Abstract
Wood ceramics (WCS) were prepared from thermo-modified poplar wood residues and untreated poplar wood. At 1000 °C sintering temperature, the ratios of wood powder and phenolic resin at 10:3, 10:6 and 10:9 were tested. The effects of materials on the properties of WCS, carbon yield and volume shrinkage were studied. With the increase in resin content, the carbon yield increased; however, the volume shrinkage decreased. Carbon yield of WCS made from 220 °C thermo-modified poplar wood was 40.45%, as the ratio of wood powder/phenolic resins was 10:6. The microstructure, chemical structure and crystallinity of WCS were analysed by scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) and X-ray diffraction (XRD), respectively. The results showed that WCS had a porous structure. WCS prepared from thermo-modified materials, amorphous carbon and hard glass carbon melted more evenly; meanwhile, there were more pores on glass carbon. The FTIR spectra showed that the stretching vibration of C-O-C weakened at ceramics made of thermo-modified poplar. The XRD pattern indicated that the raw material has no apparent influence on the graphitization degree of WCS.
1. Introduction
Wood ceramics (WCS) are new materials produced based on the idea of creating new high-performance materials from recovered wood and wood residues. In the preparation process, WCS were prepared by pyrolysis of woody materials such as wood raw materials, wood processing residues, wastepaper, bagasse and straw, impregnated with different precursor substances, such as resins and metals [1,2,3]. A composite material with both biochar and ceramic properties was obtained after carbonization [4]. WCS has excellent properties, such as abrasion resistance, corrosion resistance and high specific strength [5,6]. This material could be used for flooring in demanding applications, where aesthetic is essential. However, the expensive cost of preparing WCS and the complex process involved consumes a lot of energy, leading to limitations in application. Therefore, it is essential to find an efficient way to manufacture WCS with cheap and readily available materials.
Heat-treated wood, commonly known as “carbonized wood”, is widely used in indoor and outdoor applications, architectural decoration, and flooring among other fields, due to its aesthetics, dimensional stability, and 100% natural choice for the demands of modern wood architecture. In the past 20 years, the production capacity of thermally modified wood has considerably increased in Europe, from 0.01 million m3 in 2001 to 0.5 million m3 in 2018 [7]. Heat-treated wood production is expected to continue to rapidly increase with enhanced environmental awareness and rapid changes in aesthetic trends. At the end of its service life, large amounts of heat-treated wood are accumulated; therefore, considerable attention should be given to find reasonable ways to reuse these resources. The objective of the respective study was to elucidate whether this material is a suitable choice to use them for manufacturing WCS.
Machining and thermal modification of wood are energetically demanding processes. In addition, the disposal of wood results in the anaerobic degradation and methane emissions, thus all environmental benefits of wood, are annihilated. Therefore, the reuse and recycling of recovered heat-treated wood products reduce energy consumption and lower pressure on natural resources.
Furthermore, when the wood is thermo-modified at temperatures above 150 °C, the physical and chemical properties of wood are permanently altered. The basic principle of heat modification is to transform the hydrophilic property of wood into hydrophobic through the thermal degradation of polysaccharides (mainly unstable hemicellulose), through crosslinking, and increased crystallinity of cellulose in the wood cell wall [8,9]. The main reason for the change in wood properties is the influence of high temperature on the chemical properties of wood. Higher treatment temperature proportionately decreases the degree of polymerization of the cellulose, which results in reduced mechanical properties. It is reported that thermal modification reduces mechanical properties by 0–30% [10,11]. Therefore, in contrast to natural wood, heat-treated wood has a brittle structure and decreased strength. Therefore, a lot of energy required for production wood particles can be saved [12].
Meanwhile, heat treatment causes a gradual separation between cells and improves the permeability of wood. In addition, it also reduces the free surface energy [13], which is conducive for mixing with phenolic resin [14]. Furthermore, the moisture content of the matrix has a significant effect on the preparation of WCS. The moisture content of wood is commonly controlled below 8% when preparing the wood/phenolic composites [15]. Hence, it is necessary to reduce the moisture content before the samples are hot-pressed. The heat treatment is effective in reducing the hygroscopicity of wood, as proven by Unsal [16], where 10–25% reduction in moisture sorption was achieved. The reduction in water content was caused by several factors, such as reduced affinity for hydroxyl groups [17], increased crystallinity of cellulose [18], and further crosslinking was caused by the polycondensation in lignin [19]. Therefore, the cost for drying of wood prior WCS production can be saved as well. In addition, the improved dimensional stability is an important outcome of thermal modification as well. Besides, the microanalysis results showed a gradual increase in carbon content with an obvious decrease in oxygen content, which attributed the decrease in oxygen to the dehydration reactions during wood thermal degradation [20]. The lignin content increased, while the holocellulose content decreased due to numerous dehydration reactions demonstrated after treatment [21]. Lignin is considered to be a precursor for carbon production because lignin has a high carbon content. This variation can improve the carbon yield and mechanical properties of WCS. These advantages provided a possibility for the preparation of WCS, which offer an effective way for the recovery and utilization of a large amount of recovered wood resources.
Poplar is a fast-growing species with low density and a typical porous structure which is conducive to phenolic resin impregnation [22,23]. So, this work presents a process for manufacturing WCS from heat-treated poplar residue mixed with phenolic resin. Referring to the experimental results and methods of the previous researchers, the ratio range of wood powder to phenolic resin was selected [5]. Meanwhile, WCSs prepared from untreated poplar were used as contrast materials. The effects of weight ratio of phenolic resin to wood powder to the basic properties of manufactured WCS, were studied in more detail. The microstructure, chemical and wide-angle X-ray diffractograms’ structure were also investigated.
2. Materials and Methods
2.1. Sample Preparation
In this study, the residues of poplar wood (Populus sp.) modified at high temperature and untreated poplar wood collected from a wood products company in Zhejiang, China, were used to investigate the WCS production. The modified wood was heat-treated at 220 °C for a fixed duration of 3 h. The obtained residues of secondary wood processing were chopped and milled into small wood powders with a dimension of 0.25–0.42 mm using a grinder (GH30/20). Prior to the milling, the colour of the wood was determined according to the CIELab system. The colour of modified wood reflects the intensity of the treatment. Darker colour corresponds to more intensive treatment. The powders were dried to 10% moisture content in a laboratory oven (XMT-C2000 1010-1B). A commercial liquid phenol-formaldehyde (PF) resin with a solid content of 43% was used as a binder in the production of WCS obtained from Dynea (Guangdong, China) Co., Ltd.
2.2. Manufacturing of WCS
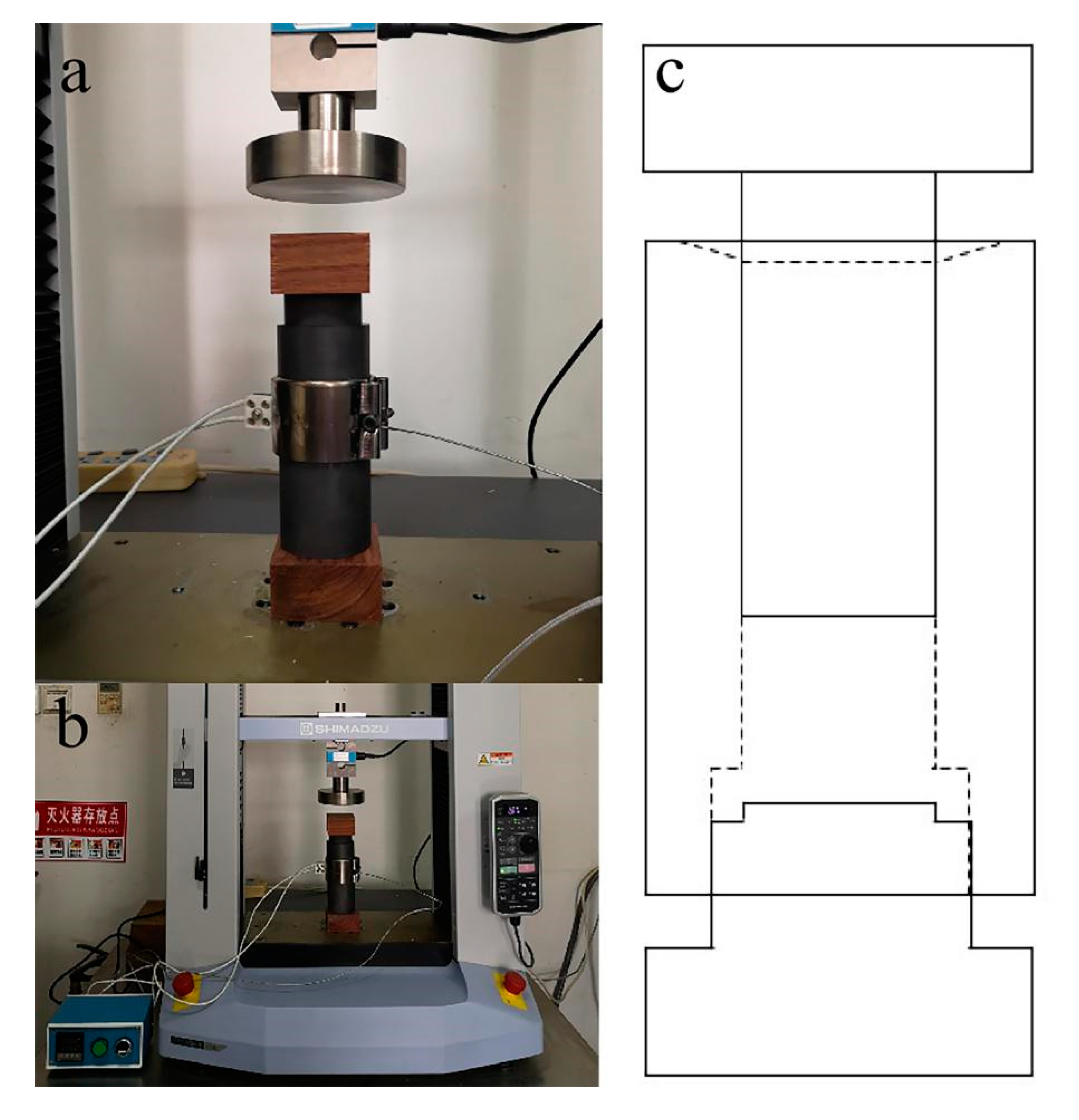
Based on the experience in the references and preliminary experiments, the technological parameters of wood ceramics were determined [3,5,6]. The wood powders were impregnated with a water solution of PF resin, and were magnetically stirred in an airtight container to obtain a homogenized mixture. The weight ratio of wood powder to the PF resin was 10:3, 10:6 and 10:9. The mixture materials were then pressed into wood/PF resin composites by an electric universal testing machine (AGS-X-20kN, Shimadzu, Japan), with a self-made metal mould surrounded by heating mantle, as shown in Figure 1. Temperature measurement was conducted using a thermocouple, fixed on the self-made metal mould. The temperature was set at 150 °C for 10 min. The samples were oven-dried at 105 °C until the constant weight was obtained. The preparation of composites was conducted in a vacuum furnace. To avoid cracking and deformation during carbonization, the temperature was slowly increased during the initial heating period. The samples were then carbonized at the target temperature of 1000 °C at a heating rate of 5 °C/min from room temperature to 350 °C and at 3 °C/min from 350 °C to 1000 °C. The samples were subsequently cooled to ambient temperature at a rate of 5 °C/min with the furnace. Each group consisted of ten duplicates.
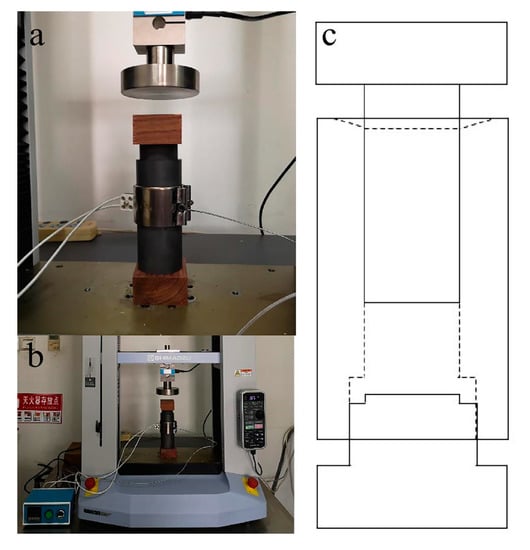
Figure 1.
Hot pressing mould and system. (a) The self-made pressing mould. (b) Hot pressing system. (c) Structure chart of pressing mould.
2.3. Characterization of WCS
To investigate the effect of the process on dimension shrinkage and weight loss on WCS from heat-treated poplar wood and PF composite, the dimensions of 10 samples were measured by using digital Vernier calipers (Mitutoyo CD-6CSX) and a digital micrometre (Mahr MarCator 1086R). The volume shrinkage was calculated using volume change of samples before and after carbonization. Similarly, the weight changes were measured using an electrical balance (VANTE WT (20002)).
The pyrolysis of poplar wood and 220 °C heat-treated poplar wood was conducted by applying to thermogravimetric analysis (TGA) (TA instrument, USA) in a high-purity N2 (99.999%) environment. Additionally, 2 mg of wood powder was heated from 25 °C to 900 °C at a heating rate of 20 °C/min and a steady nitrogen flow rate of 100 mL/min.
Using an elemental analyser (PE 2400 II Elemental Analyzer), the weight percentages of C, H, O and N elements in the wood powder and WCS were measured. The weight percentage of O was obtained by difference, that is, O (wt.%) = 100-C-H-N.
The morphology of WCS was observed and analysed by scanning electron microscopy (SEM) (FEI Quanta 200). Before the testing, all samples were dried at 100 °C for 6 h, and the observation surface of all samples was sprayed with gold using a particle sputtering apparatus. The test voltage was 15KV and the resolution of the analysis mode was 3.0 nm.
Through Fourier transform infrared spectroscopy (FTIR) (Thermo Scientific Instrument Co. U.S.A, Nicolet 8700), the chemical structure of WCS was characterized. All specimens were dried at 100 °C for 6h before detection, approximately 2 mg of sample uniformly mixed with 200 mg KBr powder and compressed to form a disk. The determination range was 400–4000 cm −1 at a resolution of 4 cm−1. The wide-angle X-ray diffraction graph of WCS was measured using an X-ray model diffractometer (Rigaku, Ultima IV) using Ni-filtered Cu Ka radiation (λ = 1.5406 A) at 40Kv and 30mA. Scattered radiation was recorded in the range of 5°–50° at a scan rate of 5 °C/min.
2.4. Statistical Analysis
The data were statistically analysed with the aid of variance (ANOVA) using SPSS Version 25.0, (IBM SPSS Statistics for Windows, IBM Corporation, means were analysed and grouped using Duncan’s multiple range test (DMRT).
3. Results and Discussion
3.1. Pyrolysis of Wood Powder
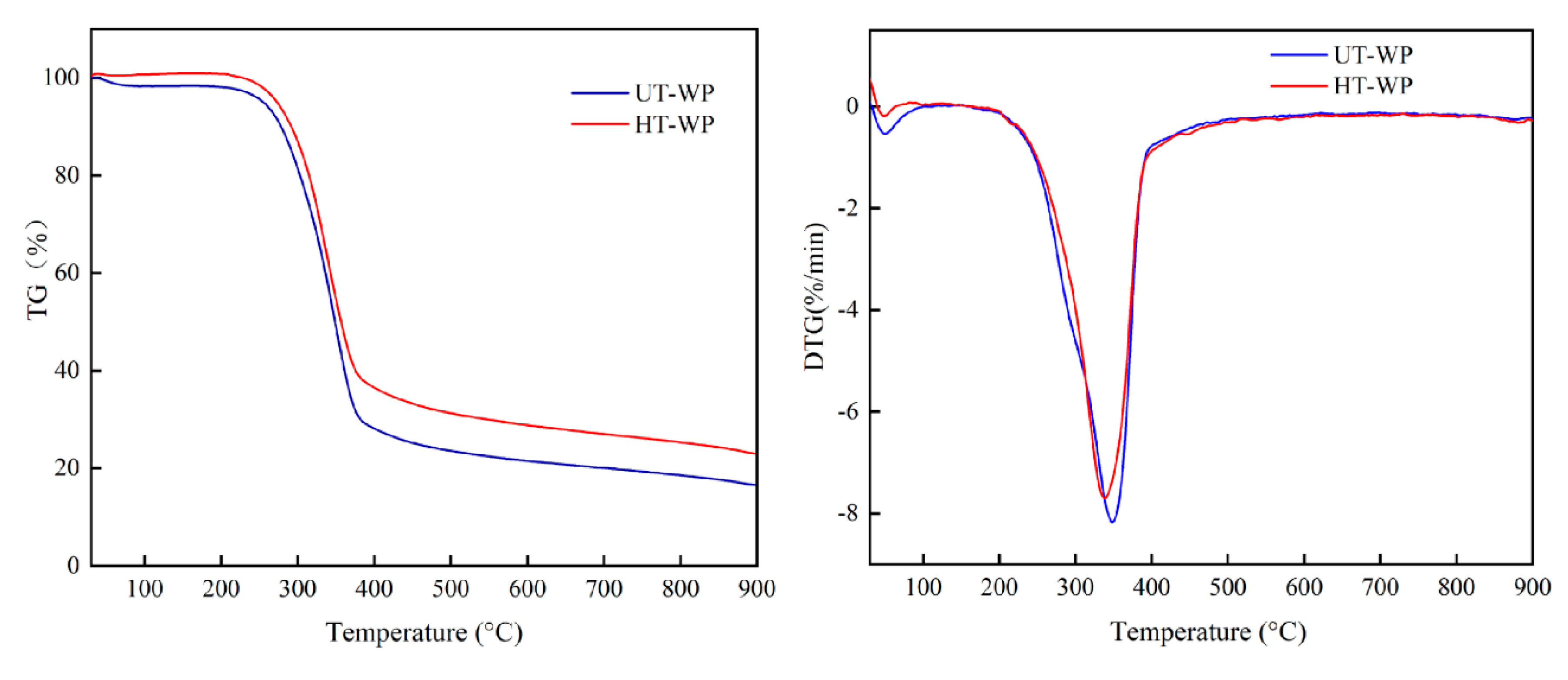
The colour of thermally modified wood reflects the intensity of the treatment. Non-modified wood was of light colour (L*—81.2; a*—3.97; b*—24.69). As expected, thermally modified wood was considerably darker (L*—28.95; a*—8.86; b*—14.23), as a consequence of the heating of poplar wood at elevated temperatures. To study the pyrolysis characteristics of poplar wood and heat-treated poplar wood, wood powders were heated by up to 900 °C in the thermal analyser in an inert atmosphere. The TGA curves of untreated wood powder (UT-WP) and heat-treated wood powder (HT-WP) are shown in Figure 2.
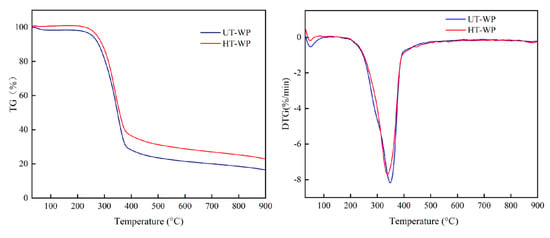
Figure 2.
The pyrolysis curves of the poplar wood (UT-WP) and 220 °C heat-treated poplar wood (HT-WP). (a) The thermogravimetric (TG) curve. (b)The differential thermogravimetric (DTG) curve.
The TG and DTG curves show the typical dynamic gravimetric curves of wood materials [24]. As seen in the figures, the TG curves of different materials have similar change tendency with increasing temperature. The weight loss of wood powder starts at about 200 °C and almost ended at 650 °C. The maximum decomposition rates of wood powder are between 240–550 °C. Heat-treated poplar wood generated more carbon than untreated poplar wood, because lignin has higher carbon yield ratios than the other main components, such as cellulose and hemicellulose in wood during carbonization [25]. Many studies revealed that the relative percentage of lignin in heat-treated wood increased compared to untreated wood [26,27,28].
It is known that wood powders shift into amorphous carbon caused by major weight loss of wood powder, due to the removal of water and evaporation of organic matters (cellulose, hemicellulose and lignin). The decomposition of wood powder consists of four major stages: moisture removal, hemicellulose decomposition, cellulose decomposition and lignin decomposition [29]. Absorbed water and structure water were desorped by up to 240 °C [24]. From 240 °C to 400 °C, the major weight loss is due to the breakdown of cellulose and lignin [30], and the structure of hydrocarbons is formed by chain reduction or depolymerization, because C-C and C-O bonds within the ring units break down. The decomposition rate of lignin picks a maximum value at 360 °C, introducing more carbon content [31]. Pyrolysis initiates the destruction and decomposition of wood (biological) polymer components. Small molecules such as H2O and CO2, together with complex fatty acids, carbonyls and alcohols, evaporate from the original macromolecular lattices [32]. Owing to methane and hydrogen removal, aromatic multicore structures begin to form at 400 °C and gradually form amorphous carbon [33]. Above 800 °C, thermally induced decomposition and rearrangement almost cease to leave the carbon structure. Furthermore, for heat-treated wood, the maximum weight loss temperature shifted to the lower side and the maximum decomposition rate reduced. This is attributed to the destruction of molecular hydrogen bonds of cellulose degradation and the hydrolysis of hemicellulose that has a catalytic effect on the pyrolysis of biomass components to a certain extent, such that the pyrolysis temperature can be reduced [30]. The maximum decomposition rate is also reduced, which is attributed to the pyrolysis rate of lignin with a stable benzene ring structure, slower than that of cellulose and hemicellulose [34].
3.2. Properties of WCS
3.2.1. The Carbon Yield and Volume Shrinkage of WCS
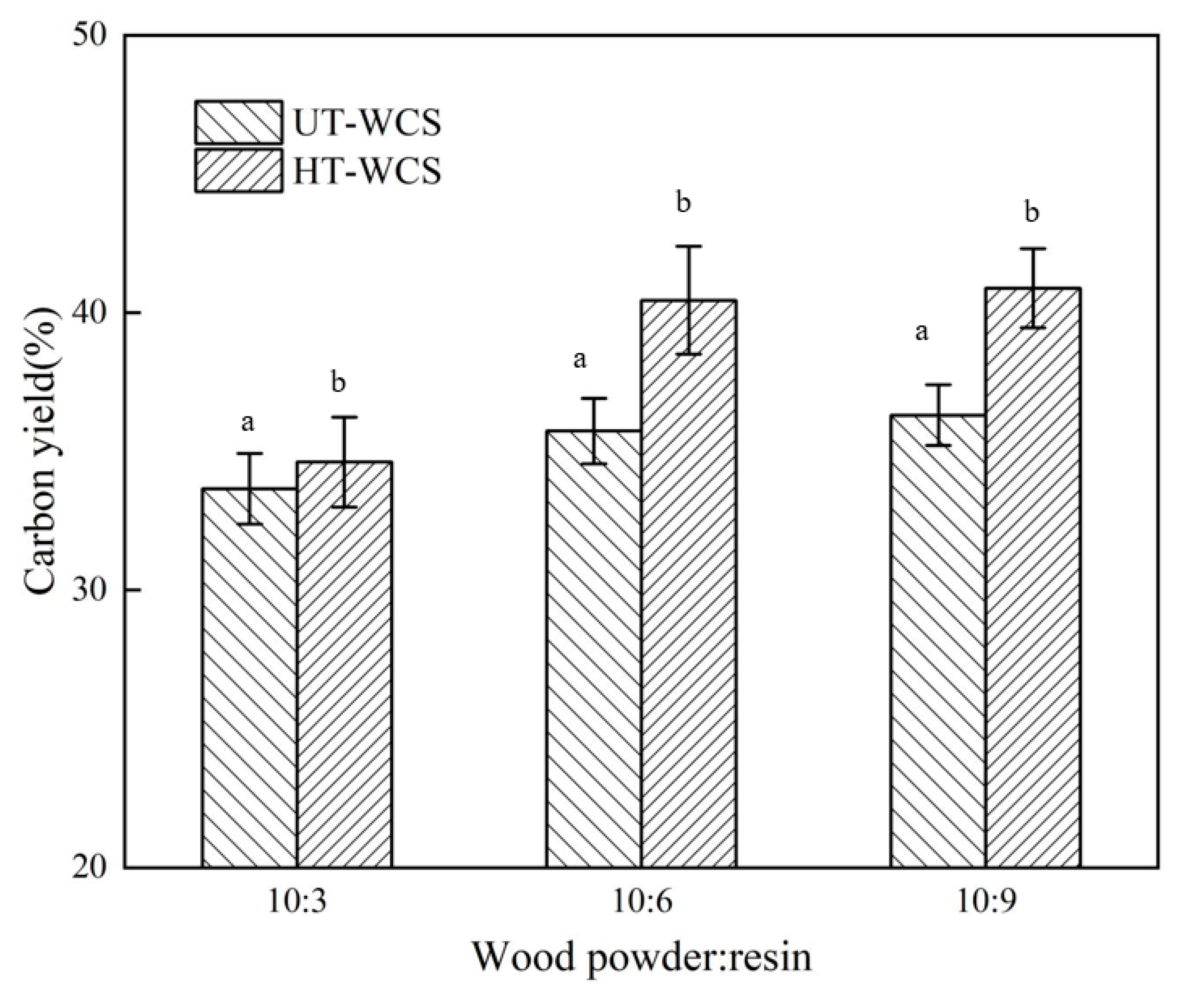
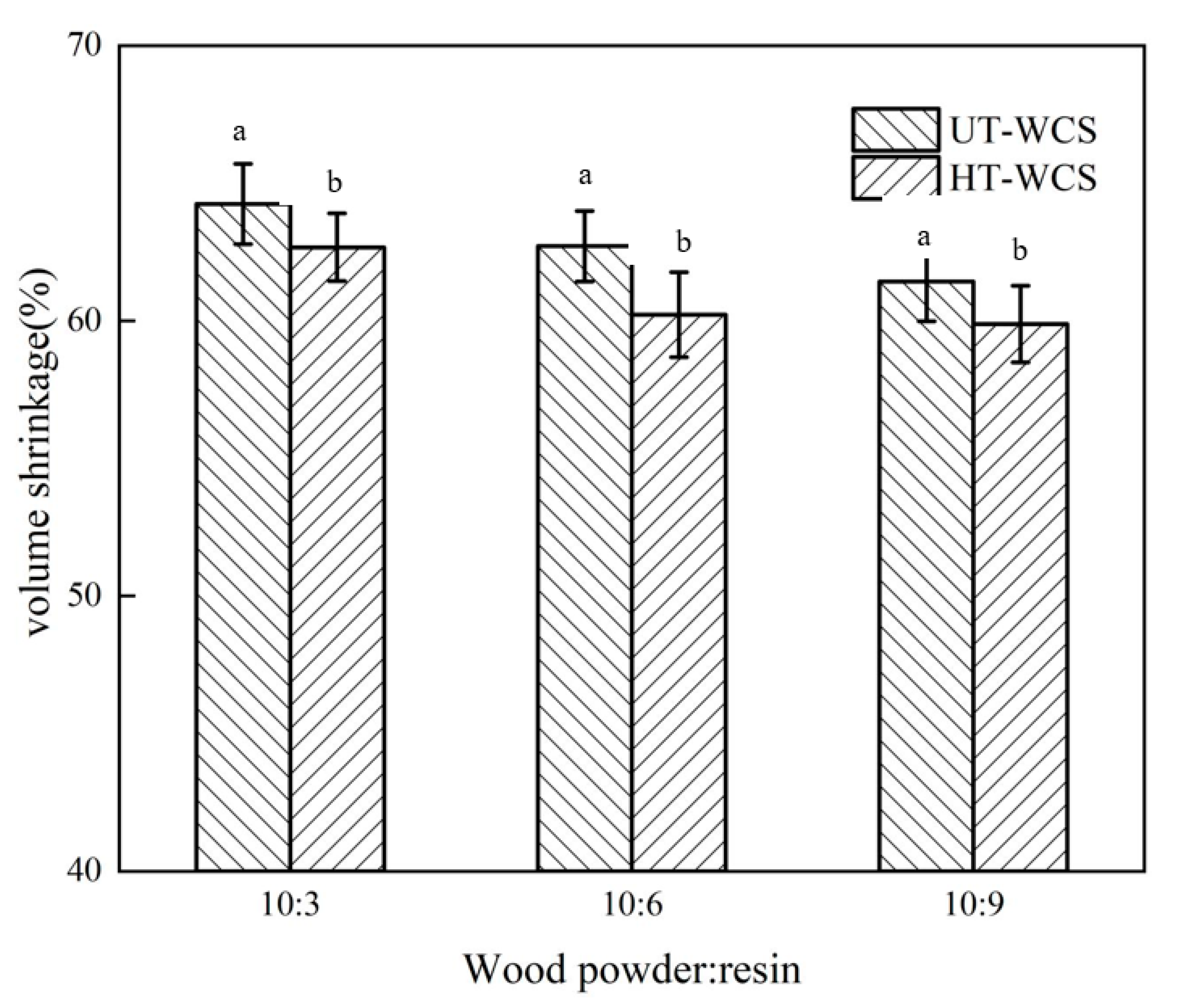
Since both wood powder and phenolic resin considerably shrink during carbonization and a large number of small molecular substances are released; the mass ratio between wood powder and phenolic resin is an important factor that affects the quality and volume shrinkage of WCS. The carbon yield and volume shrinkage of WCS are shown in Figure 3 and Figure 4.
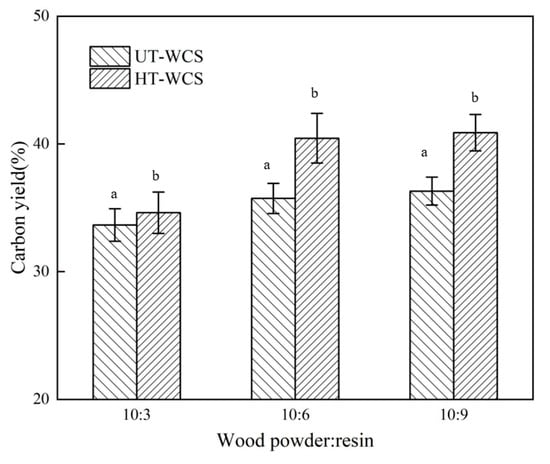
Figure 3.
(a) The carbon yield of UT-WCS; (b) The carbon yield of HT-WCS. Different letter (a,b) indicate significant difference between carbon yield (%) at 99% confidence level.
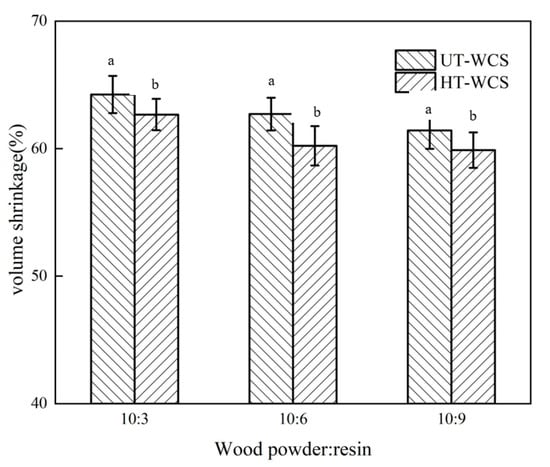
Figure 4.
(a) The volume shrinkage of UT-WCS; (b) The volume shrinkage of HT-WCS. Different letter (a,b) indicate significant difference between volume shrinkage (%) at 99% confidence level.
As the proportion of phenolic resin increased, the carbon yield of WCS increased, and the volume shrinkage decreased, as shown in Figure 1. When the ratios of wood powder/resin were 10:3, 10:6 and 10:9, the carbon yield ratio of WCS made from untreated wood (UT-WCS) was 33.65%, 35.74% and 36.31%, respectively. The carbon yield ratio of WCS made from untreated poplar wood increased by 6.2% as the ratio of wood powder/phenolic resins increases from 10:3 to 10:6, increased by 1.6% as the ratio of wood powder/phenolic resins increases from 10:6 to 10:9. The carbon yield ratios of WCS made from heat-treated wood (UT-WCS) were 34.62%, 40.45% and 40.88%. When the ratio of wood powder/phenolic resin increased from 10:3 to 10:6, the carbon yield increased by 16.8%. When the ratio of wood powder/phenolic resin increased from 10:6 to 10:9, the carbon yield increased by 1.1%. Compared to UT-WCS, the carbon yield of WCS made from heat-treated wood (HT-WCS) had the same increasing tendency and higher carbon yield. This can be seen from the TGA of heat-treated wood due to its relatively increased content of lignin. For UT-WCS, when the ratios of wood powder/resin were 10:3, 10:6 and 10:9, the volume shrinkage ratio was 64.25%, 62.71%, 61.43%, respectively. The volume shrinkage ratio decreased by 2.4% as the ratio of phenolic resins increases from 10:3 to 10:6 and 2% as the ratio of phenolic resins increases from 10:6 to 10:9, respectively. For HT-WCS, the volume shrinkage ratios were 62.67%, 60.22%, 59.88%, respectively. The volume shrinkage ratio decreased by 3.9% as the ratio of phenolic resins increases from 10:3 to 10:6 and 0.6% as the ratio of phenolic resins increases from 10:6 to 10:9, respectively. The volume shrinkage of WCS mainly accounted for the decomposition of wood powder, resin and the formation of carbon/carbon composite. The higher the content of wood powder, the higher the volume shrinkage. Moreover, the volume shrinkage rate of UT-WCS was higher than that of HT-WCS. This is because untreated wood is more gasified than heat-treated wood, which can be proved by the TG graph. ANOVA test results indicated that there was a significant difference between the carbon yield and volumetric shrinkage of untreated and heat-treated of WCS at the 99% confidence interval (p < 0.01). Duncan’s test classified the value of the carbon yield and volumetric shrinkage into two groups (Figure 3 and Figure 4). Considering the change rate of carbon yield and volume shrinkage with increasing wood powder/resin ratio from 10:6 to 10:9, it is suitable to use the 10:6 ratio of wood powder/resin to prepare, such that the WCS is manufactured with higher carbon yield, lower shrinkage and lower cost of PF resin. Therefore, the ratio of wood powder/resin with 10:6 was employed in the following analysis.
3.2.2. Elemental Analysis of WCS
The C, H, O and N elements of wood powder and WCS are shown in Table 1.

Table 1.
Elemental composition of different samples.
For the wood powder, the elemental analysis shows that heat-treated poplar wood has higher carbon content and lower O and H content than untreated wood. This is mainly due to the dehydration of the carbohydrates to produce aldehydes and the decarboxylation (cleavage of acetic acid from hemicelluloses) during heat treatment, which resulted in a reduction of O and H content [35,36]. Therefore, the C content increases after heat treatment. The result can further explain the higher carbon yield of heat-treated wood in the TG graph. After sintering, for wood powder, the breaking down of C-O and C-C bonds in the ring could confirm the decomposition of cellulose and lignin, and result to releasing CO2, CO and other volatile organic gases, then further developing the aromatic polynuclear structures after sintering at 1000 °C [37]. For phenolic resin, the first step is to finish the dehydration reaction, including the thermal curing reaction of hydroxy groups and hydrogen groups within the aromatic ring and the condensation between methylene and hydroxyl groups, releasing the volatile substances such as CH4, CO2 and CO. Finally, the condensation aromatic polynuclear structure is formed [38]. As a result, the C-content of WCS greatly increases, and the O and H content sharply decreased compared to wood powder. For the WCS, these results show that the carbon element of WCS made from heat-treated wood (HT-WCS) was much higher than that of WCS made from untreated wood (UT-WCS), which is in agreement with the regular carbon yield. Besides, the contents of H, O and N are lower than that of UT-WCS. This is due to HT-WCS having a more stable aromatic structure.
3.3. SEM Micrographs of WCS
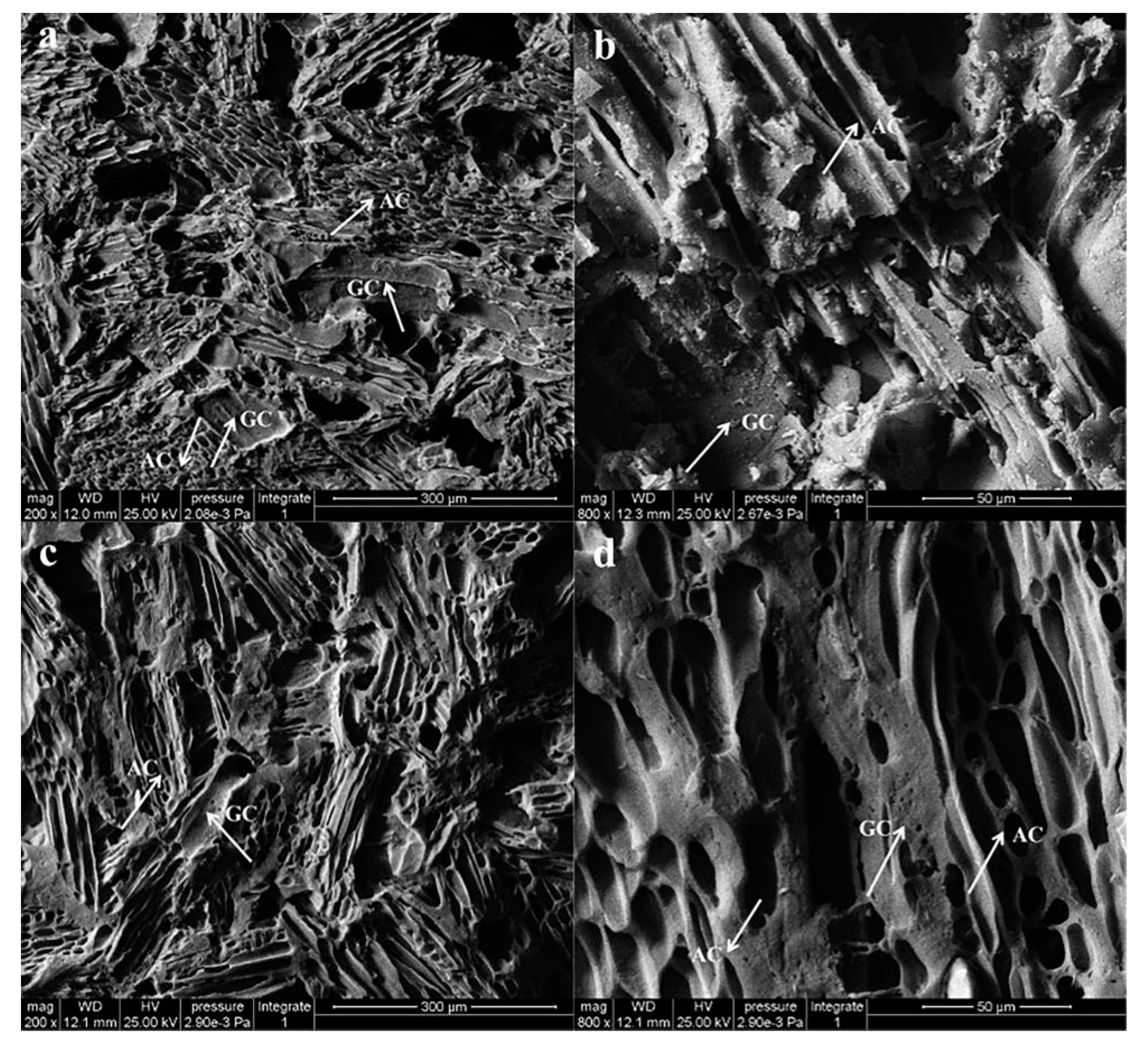
As Figure 5 showed, it displays the SEM micrographs of WCS made from poplar powder phenolic resin composite with a weight ratio of 10:6 carbonized at 1000 °C. Normally, the carbonization progress of organic compounds involves the changes of chemical performance and structural organization. The chemical changes include carbonization, dehydrogenation and deoxidization under low temperatures condition, and the graphite precipitation at high temperatures is the main characteristic of structural changes. Wood powder generated amorphous carbon with porous structure, and the phenolic resin was translated into graphite at high temperatures [39]. It is well known that cellulose, the main component of wood, is a long chain macromolecule and phenolic resin that contains phenyl groups in its molecular structure. After dehydrogenation and deoxidization, the benzene ring group of the composites is likely to precipitate out of the carbon phase, forming its ordered carbon network structure, and then forming a well-stacked graphite structure at high temperatures.
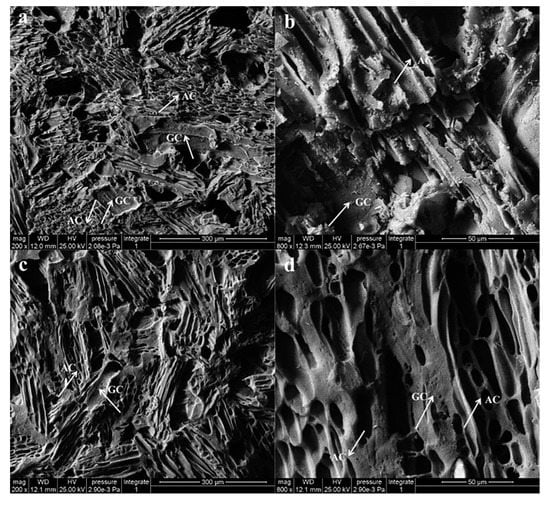
Figure 5.
(a,b): UT-WCS; (c,d): HT-WCS (a,c): magnified 200 times; (b,d): magnified 800 times) (AC indicates amorphous carbon from wood powder and GC indicates glassy carbon from phenolic resin).
The WCS is a typical porous material (Figure 5a–d). Carbonized wood powder and phenolic resin produced pores with different shrinkage rates and uneven structures during carbonization. The larger pores have a regular shape and size, which depend on the diameter and density of the wood powder. The wood powder has the original cellular structure and texture with a regular shape, retained from wood. During the carbonization process, wood carbon existed as particles, maintaining the texture and porous structure of the wood powder. Other tiny pores in WCS are formed by emitting volatile gas products [5]. From Figure 5b,d, more pores exist on the glassy carbon of HT-WCS, which indicates that more gas escaped from the phenolic resin during the carbonization process. For the thermo-modified wood, lignin and carbohydrates are gradually converted into volatiles and ‘extractive like material’ during pyrolysis, which in turn is partly degraded, together with the original extractives into various volatile products [11].
After being sintered at 1000 °C, partial glassy carbon, which comes from phenolic resin, is keeping moving on with continuous structure, and the phenolic resin-poplar powder is carbonized into WCS. Figure 5a,c figured that the carbonized wood powders get in touch with other through forming glassy carbon bridges. HT-WCS (WCS made from heat-treated wood) exhibits denser and smoother surface along with more pores. As shown in Figure 5a, UT-WCS (WCS made from untreated wood) is of heterogeneous laminated microstructure due to untreated wood powder, which tends to aggregate into smaller particles or even agglomerate when mixed with phenolic resins. This is determined by the higher density and the compact structure of wood powder. The glass carbon produced by phenolic resin is separated from the amorphous carbon produced by wood powder. However, HT-WCS exhibits a topologically uniform microstructure. The phenolated wood carbon formed a continuous part, and the interconnected open form network structure is obvious. Small glassy carbon regions are dispersed among the wood carbon particles, as shown in Figure 5c,d. Glass carbon originating from phenolic resin and amorphous carbon originating from wood powder grow together, and the interface gradually disappears. The lower density and moisture content and looser structure facilitate the mixing of heat-treated wood powder with phenolic resin. As a result, the surface of the wood carbon was almost covered by the phenolated wood carbon, due to a large amount of accumulated phenol resin in the vessels of heat-treated wood, which strengthened the cell wall and improved the bonding strength between wood particles. In addition, the heat-treated wood powder contains more lignin that can play the role of an adhesive. Higher content of phenolic resin could result to a higher content of glassy carbon in WCS, then strengthen the contact between the carbonized wood powders [40,41].
3.4. FTIR Analysis
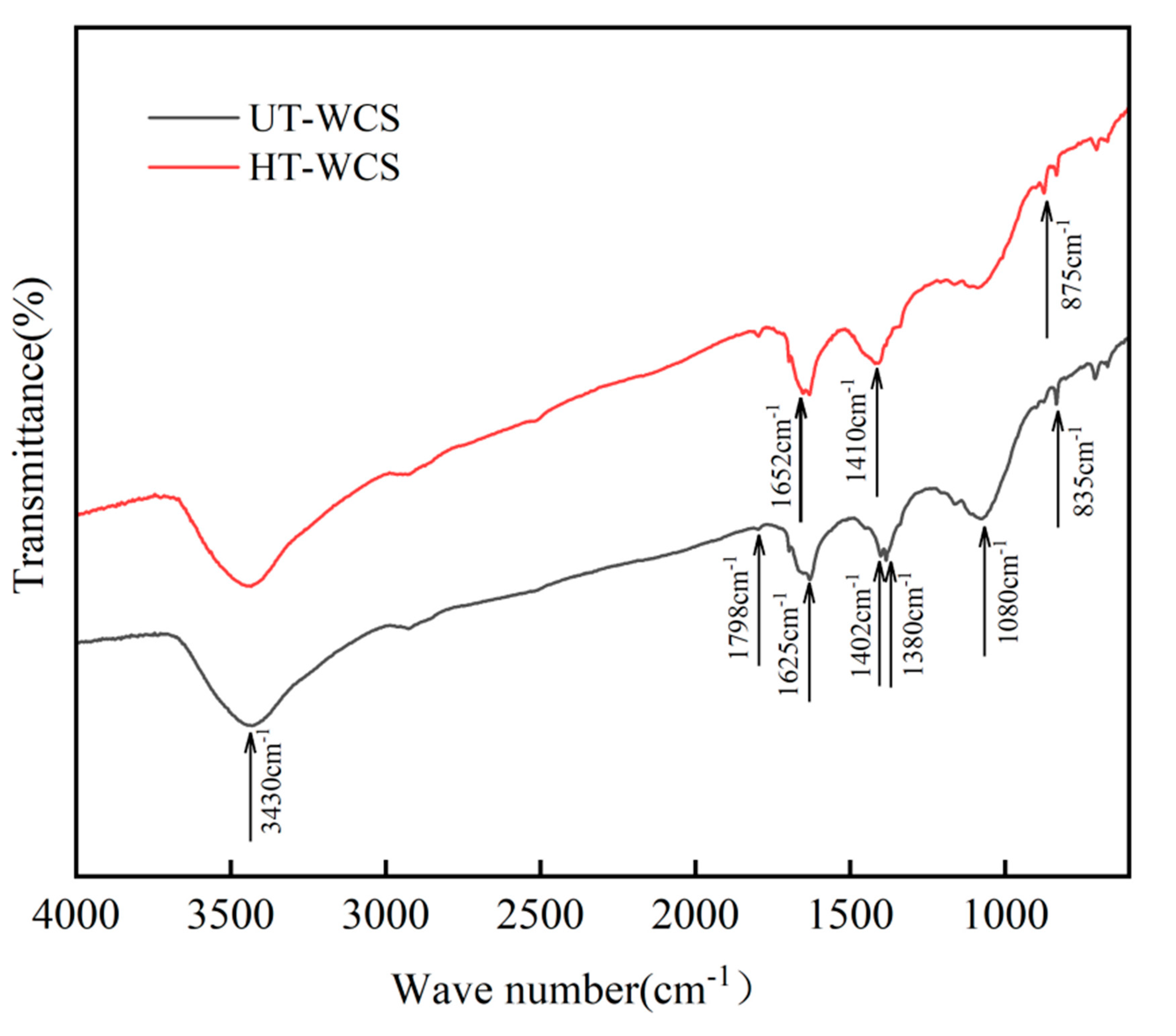
Popescu et al. [42] confirmed that the intensity of the hydroxyl group (-OH) attributed to 3360 cm−1 was reduced after heat treatment due to partial esterification of cellulose in the amorphous zone and the deacetylation reaction of hemicellulose. This result is consistent with Tjeerdsma [35]. The acetyl group of hemicellulose partially hydrolyzes to form acetyl, which in turn promotes hemicellulose hydrolysis. Therefore, the absorption intensity of xylan (peaks at 1740 cm−1 related to the C = O stretching) was reduced. The vibration of the aromatic ring between 1543 cm−1 and 1507 cm−1, and the stretching vibration of C-O-C of lignin at 1242 cm−1 increased. In addition, the peak strength of tetrad substitution on the benzene ring increased, indicating that the relative content of lignin increased. The FTIR spectra of WCS prepared from different wood powders are shown in Figure 6.
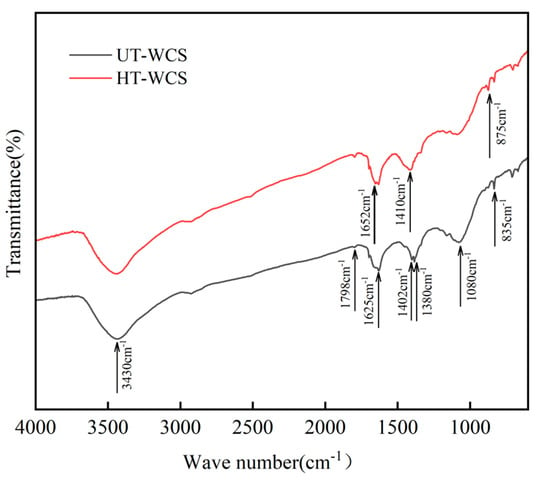
Figure 6.
The FTIR spectra of WCS prepared from the different wood powders.
The sintering of WCS is a process of fracture, reaction and recombination of functional groups of wood powder and phenolic resin at a certain temperature. The C = O bond for wood at 1740 cm−1 was not found in the spectra of WCS, indicating that hemicellulose is completely degraded after sintering. The peak at 3430 cm−1 belongs to the hydroxyl (-OH). The absorption peak width of HT-WCS is narrower than that of UT-WCS. For the HT-WCS, peaks at 1798 cm−1 are attributed to C = O telescopic vibration or the acetyl group. The peaks are higher than those of UT-WCS due to the degradation of more acetyl groups by hemicellulose polysaccharides. In HT-WCS, the C-H stretching in methyl was observed at 1650 cm−1. However, the peaks that do not appear in UT-WCS might be due to insufficient pyrolysis of phenols produced by lignin. The C = C stretching of the aromatic ring band was observed at 1625 cm−1. For WCS, around 1400 cm−1 is the expansion of aromatic hydrocarbon C = C, which is much stronger than wood, indicating that WCS has obvious aromatization after sintering. The absorption of phenolic hydroxyl groups happens at 1380 cm−1. The peak related to the stretching of C-O in aromatic structure connected with the alkyl group is clearly visible at 1080 cm−1. The absorption peak of UT-WCS is stronger than that of HT-WCS, due to reduced cellulose and hemicellulose degradation after sintering [5]. Peaks at 875 cm−1 and 835 cm−1 are attributed to C-H expansion outside the benzene ring, due to the hydroxymethyl synthesis reaction of formaldehyde and phenol. Since the heat treatment sample contains more phenols and aldehydes, hydroxylation reaction can also be completed at high temperatures, similar to the carbonization of phenolic resin.
3.5. XRD Analysis
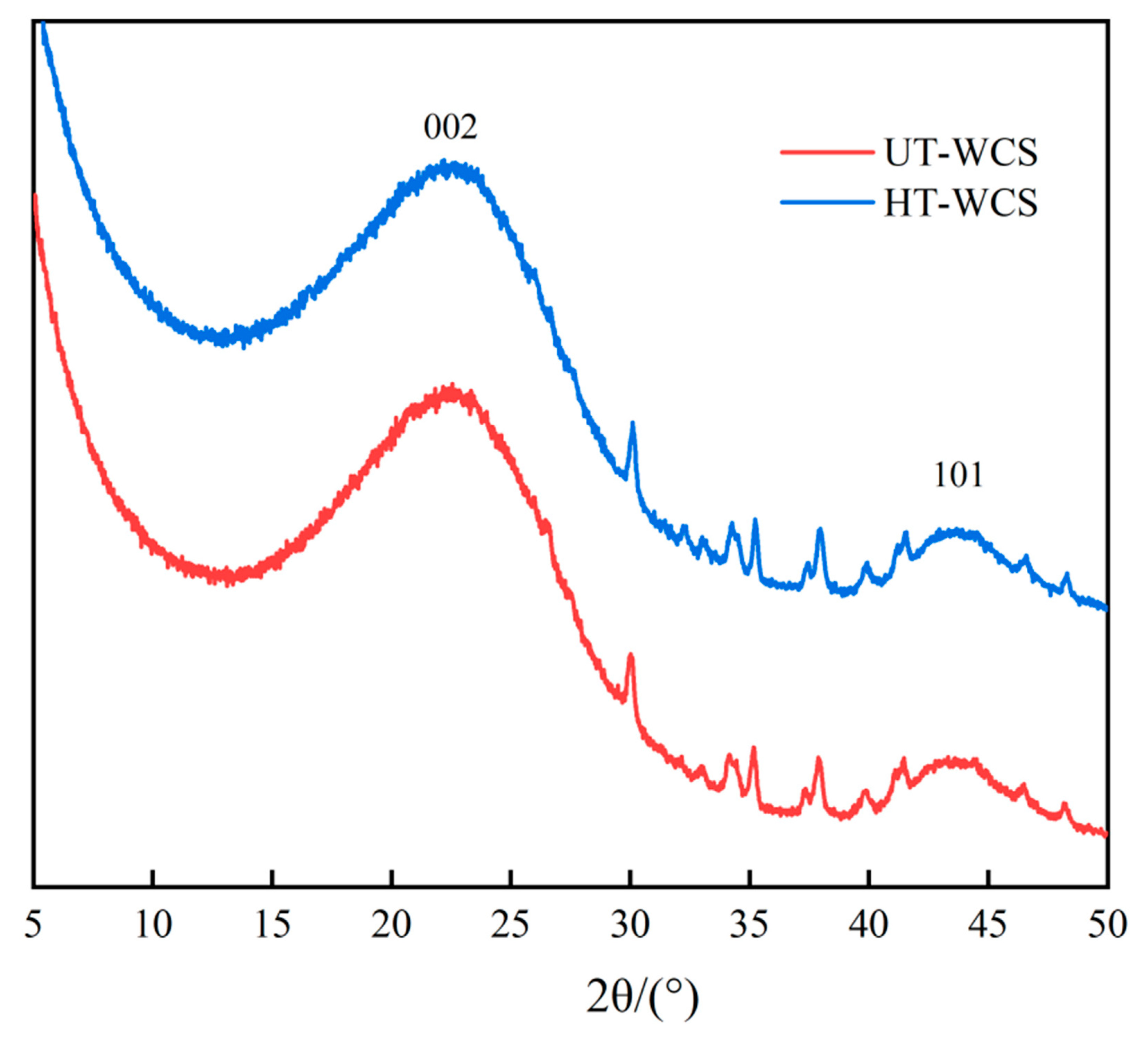
WCS undergoes a series of chemical reactions during high temperature carbonization, accompanied by physical and structural changes. Amorphous carbon was formed by the chemical reaction of lignin, cellulose and hemicellulose in wood, such as pyrolysis, condensation and rearrangement [38,39]. Figure 7 shows the XRD patterns of WCS.
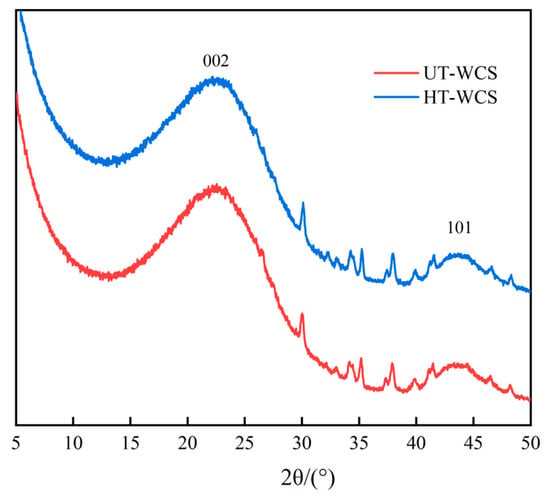
Figure 7.
The wide-angle X-ray diffraction graph of the WCS.
The patterns showing two obvious graphite peaks, attributed to broad (002) and low intensity (101) planes [43], indicate that the development of the hexagonal network layer stack is roughly parallel to each other, called a turbine structure [44], while specific broad peaks of cellulose and other linear molecules in the wood did not appear at 2θ = 18.15° [45]. The shape and strength of the (002) peak indicate that WCS contains amorphous carbon, turbine structure and graphite [5]. As shown in Figure 7, the position and intensity of the diffraction peaks in the spectra of HT-WCS and UT-WCS are almost similar, which indicates that the order degree of charcoal structure and glassy carbon structure from two different materials are analogous, and the detectable effect of different wood powders on the XRD pattern of WCS is not found.
4. Conclusions
In this work, a new WCS was prepared from phenolic resin-poplar powder composite material. The WCS made from thermal treated poplar residue may apply a new method for the application of heavy metal adsorption in sewage; meanwhile, WCS are also expected to be used as thermal insulation materials. The effect of different wood powders, the weight ratio of wood powders and phenolic resin on the basic properties of WCS was studied. The microstructure and chemical structure were investigated at a ratio of 10:6. To summarize, the above-mentioned results are shown as follows:
- With the increase in resin content, the carbon yield of WCS increased, and the volume shrinkage decreased. Considering the resource cost, 10:6 is the optimum proportion for preparing WCS. At the same ratio of wood powder/phenolic resins of 10:6 and carbonization temperature, the carbon yield of HT-WCS was 40.45% and of UT-WCS was 35.74%.
- The WCS has an interconnected porous network microstructure composed of amorphous carbon and glass carbon. At the same pyrolysis temperature, the microstructure of HT-WCS is more rigid and homogeneous. This indicates that the thermos-modified wood significantly helps to increase the performance of phenolic resin to mix wood powders, and leads to construct a more uniform porous microstructure, strengthening the bonding between the two types of carbon.
- There is typical non-graphitizable carbon-containing C = C bonds, C-O-C bonds and C–H structure. HT-WCS and UT-WCS have analogous chemical structures. For UT-WCS, the absorption peak of stretching C-O in the aromatic structure connected with the alkyl group is stronger than that of HT-WCS.
- The XRD patterns indicated that WCS contains amorphous carbon, turbine structure and graphite. The intensity and position of diffraction peaks in the pattern were almost similar with different materials, which indicate that the effect of different wood powders on the XRD pattern of WCS is minimal.
Author Contributions
H.C., Methodology, Software, Data curation, Writing-Original draft preparation, and Investigation; M.B., Writing-Reviewing and Editing; M.H., Writing-Reviewing and Editing; D.C., Resources, Supervision, Writing-Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the National Natural Science Foundation of China (31700488), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okabe, T.; Saito, K.; Hokkirigawa, K. New porous carbon materials, Woodceramics: Development and fundamental properties. J. Porous Mater. 1996, 2, 207–213. [Google Scholar] [CrossRef]
- Wu, W.T.; Tan, F.L.; Xu, F. Preparation and Characteristic of Composites with Wheat Straw Woodceramic/Attapulgite. Appl. Mech. Mater. 2013, 330, 126–130. [Google Scholar] [CrossRef]
- Ramirez-Rico, J.; Martínez-Fernandez, J.; Singh, M. Biomorphic ceramics from wood-derived precursors. Int. Mater. Rev. 2017, 62, 465–485. [Google Scholar] [CrossRef]
- Singh, M. Environmentally conscious ceramics (ecoceramics) from natural wood precursors. Curr. Opin. Solid State Mater. Sci. 2003, 7, 247–254. [Google Scholar] [CrossRef]
- Qian, J.; Jin, Z.; Wang, J. Structure and basic properties of woodceramics made from phenolic resin–basswood powder composite. Mater. Sci. Eng. A 2004, 368, 71–79. [Google Scholar] [CrossRef]
- Mizutani, M.; Takase, H.; Adachi, N.; Ota, T.; Daimon, K.; Hikichi, Y. Porous ceramics prepared by mimicking silicified wood. Sci. Technol. Adv. Mater. 2005, 6, 76–83. [Google Scholar] [CrossRef]
- Jones, D.; Sandberg, D.; Goli, G.; Todaro, L. Wood Modification in Europe: Processes, Products, Applications; GESAAF—University of Florence: Florence, Italy, 2018; p. 41. [Google Scholar]
- Bal, B.C.; Bektaş, I. The Effects of Heat Treatment on Some Mechanical Properties of Juvenile Wood and Mature Wood ofEucalyptus grandis. Dry. Technol. 2013, 31, 479–485. [Google Scholar] [CrossRef]
- Chung, H.; Park, Y.; Yang, S.-Y.; Kim, H.; Han, Y.; Chang, Y.-S.; Yeo, H. Effect of heat treatment temperature and time on sound absorption coefficient of Larix kaempferi wood. J. Wood Sci. 2017, 63, 575–579. [Google Scholar] [CrossRef]
- Santos, J.A. Mechanical behaviour of Eucalyptus wood modified by heat. Wood Sci. Technol. 2000, 34, 39–43. [Google Scholar] [CrossRef]
- Yildiz, S.; Gezer, E.D.; Yildiz, U.C. Mechanical and chemical behavior of spruce wood modified by heat. Build. Environ. 2006, 41, 1762–1766. [Google Scholar] [CrossRef]
- Klingner, R.; Sell, J.; Zimmermann, T.; Herzog, A.; Vogt, U.; Graule, T.; Thurner, P.; Beckmann, F.; Müller, B. Wood-Derived Porous Ceramics via Infiltration of SiO2-Sol and Carbothermal Reduction. Holzforschung 2003, 57, 440–446. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, Y.C.; Liu, H.Z.; Li, Y.J. Study of Physical-mechanical Properties of Bamboos through High Temperature Heat Treatment. For. Mach. Woodwork. Equip. 2012, 40, 22–24. [Google Scholar]
- Qian, J.-M.; Wang, J.-P.; Qiao, G.-J.; Jin, Z.-H. Preparation of porous SiC ceramic with a woodlike microstructure by sol-gel and carbothermal reduction processing. J. Eur. Ceram. Soc. 2004, 24, 3251–3259. [Google Scholar] [CrossRef]
- Shi, J.Y.; She, Y.; Wang, X.J. Study on modified phenolic resin bonded high moisture content veneer. J. Jilin Forest. Univ. 1997, 1, 17–21. [Google Scholar]
- Unsal, O.; Korkut, S.; Atik, C. The effect of heat treatment on some properties and colour in eucalyptus (Eucalyptus camaldulensis dehn.) wood. Maderas. Cienc. Technol. 2003, 5, 145–152. [Google Scholar] [CrossRef]
- Mitsui, K.; Takada, H.; Sugiyama, M.; Hasegawa, R. Changes in the Properties of Light-Irradiated Wood with Heat Treatment. Part 1. Effect of Treatment Conditions on the Change in Color. Holzforschung 2001, 55, 601–605. [Google Scholar] [CrossRef]
- Lekounougou, S.; Petrissans, M.; Jacquot, J.-P.; Gelhaye, E.; Gérardin, P. Effect of heat treatment on extracellular enzymatic activities involved in beech wood degradation by Trametes versicolor. Wood Sci. Technol. 2008, 43, 331–341. [Google Scholar] [CrossRef]
- Kocaefe, D.; Poncsak, S.; Tang, J.; Bouazara, M. Effect of heat treatment on the mechanical properties of North American jack pine: Thermogravimetric study. J. Mater. Sci. 2010, 45, 681–687. [Google Scholar] [CrossRef]
- Chaouch, M.; Pétrissans, M.; Petrissans, A.; Gerardin, P. Use of wood elemental composition to predict heat treatment intensity and decay resistance of different softwood and hardwood species. Polym. Degrad. Stab. 2010, 95, 2255–2259. [Google Scholar] [CrossRef]
- Bourgois, J.; Bartholin, M.-C.; Guyonnet, R. Thermal treatment of wood: Analysis of the obtained product. Wood Sci. Technol. 1989, 23, 303–310. [Google Scholar] [CrossRef]
- Dong, H.; Bahmani, M.; Rahimi, S.; Humar, M. Influence of Copper and Biopolymer/Saqez Resin on the Properties of Poplar Wood. Forests 2020, 11, 667. [Google Scholar] [CrossRef]
- Gao, J.M.; Meng, L.X.; Qi, J.K.; Ma, T.; Yan, Z.L.; Zhou, Z.L. Research on Silicon Carbide Woodceramics from Poplar Fiber. Rare Met. Mater. Eng. 2011, 40, 223–226. [Google Scholar]
- Mar’yandyshev, P.A.; Chernov, A.A.; Lyubov, V.K. Analysis of thermogravimetric data for different forms of wood. Solid Fuel Chem. 2015, 49, 117–122. [Google Scholar] [CrossRef]
- Qu, T.; Guo, W.; Shen, L.; Xiao, J.; Zhao, K. Experimental Study of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose, and Lignin. Ind. Eng. Chem. Res. 2011, 50, 10424–10433. [Google Scholar] [CrossRef]
- Zaman, A.; Alén, R.; Kotilainen, R. Thermal behavior of Scots pine (Pinus sylvestris) and silver birch (Betula pendula) at 200–230 °C. Wood Fiber Sci. 2000, 32, 138–143. [Google Scholar]
- Runkel, R.O.H.; Wilke, K.D. Zur Kenntnis des thermoplastischen Verhaltens von Holz. Eur. J. Wood Wood Prod. 1951, 9, 260–270. [Google Scholar] [CrossRef]
- Kollmann, F.; Fengel, D. Änderungen der chemischen Zusammensetzung von Holz durch thermische Behandlung. Eur. J. Wood Wood Prod. 1965, 23, 461. [Google Scholar] [CrossRef]
- Liu, G.; Song, H.; Wu, J. Thermogravimetric study and kinetic analysis of dried industrial sludge pyrolysis. Waste Manag. 2015, 41, 128–133. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose–hemicellulose and cellulose–lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Islamova, S.I.; Khamatgalimov, A. Thermogravimetric and kinetic analyses of the thermal decomposition of fuel wood. Solid Fuel Chem. 2017, 51, 83–87. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, M.; Zhao, L.; Zhu, L. Influence of Interactions among Three Biomass Components on the Pyrolysis Behavior. Ind. Eng. Chem. Res. 2018, 57, 5241–5249. [Google Scholar] [CrossRef]
- Chen, T.; Li, L.; Zhao, R.; Wu, J. Pyrolysis kinetic analysis of the three pseudocomponents of biomass–cellulose, hemicellulose and lignin. J. Therm. Anal. Calorim. 2016, 128, 1825–1832. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Militz, H. Chemical changes in hydrothermal treated wood: FTIR analysis of combined hydrothermal and dry thermaly modified wood. Eur. J. Wood Wood Prod. 2005, 63, 102–111. [Google Scholar] [CrossRef]
- Windeisen, E.; Strobel, C.; Wegener, G. Chemical changes during the production of thermo-treated beech wood. Wood Sci. Technol. 2007, 41, 523–536. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, D.K. Fractionated Pyrolysis of Biomass and its Components. Appl. Mech. Mater. 2014, 535, 734–737. [Google Scholar] [CrossRef]
- Suda, T.; Kondo, N.; Okabe, T.; Saito, K. Electrical Properties of Woodceramics. J. Porous Mater. 1999, 6, 255–258. [Google Scholar] [CrossRef]
- Fan, T.; Hirose, T.; Okabe, T.; Zhang, D. Surface Area Characteristics of Woodceramics. J. Porous Mater. 2001, 8, 211–217. [Google Scholar] [CrossRef]
- Pan, J.; Cheng, X.-N.; Yan, X.; Zhang, C. Preparation and hierarchical porous structure of biomorphic woodceramics from sugarcane bagasse. J. Eur. Ceram. Soc. 2013, 33, 575–581. [Google Scholar] [CrossRef]
- Oh, S.W.; Hirose, T.; Okabe, T. Manufacturing Characteristics of Wood ceramics from Thinned Small Logs (I)—Resin Impregnation Rate and Bending Strength. J. Korean Wood Sci. Technol. 2000, 28, 56–60. [Google Scholar]
- Popescu, M.-C.; Froidevaux, J.; Navi, P.; Popescu, C.-M. Structural modifications of Tilia cordata wood during heat treatment investigated by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2013, 1033, 176–186. [Google Scholar] [CrossRef]
- Kercher, A.; Nagle, D.C. Evaluation of carbonized medium-density fiberboard for electrical applications. Carbon 2002, 40, 1321–1330. [Google Scholar] [CrossRef]
- Onodera, A.; Terashima, K.; Urushihara, T.; Suito, K.; Sumiya, H.; Satoh, S. High-pressure synthesis of diamond from phenolic resin. J. Mater. Sci. 1997, 32, 4309–4318. [Google Scholar] [CrossRef]
- Tzeng, S.-S.; Chr, Y.-G. Evolution of microstructure and properties of phenolic resin-based carbon/carbon composites during pyrolysis. Mater. Chem. Phys. 2002, 73, 162–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).