Management Intensity and Forest Successional Stages as Significant Determinants of Small Mammal Communities in a Lowland Floodplain Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Sampling
2.3. Evaluation of Environmental Characteristics
2.4. Statistical Analyses
2.4.1. Regression Analysis
2.4.2. Multivariate Data Analysis
3. Results
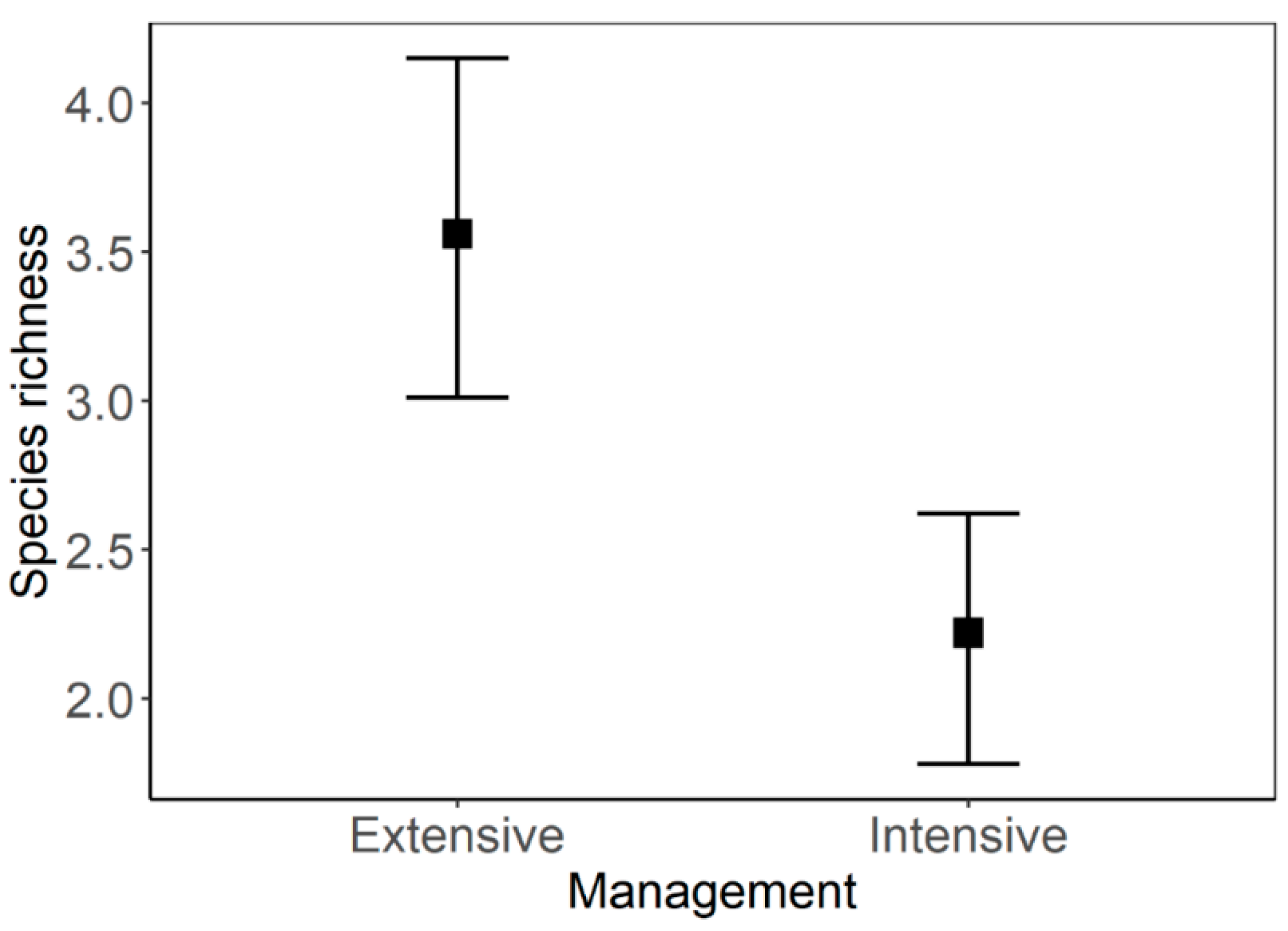
3.1. Species Richness and Abundance
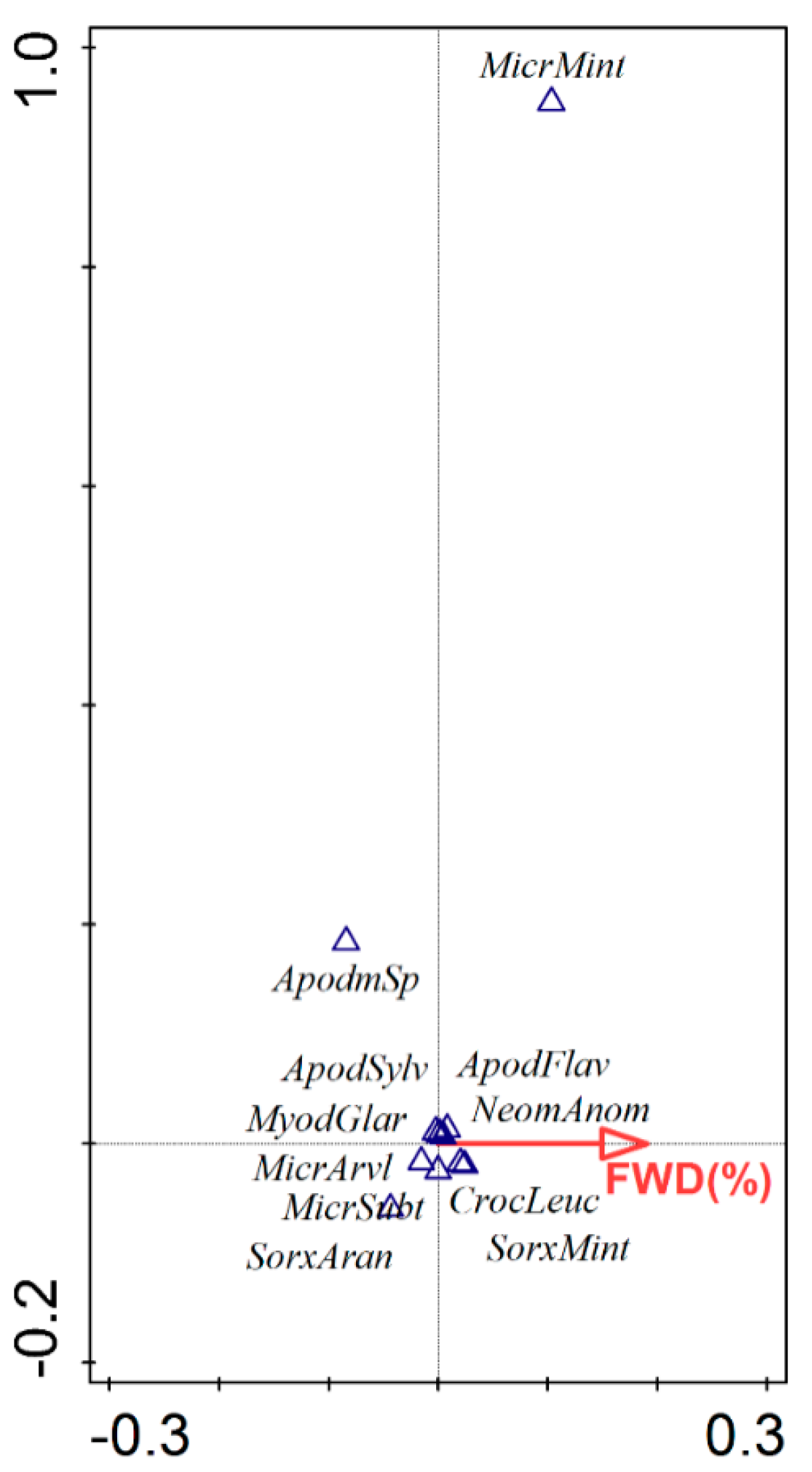
3.2. Species Assemblage
4. Discussion
4.1. Responses of Species Richness and Abundance to Forest Managements
4.2. Responses of Species Assemblage to Forest Managements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stachová, J. Forests in the Czech public discourse. J. Landsc. Ecol. 2019, 11, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.B.; Franklin, J.F. Conserving Forest Biodiversity: A Comprehensive Multiscaled Approach; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Anthony, R.G.; Forsman, E.D.; Green, G.A.; Witmer, G.; Nelson, S.K. Small mammal populations in riparian zones of different-aged coniferous forests. Murrelet 1987, 68, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Mullen, K.; O’Halloran, J.; Breen, J.; Giller, P.; Pithon, J.; Kelly, T. Distribution and composition of carabid beetle (Coleoptera, Carabidae) communities across the plantation forest cycle—Implications for management. For. Ecol. Manag. 2008, 256, 624–632. [Google Scholar]
- Purchart, L.; Tuf, I.H.; Hula, V.; Suchomel, J. Arthropod assemblages in Norway spruce monocultures during a forest cycle—A multi-taxa approach. For. Ecol. Manag. 2013, 306, 42–51. [Google Scholar] [CrossRef]
- Lešo, P.; Lešová, A.; Kropil, R.; Kanuch, P. Response of the dominant rodent species to close-to nature logging practices in a temperate mixed forest. Ann. For. Sci. 2016, 59, 259–268. [Google Scholar]
- Gallé, R.; Gallé-Szpisjak, N.; Torma, A. Habitat structure influences the spider fauna of short-rotation poplar plantations more than forest age. Eur. J. For. Res. 2017, 136, 51–58. [Google Scholar] [CrossRef]
- Niemelä, J.; Haila, Y.; Halme, E.; Lahti, T.; Pajunen, T.; Punttila, P. The distribution of carabid beetles in fragments of old coniferous taiga and adjacent managed forest. Ann. Zool. Fenn. 1988, 25, 107–119. [Google Scholar]
- Elek, Z.; Magura, T.; Tóthmérész, B. Impacts of non-native Norway spruce plantation on abundance and species richness of ground beetles (Coleoptera: Carabidae). Web Ecol. 2001, 2, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Magura, T.; Bogyó, D.; Mizser, S.; Nagy, D.D.; Tóthmérész, B. Recovery of ground-dwelling assemblages during reforestation with native oak depends on the mobility and feeding habits of the species. For. Ecol. Manag. 2015, 339, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.B.; Wood, J.; McBurney, L.; Blair, D.; Banks, S.C. Single large versus several small: The SLOSS debate in the context of bird responses to a variable retention logging experiment. For. Ecol. Manag. 2015, 339, 1–10. [Google Scholar] [CrossRef]
- Manuwal, D.A.; Huff, M.H. Spring and winter bird populations in a Douglas-fir forest sere. J. Wildl. Manage. 1987, 51, 586–595. [Google Scholar] [CrossRef]
- Luque, C.A.; Gers, C.; Lauga, J.; Mariano, N.; Wink, M.; Legal, L. Analysis of forestry impacts and biodiversity in two Pyrenean forests through a comparison of moth communities (Lepidoptera, Heterocera). Insect Sci. 2007, 14, 323–338. [Google Scholar] [CrossRef]
- Košulič, O.; Hamřík, T.; Lvončík, S. Patterns of change in the species composition of vascular plants during different succession stages and management intensity of a lowland floodplain forest. Biologia 2020, 75, 1801–1813. [Google Scholar] [CrossRef]
- Niemelä, J.; Langor, D.; Spence, J.R. Effects of clear-cut harvesting on boreal ground-beetle assemblages (Coleoptera: Carabidae) in western Canada. Conserv. Biol. 1993, 7, 551–561. [Google Scholar]
- Sipos, J.; Hodecek, J.; Kuras, T.; Dolny, A. Principal determinants of species and functional diversity of carabid beetle assemblages during succession at post-industrial sites. Bull. Entomol. Res. 2017, 107, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2020, 132, 39–50. [Google Scholar] [CrossRef]
- Hannah, L.; Carr, J.L.; Lankerani, A. Human disturbance and natural habitat: A biome level analysis of a global data set. Biodiv. Conserv. 1995, 4, 128–155. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change. A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009. [Google Scholar]
- Hodecek, J.; Kuras, T.; Sipos, J.; Dolny, A. Role of reclamation in the formation of functional structure of beetle communities: A different approach to restoration. Ecol. Eng. 2016, 94, 537–544. [Google Scholar] [CrossRef]
- Khanaposhtani, M.G.; Kaboli, M.; Karami, M.; Etemad, V. Effect of habitat complexity on richness, abundance and distributional pattern of forest birds. Environ. Manag. 2012, 50, 296–303. [Google Scholar] [CrossRef]
- McElhinny, C.; Gibbons, P.; Brack, C.; Bauhus, J. Forest and woodland stand structural complexity: Its definition and measurement. For. Ecol. Manag. 2005, 218, 1–24. [Google Scholar] [CrossRef]
- Niemelä, J. Management in relation to disturbance in the boreal forest. For. Ecol. Manag. 1999, 115, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Gallé, R.; Maák, I.; Szpisjak, N. The effects of habitat parameters and forest age on the ground dwelling spiders of lowland poplar forests (Hungary). J. Insect Conserv. 2014, 18, 791–799. [Google Scholar] [CrossRef]
- Hédl, R.; Šipoš, J.; Chudomelová, M.; Utinek, D. Dynamics of herbaceous vegetation during four years of experimental coppice introduction. Folia Geobot. 2017, 52, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Machar, I.; Schlossarek, M.; Pechanec, V.; Uradnicek, L.; Praus, L.; Sıvacıoğlu, A. Retention forestry supports bird diversity in managed, temperate hardwood floodplain forests. Forests 2020, 10, 300. [Google Scholar] [CrossRef] [Green Version]
- Šipoš, J.; Hédl, R.; Hula, V.; Chudomelová, M.; Košulič, O.; Niedobová, J.; Riedl, V. Patterns of functional diversity of two trophic groups after canopy thinning in an abandoned coppice. Folia Geobot. 2017, 52, 45–58. [Google Scholar]
- Bogdziewicz, M.; Zwolak, R. Responses of small mammals to clear-cutting in temperate and boreal forests of Europe: A meta-analysis and review. Eur. J For. Res. 2014, 133, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, lichen, and algal communities. Appl. Veg.Sci. 2016, 19, 1–264. [Google Scholar] [CrossRef]
- Quitt, E. Climatic regions of Czechoslovakia. Studia Geographica 1971, 16, 1–73. [Google Scholar]
- Castro, A.; Wise, D.H. Influence of fine woody debris on spider diversity and community structure in forest leaf litter. Biodivers. Conserv. 2009, 18, 3705–3731. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs. User’s Manual and Program Documentation; Simon Fraser University, Institute of Ecosystem Studies: Burnaby, BC, Canada, 1999; 40p. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2020, Vienna. Available online: https://www.r-project.org/ (accessed on 6 June 2020).
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0.; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Krojerová-Prokešová, J.; Homolka, M.; Barančeková, M.; Heroldová, M.; Baňař, P.; Kamler, J.; Purchart, L.; Suchomel, J.; Zejda, J. Structure of small mammal communities on small-sized clearings in managed central European forests. For. Ecol. Manag. 2016, 367, 41–51. [Google Scholar] [CrossRef]
- Hoffmeyer, I. Interaction and habitat selection in the mice Apodemus flavicollis and A. sylvaticus. Oikos 1973, 24, 108–116. [Google Scholar] [CrossRef]
- Gasperini, S.; Mortelliti, A.; Bartolommei, P.; Bonacchi, A.; Manzo, E.; Cozzolino, R. Effects of forest management on density and survival in three forest rodent species. For. Ecol. Manag. 2016, 382, 151–160. [Google Scholar] [CrossRef]
- Suchomel, J.; Čepelka, L.; Purchart, L. Structure and diversity of small mammal communities of lowland forests in the rural central European landscape. Eur. J For. Res. 2012, 131, 1933–1941. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small mammal community response to early meadow–forest succession. For. Ecosyst. 2017, 4, 11. [Google Scholar] [CrossRef]
- Briani, D.C.; Palma, A.R.T.; Vieira, E.M. Post-fire succession of small mammals in the Cerrado of central Brazil. Biodivers. Conserv. 2004, 13, 1023–1037. [Google Scholar] [CrossRef]
- Schoonmaker, P.; McKee, A. Species composition and diversity during secondary succession of coniferous forest in the western cascade mountains of Oregon. For. Sci. 1988, 34, 960–979. [Google Scholar]
- Perrin, A. Alternative life-history styles of small mammals. In Alternative Life-History Styles of Animals (Perspectives in Vertebrate Science); Burton, M.N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 209–242. [Google Scholar]
- Šipoš, J.; Suchomel, J.; Purchart, L.; Kindlmann, P. Main determinants of rodent population fuctuations in managed Central European temperate lowland forests. Mammal Res. 2017, 62, 283–295. [Google Scholar] [CrossRef]
- Fitzgibbon, C.D. Small mammals in farm woodlands: The effects of habitat, isolation and surrounding land-use patterns. J. Appl. Ecol. 1997, 34, 530–539. [Google Scholar] [CrossRef]
- Schlinkert, H.; Ludwig, M.; Batáry, P.; Holzschuh, A.; Kovács-Hostyánszki, A.; Tscharntke, T.; Fischer, C. Forest specialist and generalist small mammals in forest edges and Hedges. Wildlife Biol. 2016, 22, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Butet, A.; Delettre, Y.R. Diet diferentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Kara, Ö.; Bolat, İ.; Çakiroğlu, K.; Öztürk, M. Plant canopy effect on litter accumulation and soil microbial biomass in two temperate forests. Biol. Fertil. Soils 2008, 45, 193–198. [Google Scholar] [CrossRef]
- Montgomery, W.I.; Gurnell, J. The behaviour of Apodemus. In The Ecology of Woodland Rodents Bank Voles and Wood Mice; Flowerdew, S.R., Gurnell, J., Gipps, J.M.W., Eds.; Oxford University Press: Oxford, UK, 1985; pp. 89–115. [Google Scholar]
- Čepelka, L.; Šipoš, J.; Suchomel, J.; Heroldová, M. Can we detect response differences among dominant rodent species to climate and acorn crop in a Central European forest environment? Eur. J For. Res. 2020, 139, 539–548. [Google Scholar] [CrossRef]
- Montgomery, W.I. Intra- and interspecific interactions of Apodemus sylvaticus (L.) and A. flavicollis (Melchior) under laboratory conditions. Anim. Behav. 1978, 26, 1247–1254. [Google Scholar] [CrossRef]
- Kraus, D.; Krumm, F. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; European Forest Institute: Joensuu, Finland, 2013; 284p. [Google Scholar]


| Old-Growth Forest | ||||||
|---|---|---|---|---|---|---|
| Species | Abundance | Scores on the First CCA Axis | Explained Variance | F | p | |
| Intensive | Extensive | |||||
| Apodemus flavicollis | 35 | 9 | −0.216 | 16.4 | 5.2 | <0.01 |
| Apodemus sylvaticus | 2 | 7 | 0.987 | 16.6 | 3.5 | 0.037 |
| Clethrionomys glareolus | 44 | 29 | 0.016 | 2.8 | 0.84 | 0.599 |
| Crocidura leucodon | 0 | 6 | 1.062 | 32.3 | 9.0 | <0.001 |
| Microtus arvalis | 8 | 3 | −0.325 | 5.4 | 1.3 | 0.288 |
| Sorex araneus | 5 | 3 | −0.078 | 2.4 | 0.43 | 0.651 |
| Sorex minutus | 14 | 11 | −0.109 | 9.0 | 3.2 | 0.047 |
| Clearings | ||||||
| Apodemus flavicollis | 6 | 14 | 0.041 | 0.1 | 0.03 | 0.852 |
| Apodemus sylvaticus | 46 | 11 | 1.092 | 18.3 | 12.6 | <0.001 |
| Clethrionomys glareolus | 0 | 12 | −0.771 | 35.1 | 21.4 | <0.001 |
| Crocidura leucodon | 1 | 18 | −0.695 | 25.2 | 0.63 | 0.571 |
| Micromys minutus | 0 | 2 | −0.262 | 1.8 | 0.32 | 0.572 |
| Microtus arvalis | 27 | 103 | −0.095 | 3.2 | 3.7 | 0.056 |
| Microtus subteraneus | 1 | 3 | 0.157 | 0 | 0.008 | 0.928 |
| Neomys anomalus | 0 | 3 | −0.668 | 14.1 | 5.2 | 0.024 |
| Sorex araneus | 0 | 27 | −0.366 | 7.3 | 5.7 | 0.018 |
| Sorex minutus | 1 | 34 | −0.501 | 12.8 | 11.2 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchomel, J.; Šipoš, J.; Košulič, O. Management Intensity and Forest Successional Stages as Significant Determinants of Small Mammal Communities in a Lowland Floodplain Forest. Forests 2020, 11, 1320. https://doi.org/10.3390/f11121320
Suchomel J, Šipoš J, Košulič O. Management Intensity and Forest Successional Stages as Significant Determinants of Small Mammal Communities in a Lowland Floodplain Forest. Forests. 2020; 11(12):1320. https://doi.org/10.3390/f11121320
Chicago/Turabian StyleSuchomel, Josef, Jan Šipoš, and Ondřej Košulič. 2020. "Management Intensity and Forest Successional Stages as Significant Determinants of Small Mammal Communities in a Lowland Floodplain Forest" Forests 11, no. 12: 1320. https://doi.org/10.3390/f11121320
APA StyleSuchomel, J., Šipoš, J., & Košulič, O. (2020). Management Intensity and Forest Successional Stages as Significant Determinants of Small Mammal Communities in a Lowland Floodplain Forest. Forests, 11(12), 1320. https://doi.org/10.3390/f11121320






