D, Sewdien A, Rasdan J, Critchlow S, Tjong-A-Hung N, Ospina A, Wortel V, Putz FE. Stump Sprout Characteristics of Three Commercial Tree Species in Suriname. Forests. 2020; 11(11):1130. https://doi.org/10.3390/f11111130
Chicago/Turabian StyleRamdial, Donna, Artie Sewdien, Jerry Rasdan, Shermaine Critchlow, Noraisah Tjong-A-Hung, Alejandra Ospina, Verginia Wortel, and Francis E Putz. 2020. "Stump Sprout Characteristics of Three Commercial Tree Species in Suriname" Forests 11, no. 11: 1130. https://doi.org/10.3390/f11111130
APA StyleRamdial, D., Sewdien, A., Rasdan, J., Critchlow, S., Tjong-A-Hung, N., Ospina, A., Wortel, V., & Putz, F. E. (2020). Stump Sprout Characteristics of Three Commercial Tree Species in Suriname. Forests, 11(11), 1130. https://doi.org/10.3390/f11111130






