Stomatal and Leaf Morphology Response of European Beech (Fagus sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Locality Description and Plant Material
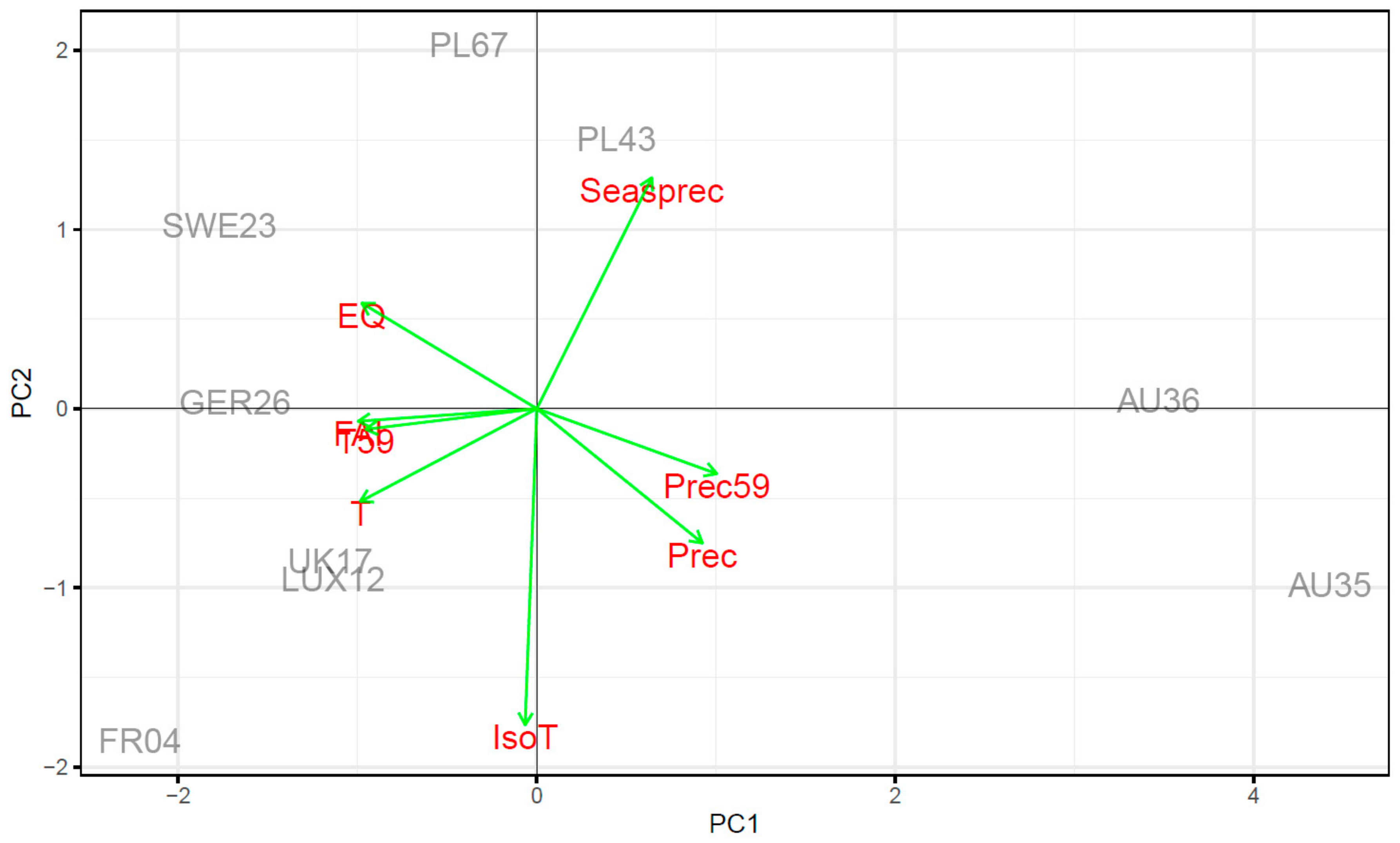
- Ellenberg quotient (EQ) [47]Th—mean temperature of the hottest month (here July), Prec—annual sum of precipitation.
- Forest Aridity Index (FAI) [48]T7–8—mean temperature of July and August, Prec5–7—precipitation sum for May to July, Prec7–8—precipitation sum for July to August.
- Isothermality (IsoT)T—annual mean temperature, Th—mean temperature of warmest month, Tc—mean temperature of coldest month, TMAX—max temperature of warmest month, TMIN—min temperature of coldest month.
- Precipitation seasonality (SeasPrec)σPrec—annual standard deviation of precipitation, Prec—annual sum of precipitation.
2.2. Stomatal and Leaf Morphological Traits
2.3. Quantification of Phenotypic Plasticity
2.4. Statistical Analysis
3. Results
3.1. Stomatal and Leaf Morphological Traits
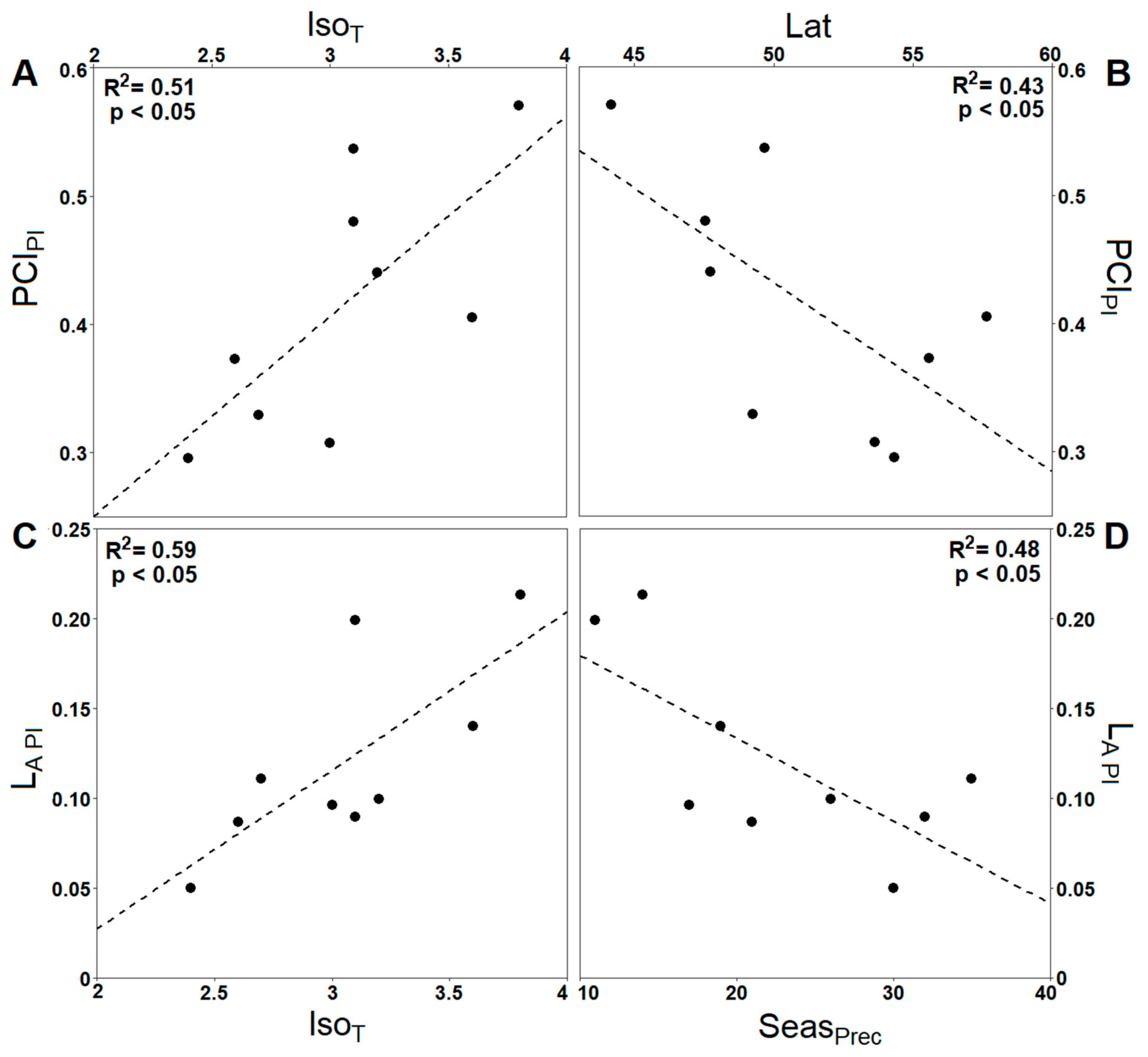
3.2. Impact of Climate Ecodistance on Stomatal and Leaf Morphological Traits
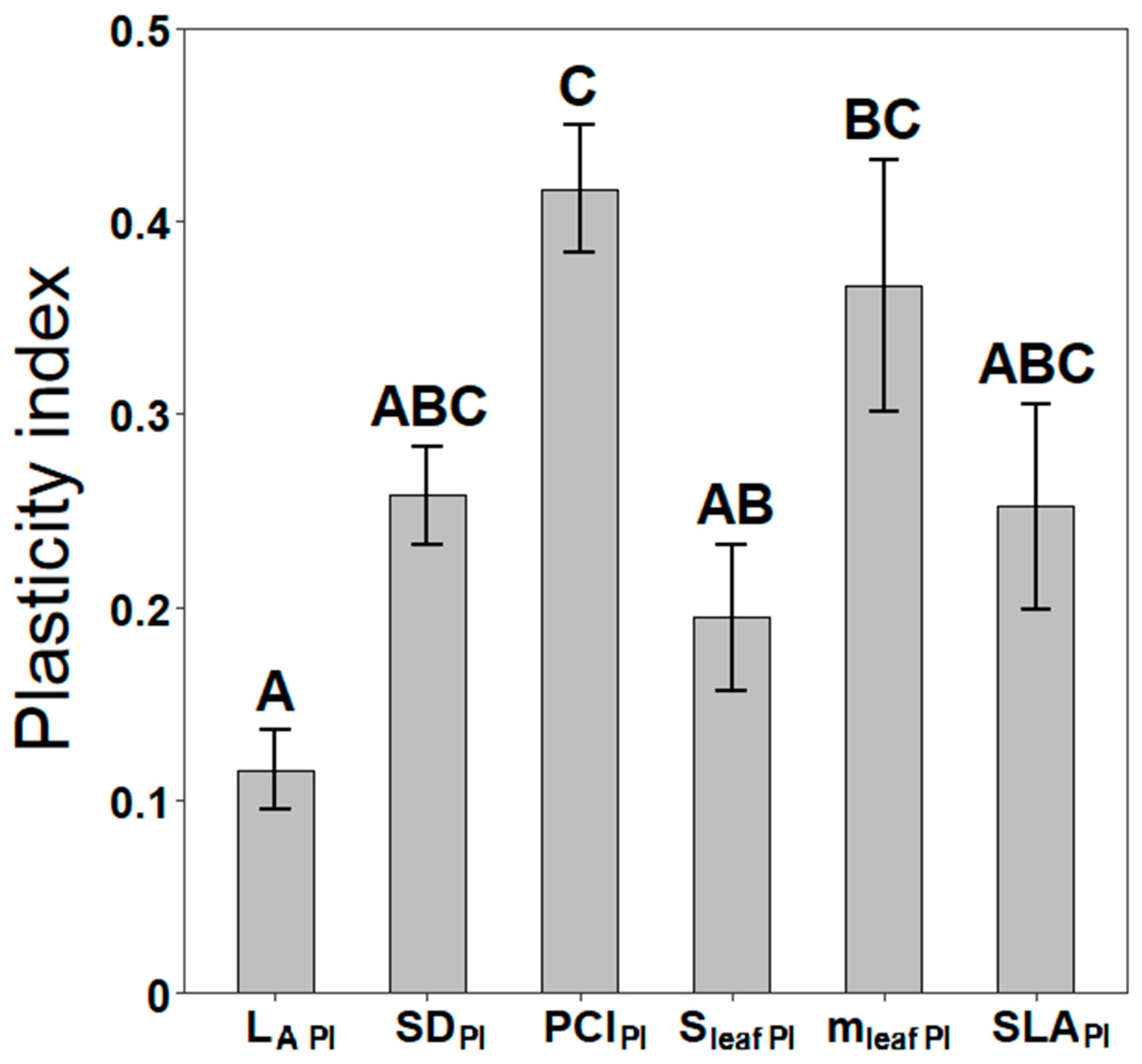
3.3. Phenotypic Plasticity of Stomatal and Leaf Morphological Traits
4. Discussion
4.1. Functional Aspects of the Adaptive Response
4.2. Climate Ecodistance as an Effective Tool for Provenance Research
4.3. Phenotypic Plasticity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| SK | LA | SD | PCI | Sleaf | mleaf | SLA |
| FR04 | BC | AC | AB | B | A | AB |
| LUX12 | AB | B | A | AB | ABC | BC |
| UK17 | AB | AB | AB | AB | AB | ABC |
| SWE23 | AB | C | B | AB | ABCD | A |
| GER26 | A | ABC | AB | A | ABC | C |
| AU35 | A | ABC | AB | AB | BCD | A |
| AU36 | AB | ABC | AB | A | D | AB |
| PL43 | AB | AB | A | AB | A | BC |
| PL67 | C | AC | A | A | CD | AB |
| CZ | LA | SD | PCI | Sleaf | mleaf | SLA |
| FR04 | C | ABCD | AB | B | D | A |
| LUX12 | C | AB | B | AB | ACD | A |
| UK17 | AB | ABCD | ACD | A | ABC | A |
| SWE23 | AB | D | CD | A | B | A |
| GER26 | B | CD | D | A | AB | A |
| AU35 | AB | ABC | AC | A | AB | A |
| AU36 | AB | A | AB | A | ABC | A |
| PL43 | AC | BCD | ACD | AB | ABCD | A |
| PL67 | AC | ABCD | AB | AB | CD | A |
| Trait | EQED | LongED | LatED | EQED + LongED | EQED + LatED | LongED + LatED | Full Model |
| SD | 0.704 | 0.014 | 0.007 | 0.125 | 0.131 | 0.002 | 0.016 |
| LA | 0.222 | 0.31 | 0.168 | 0.202 | 0.031 | 0.047 | 0.021 |
| PCI | 0.211 | 0.461 | 0.151 | 0.065 | 0.032 | 0.073 | 0.007 |
| SLA | 0.312 | 0.239 | 0.292 | 0.044 | 0.06 | 0.046 | 0.006 |
| Sleaf | 0.16 | 0.472 | 0.152 | 0.069 | 0.031 | 0.102 | 0.014 |
| FAIED | LongED | LatED | FAIED + LongED | FAIED + LatED | LongED + LatED | Full model | |
| SD | 0.263 | 0.056 | 0.029 | 0.046 | 0.532 | 0.009 | 0.065 |
| LA | 0.286 | 0.284 | 0.154 | 0.163 | 0.049 | 0.043 | 0.021 |
| PCI | 0.177 | 0.482 | 0.158 | 0.068 | 0.031 | 0.076 | 0.008 |
| SLA | 0.316 | 0.241 | 0.296 | 0.047 | 0.048 | 0.047 | 0.005 |
| Sleaf | 0.149 | 0.345 | 0.111 | 0.273 | 0.021 | 0.075 | 0.027 |
| SD | LA | PCI | SLA | Sleaf | ||||||
| R2m | R2c | R2m | R2c | R2m | R2c | R2m | R2c | R2m | R2c | |
| EQED | 0.38 | 0.76 | 0.03 | 0.87 | 0.01 | 0.89 | 0.04 | 0.86 | 0.02 | 0.38 |
| LongED | 0.01 | 0.84 | 0.02 | 0.78 | 0.01 | 0.91 | 0.01 | 0.75 | 0.12 | 0.18 |
| LatED | 0.00 | 0.84 | 0.00 | 0.78 | 0.00 | 0.91 | 0.01 | 0.77 | 0.03 | 0.22 |
| EQED + LongED | 0.38 | 0.75 | 0.07 | 0.92 | 0.01 | 0.91 | 0.04 | 0.86 | 0.19 | 0.75 |
| EQED + LatED | 0.37 | 0.77 | 0.03 | 0.86 | 0.01 | 0.88 | 0.04 | 0.86 | 0.05 | 0.40 |
| LongED + LatED | 0.02 | 0.84 | 0.02 | 0.78 | 0.01 | 0.91 | 0.02 | 0.77 | 0.15 | 0.29 |
| Full model | 0.37 | 0.76 | 0.07 | 0.91 | 0.01 | 0.91 | 0.04 | 0.86 | 0.21 | 0.77 |
| SD | LA | PCI | SLA | Sleaf | ||||||
| R2m | R2c | R2m | R2c | R2m | R2c | R2m | R2c | R2m | R2c | |
| FAIED | 0.06 | 0.82 | 0.02 | 0.83 | 0.00 | 0.90 | 0.02 | 0.80 | 0.09 | 0.43 |
| LongED | 0.01 | 0.84 | 0.02 | 0.78 | 0.01 | 0.91 | 0.01 | 0.75 | 0.12 | 0.18 |
| LatED | 0.00 | 0.84 | 0.00 | 0.78 | 0.00 | 0.91 | 0.01 | 0.77 | 0.03 | 0.22 |
| FAIED + LongED | 0.06 | 0.82 | 0.04 | 0.85 | 0.01 | 0.91 | 0.02 | 0.79 | 0.23 | 0.58 |
| FAIED + LatED | 0.11 | 0.86 | 0.02 | 0.83 | 0.00 | 0.90 | 0.02 | 0.79 | 0.09 | 0.40 |
| LongED + LatED | 0.02 | 0.84 | 0.02 | 0.78 | 0.01 | 0.91 | 0.02 | 0.77 | 0.15 | 0.29 |
| Full model | 0.11 | 0.86 | 0.05 | 0.85 | 0.01 | 0.90 | 0.02 | 0.78 | 0.23 | 0.56 |
References
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought responses at leaf, stem and fine root levels of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees in dry and wet years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2006, 21, 1–11. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; De Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016, 135, 897–909. [Google Scholar] [CrossRef] [Green Version]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Charru, M.; Seynave, I.; Morneau, F.; Bontemps, J.-D. Recent changes in forest productivity: An analysis of national forest inventory data for common beech (Fagus sylvatica L.) in north-eastern France. For. Ecol. Manag. 2010, 260, 864–874. [Google Scholar] [CrossRef]
- Rita, A.; Gentilesca, T.; Ripullone, F.; Todaro, L.; Borghetti, M. Differential climate–growth relationships in Abies alba Mill. and Fagus sylvatica L. in Mediterranean mountain forests. Dendrochronologia 2014, 32, 220–229. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur. J. For. Res. 2009, 128, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Schuldt, B.; Knutzen, F.; Delzon, S.; Jansen, S.; Müller-Haubold, H.; Burlett, R.; Clough, Y.; Leuschner, C. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 2015, 210, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Aranda, I.; Bahamonde, H.A.; Sánchez-Gómez, D. Intra-population variability in the drought response of a beech (Fagus sylvatica L.) population in the southwest of Europe. Tree Physiol. 2017, 37, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; BeechCOSTe52 Database Consortium; Garzón, M.B. Phenotypic trait variation measured on European genetic trials of Fagus sylvatica L. Sci. Data 2018, 5, 180149. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, D.; Robson, T.M.; Gascó, A.; Gil-Pelegrín, E.; Aranda, I. Differences in the leaf functional traits of six beech (Fagus sylvatica L.) populations are reflected in their response to water limitation. Environ. Exp. Bot. 2013, 87, 110–119. [Google Scholar] [CrossRef] [Green Version]
- García-Plazaola, J.I.; Becerril, J. Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees 2000, 14, 485–490. [Google Scholar] [CrossRef]
- Peuke, A.D.; Schraml, C.; Hartung, W.; Rennenberg, H. Identification of drought-sensitive beech ecotypes by physiological parameters. New Phytol. 2002, 154, 373–387. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, Ĺ.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) Populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef] [Green Version]
- Bresson, C.C.; Vitasse, Y.; Kremer, A.; Delzon, S. To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol. 2011, 31, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Dounavi, A.; Netzer, F.; Ćelepirović, N.; Ivanković, M.; Burger, J.; Figueroa, A.; Schön, S.; Simon, J.; Cremer, E.; Fussi, B.; et al. Genetic and physiological differences of European beech provenances (F. sylvatica L.) exposed to drought stress. For. Ecol. Manag. 2016, 361, 226–236. [Google Scholar] [CrossRef]
- Thiel, D.; Kreyling, J.; Backhaus, S.; Beierkuhnlein, C.; Buhk, C.; Egen, K.; Huber, G.; Konnert, M.; Nagy, L.; Jentsch, A. Different reactions of central and marginal provenances of Fagus sylvatica to experimental drought. Eur. J. For. Res. 2014, 133, 247–260. [Google Scholar] [CrossRef]
- Aranda, I.; Cano, F.J.; Gascó, A.; Cochard, H.; Nardini, A.; Mancha, J.A.; López, R.; Sánchez-Gómez, D. Variation in photosynthetic performance and hydraulic architecture across European beech (Fagus sylvatica L.) populations supports the case for local adaptation to water stress. Tree Physiol. 2015, 35, 34–46. [Google Scholar] [CrossRef]
- De Villemereuil, P.; Gaggiotti, O.E.; Mouterde, M.; Till-Bottraud, I. Common garden experiments in the genomic era: New perspectives and opportunities. Heredity 2016, 116, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyas, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Konôpková, A.; Kurjak, D.; Kmeť, J.; Klumpp, R.; Longauer, R.; Ditmarová, Ľ.; Gömöry, D. Differences in photochemistry and response to heat stress between silver fir (Abies alba Mill.) provenances. Trees 2017, 32, 73–86. [Google Scholar] [CrossRef]
- Kurjak, D.; Konôpková, A.; Kmeť, J.; Macková, M.; Frýdl, J.; Zivcak, M.; Palmroth, S.; Ditmarová, Ľ.; Gömöry, D. Variation in the performance and thermostability of photosystem II in European beech (Fagus sylvatica L.) provenances is influenced more by acclimation than by adaptation. Eur. J. For. Res. 2018, 138, 79–92. [Google Scholar] [CrossRef]
- Lavilla, M.; Seral, A.; Murciano, A.; Molino, S.; De-La-Fuente-Redondo, P.; Galan, J.M.G.Y. Stomatal traits in Iberian populations of Osmunda regalis (Osmundaceae, Polypodiopsida) and its relationship with bioclimatic variables. Acta Bot. Malacit. 2018, 42, 5–13. [Google Scholar] [CrossRef]
- Durand, M.; Brendel, O.; Buré, C.; Le Thiec, D. Changes in irradiance and vapour pressure deficit under drought induce distinct stomatal dynamics between glasshouse and field-grown poplars. New Phytol. 2020, 227, 392–406. [Google Scholar] [CrossRef]
- Gindel, I. Stomatal Number and Size as Related to Soil Moisture in Tree Xerophytes in Israel. Ecology 1969, 50, 263–267. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [Green Version]
- McCree, K.J.; Davis, S.D. Effect of Water Stress and Temperature on Leaf Size and on Size and Number of Epidermal Cells in Grain Sorghum 1. Crop. Sci. 1974, 14, 751–755. [Google Scholar] [CrossRef]
- Cutler, J.M.; Rains, D.W.; Loomis, R.S. The Importance of Cell Size in the Water Relations of Plants. Physiol. Plant. 1977, 40, 255–260. [Google Scholar] [CrossRef]
- Hepworth, C.; Doheny-Adams, T.; Hunt, L.; Cameron, D.D.; Gray, J.E. Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytol. 2015, 208, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2018, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Wellstein, C.; Chelli, S.; Campetella, G.; Bartha, S.; Galiè, M.; Spada, F.; Canullo, R. Intraspecific phenotypic variability of plant functional traits in contrasting mountain grasSLAnds habitats. Biodivers. Conserv. 2013, 22, 2353–2374. [Google Scholar] [CrossRef] [Green Version]
- Wellstein, C.; Poschlod, P.; Gohlke, A.; Chelli, S.; Campetella, G.; Rosbakh, S.; Canullo, R.; Kreyling, J.; Jentsch, A.; Beierkuhnlein, C. Effects of extreme drought on specific leaf area of grasSLAnd species: A meta-analysis of experimental studies in temperate and sub-Mediterranean systems. Glob. Chang. Biol. 2017, 23, 2473–2481. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Merilä, J.; Hendry, A.P. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. Evol. Appl. 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Stamp, M.A.; Hadfield, J.D. The relative importance of plasticity versus genetic differentiation in explaining between population differences; a meta-analysis. Ecol. Lett. 2020, 23, 1432–1441. [Google Scholar] [CrossRef]
- Palacio-López, K.; Beckage, B.; Scheiner, S.M.; Molofsky, J. The ubiquity of phenotypic plasticity in plants: A synthesis. Ecol. Evol. 2015, 5, 3389–3400. [Google Scholar] [CrossRef]
- Blackman, C.J.; Aspinwall, M.J.; Tissue, D.T.; Rymer, P.D. Genetic adaptation and phenotypic plasticity contribute to greater leaf hydraulic tolerance in response to drought in warmer climates. Tree Physiol. 2017, 37, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, K.; Ducousso, A.; Gömöry, D.; Hansen, J.K.; Ionita, L.; Liesebach, M.; Lorenţ, A.; Schueler, S.; Sułkowska, M.; De Vries, S.; et al. Chilling and forcing requirements for foliage bud burst of European beech (Fagus sylvatica L.) differ between provenances and are phenotypically plastic. Agric. For. Meteorol. 2017, 2017, 172–181. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]
- Michalski, S.; Malyshev, A.V.; Kreyling, J. Trait variation in response to varying winter temperatures, diversity patterns and signatures of selection along the latitudinal distribution of the widespread grasSLAnd plantArrhenatherum elatius. Ecol. Evol. 2017, 7, 3268–3280. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, J.; Puechmaille, S.J.; Malyshev, A.V.; Valladares, F. Phenotypic plasticity closely linked to climate at origin and resulting in increased mortality under warming and frost stress in a common grass. Ecol. Evol. 2019, 9, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK; New York, NY, USA; New Rochelle, NY, USA; Melbourne, VIC, Australia; Sydney, NSW, Australia, 1988. [Google Scholar]
- Führer, E.; Horváth, L.; Jagodics, A.; Machon, A.; Pödör, Z. Application of a new aridity index in Hungarian forestry practice. Idojaras 2011, 3, 205–216. [Google Scholar]
- Fang, J.; Lechowicz, M.J. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J. Biogeogr. 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Kržič, A.; Matović, B.; Orlović, S.; Duputié, A.; Djurdjević, V.; Galic, Z.; Stojnić, S. Prediction of the European beech (Fagus sylvatica L.) xeric limit using a regional climate model: An example from southeast Europe. Agric. For. Meteorol. 2013, 176, 94–103. [Google Scholar] [CrossRef]
- Gavrilov, M.B.; Lukić, T.; Janc, N.; Basarin, B.; Marković, S.B. Forestry Aridity Index in Vojvodina, North Serbia. Open Geosci. 2019, 11, 367–377. [Google Scholar] [CrossRef]
- Von Wühlisch, G.; Liesebach, M.; Muhs, H.J.; Stephan, B.R. A network of international beech provenance trials. In First EUFORGEN Meeting on Social Broadleaves; Turok, J., Kremer, A., de Vries, S., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1998; pp. 164–172. [Google Scholar]
- Vega, C.; González, G.; Bahamonde, H.A.; Valbuena-Carabaña, M.; Gil, L.; Fernández, V. Effect of irradiation and canopy position on anatomical and physiological features of Fagus sylvatica and Quercus petraea leaves. Plant Physiol. Biochem. 2020, 152, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Pardo, F.; Gil, L.; Pardos, J. Anatomical basis of the change in leaf mass per area and nitrogen investment with relative irradiance within the canopy of eight temperate tree species. Acta Oecologica 2004, 25, 187–195. [Google Scholar] [CrossRef]
- Wolf, L. Mikroskopicka Tehnica; National Health Publishing: Praha, Czech Republic, 1950. [Google Scholar]
- Holland, N.; Richardson, A.D. Stomatal Length Correlates with Elevation of Growth in Four Temperate Species†. J. Sustain. For. 2009, 28, 63–73. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The “hydrology” of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Aranda, I.; Bergasa, L.F.; Gil, L. Effects of relative irradiance on the leaf structure of Fagus sylvatica L. seedlings planted in the understory of a Pinus sylvestris L. stand after thinning. Ann. For. Sci. 2001, 58, 673–680. [Google Scholar] [CrossRef]
- Mátyás, C.; Fady, B.; Vendramin, G. Forests at the limit: Evolutionary—genetic consequences of environmental changes at the receding (xeric) edge of distribution. Report from a researcher workshop. Acta Silv Lign Hung 2009, 66, 201–204. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-150; 2020; Available online: https://cran.r-project.org (accessed on 28 October 2020).
- Bartoń, K. MuMIn: Multi-Model Inference, R package version 1.43.17; 2020; Available online: https://cran.r-project.org (accessed on 28 October 2020).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtainingR2from generalized linear mixed-effects models. Methods Ecol. Evol. 2012, 4, 133–142. [Google Scholar] [CrossRef]
- Marron, N.; Dreyer, E.; Boudouresque, E.; DeLay, D.; Petit, J.-M.; Delmotte, F.M.; Brignolas, F. Impact of successive drought and re-watering cycles on growth and specific leaf area of two Populus x canadensis (Moench) clones, “Dorskamp” and “Luisa_Avanzo”. Tree Physiol. 2003, 23, 1225–1235. [Google Scholar] [CrossRef] [Green Version]
- Robson, T.M.; Sánchez-Gómez, D.; Cano, F.J.; Aranda, I. Variation in functional leaf traits among beech provenances during a Spanish summer reflects the differences in their origin. Tree Genet. Genomes 2012, 8, 1111–1121. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Genotypic variation and phenotypic plasticity in the drought response of fine roots of European beech. Tree Physiol. 2008, 28, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Aspelmeier, S.; Leuschner, C. Genotypic variation in drought response of silver birch (Betula pendula Roth): Leaf and root morphology and carbon partitioning. Trees 2005, 20, 42–52. [Google Scholar] [CrossRef]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Al Meselmani, M.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Matthews, J. Guard Cell Metabolism and Stomatal Function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussotti, F.; Prancrazi, M.; Matteucci, G.; Gerosa, G. Leaf morphology and chemistry in Fagus sylvatica (beech) trees as affected by site factors and ozone: Results from CONECOFOR permanent monitoring plots in Italy. Tree Physiol. 2005, 25, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojnić, S.; Orlovic, S.; Trudić, B.; Živković, U.; Von Wuehlisch, G.; Miljkovic, D. Phenotypic plasticity of European beech (Fagus sylvatica L.) stomatal features under water deficit assessed in provenance trial. Dendrobiology 2015, 73, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galić, Z.; Kebert, M.; Von Wuehlisch, G. Provenance plasticity of European beech leaf traits under differing environmental conditions at two Serbian common garden sites. Eur. J. For. Res. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop. J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R.; Yu, L.; Shi, D.; Li, J.; Kong, Y.; Yu, Y.; Chai, G.; Hu, R.; Wang, J.; et al. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [Green Version]
- Diazlopez, L.; Gimeno, V.; Simón, I.; Martínez, V.; Rodríguez-Ortega, W.; García-Sánchez, F. Jatropha curcas seedlings show a water conservation strategy under drought conditions based on decreasing leaf growth and stomatal conductance. Agric. Water Manag. 2012, 105, 48–56. [Google Scholar] [CrossRef]
- George-Jaeggli, B.; Mortlock, M.Y.; Borrell, A.K. Bigger is not always better: Reducing leaf area helps stay-green sorghum use soil water more slowly. Environ. Exp. Bot. 2017, 138, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Anyia, A. Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. Eur. J. Agron. 2004, 20, 327–339. [Google Scholar] [CrossRef]
- Songsri, P.; Jogloy, S.; Holbrook, C.; Kesmala, T.; Vorasoot, N.; Akkasaeng, C.; Patanothai, A. Association of root, specific leaf area and SPAD chlorophyll meter reading to water use efficiency of peanut under different available soil water. Agric. Water Manag. 2009, 96, 790–798. [Google Scholar] [CrossRef]
- Poorter, L.; Markesteijn, L. Seedling Traits Determine Drought Tolerance of Tropical Tree Species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Slot, M.; Poorter, L. Diversity of Tropical Tree Seedling Responses to Drought. Biotropica 2007, 39, 683–690. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Harrison, E.L.; Cubas, L.A.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.A.; Wrage, K.J.; Reich, P.B.; Tjoelker, M.G.; Schaeffer, T.; Muermann, C. Survival, growth, and photosynthesis of tree seedlings competing with herbaceous vegetation along a water-light-nitrogen gradient. Plant Ecol. 1999, 145, 341–350. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [Green Version]
- Fanourakis, D.; Briese, C.; Max, J.; Kleinen, S.; Putz, A.; Fiorani, F.; Ulbrich, A.; Schurr, U. Rapid determination of leaf area and plant height by using light curtain arrays in four species with contrasting shoot architecture. Plant Methods 2014, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vráblová, M.; Vrábl, D.; Hronková, M.; Kubásek, J.; Šantrůček, J. Stomatal function, density and pattern, and CO2 assimilation in Arabidopsis thaliana tmm1 and sdd1-1 mutants. Plant Biol. 2017, 19, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.F.; Auerswald, K.; Tautenhahn, S.; Geiger, A.; Otto, J.; Müller, A.; Römermann, C. Inter—and intraspecific variation in stomatal pore area index along elevational gradients and its relation to leaf functional traits. Plant Ecol. 2016, 217, 229–240. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Zwieniecki, M.A.; Palma, B. Leaf hydraulic capacity in ferns, conifers and angiosperms: Impacts on photosynthetic maxima. New Phytol. 2004, 165, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Barigah, T.; Saugier, B.; Mousseau, M.; Guittet, J.; Ceulemans, R.J. Photosynthesis, leaf area and productivity of 5 poplar clones during their establishment year. Ann. Sci. For. 1994, 51, 613–625. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M.; Ruiz, C.; Lissarrague, J.R. Effect of Water Stress on Leaf Area Development, Photosynthesis, and Productivity in Chardonnay and Airén Grapevines. Am. J. Enol. Vitic. 2002, 53, 138–143. [Google Scholar]
- Wortemann, R.; Herbette, S.; Barigah, T.S.; Fumanal, B.; Alia, R.; Ducousso, A.; Gomory, D.; Roeckel-Drevet, P.; Cochard, H. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiol. 2011, 31, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Čortan, D.; Nonić, M.; Šijačić-Nikolić, M. Phenotypic Plasticity of European Beech from International Provenance Trial in Serbia. In Advances in Global Change Research; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 333–351. [Google Scholar]
- Petkova, K.; Molle, E.; Konnert, M.; Knutzen, F. Comparing German and Bulgarian provenances of European beech (Fagus sylvatica L.) regarding survival, growth and ecodistance. Silva Balc. 2019, 2, 27–48. [Google Scholar]
- Sáenz-Romero, C.; Kremer, A.; Nagy, L.; Újvári-Jármay, É.; Ducousso, A.; Kóczán-Horváth, A.; Hansen, J.K.; Mátyás, C. Common garden comparisons confirm inherited differences in sensitivity to climate change between forest tree species. Peer J. 2019, 7, e6213. [Google Scholar] [CrossRef]
- Robson, T.M.; Rasztovits, E.; Aphalo, P.J.; Alía, R.; Aranda, I. Flushing phenology and fitness of European beech (Fagus sylvatica L.) provenances from a trial in La Rioja, Spain, segregate according to their climate of origin. Agric. For. Meteorol. 2013, 180, 76–85. [Google Scholar] [CrossRef]
- Frank, A.; Pluess, A.R.; Howe, G.T.; Sperisen, C.; Heiri, C. Quantitative genetic differentiation and phenotypic plasticity of European beech in a heterogeneous landscape: Indications for past climate adaptation. Perspect. Plant Ecol. Evol. Syst. 2017, 26, 1–13. [Google Scholar] [CrossRef]
- Gianoli, E.; Valladares, F. Studying phenotypic plasticity: The advantages of a broad approach. Biol. J. Linn. Soc. 2011, 105, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Hewitt, G.M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Lander, T.A.; Klein, E.K.; Roig, A.; Oddou-Muratorio, S. Weak founder effects but significant spatial genetic imprint of recent contraction and expansion of European beech populations. Heredity 2020, 1–14. [Google Scholar] [CrossRef]
- Callahan, H.S.; Maughan, H.; Steiner, U.K. Phenotypic Plasticity, Costs of Phenotypes, and Costs of Plasticity. Ann. N. Y. Acad. Sci. 2008, 1133, 44–66. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.M.C.; Ghosh, S.M.; Gascoigne, S.J.L.; Shingleton, A.W. Plasticity Through Canalization: The Contrasting Effect of Temperature on Trait Size and Growth in Drosophila. Front. Cell Dev. Biol. 2018, 6, 156. [Google Scholar] [CrossRef]
- Debat, V.; David, P. Mapping phenotypes: Canalization, plasticity and developmental stability. Trends Ecol. Evol. 2001, 16, 555–561. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Phenotypic Plasticity, Developmental Instability, and Robustness: The Concepts and How They Are Connected. Front. Ecol. Evol. 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Yeh, P.J.; Price, T.D. Adaptive Phenotypic Plasticity and the Successful Colonization of a Novel Environment. Am. Nat. 2004, 164, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.; Bonser, S.; Davidson, A.; Finnegan, E.; Mathesius, U.; Poot, P.; Purugganan, M.; Richards, C.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Anderson, J.T.; Song, B. Plant adaptation to climate change—Where are we? J. Syst. Evol. 2020, 58, 533–545. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Steiner, U.K. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 2009, 22, 852–860. [Google Scholar] [CrossRef]
- Scheiner, S.M. The genetics of phenotypic plasticity. XVI. Interactions among traits and the flow of information. Evolution 2018, 72, 2292–2307. [Google Scholar] [CrossRef]
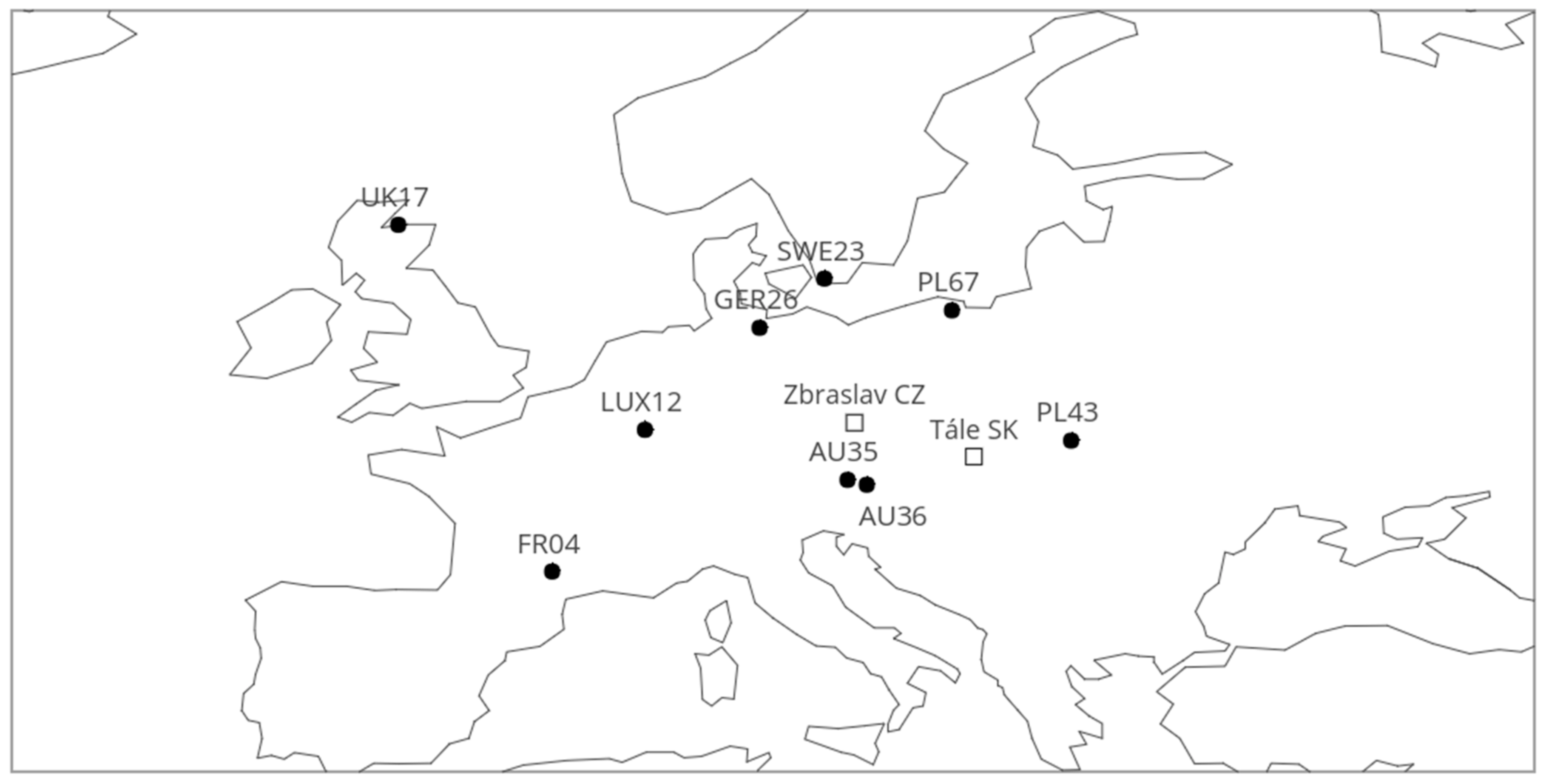



| Provenance | Long | Lat | Alt | T | T59 | Prec | Prec59 | EQ | FAI | IsoT | Seasprec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FR04 | 2.58 | 44.15 | 850 | 10.8 | 16.8 | 804 | 344 | 23.8 | 5.4 | 3.8 | 14 |
| LUX12 | 6.2 | 49.67 | 400 | 8.6 | 14.9 | 866 | 365 | 19.7 | 4.8 | 3.1 | 11 |
| UK17 | −3.42 | 57.67 | 10 | 8.2 | 12.7 | 671 | 303 | 21.8 | 5.3 | 3.6 | 19 |
| SWE23 | 13.2 | 55.57 | 40 | 7.9 | 14.3 | 640 | 286 | 25.9 | 6.2 | 2.6 | 21 |
| GER26 | 10.67 | 53.65 | 55 | 8.3 | 15 | 678 | 319 | 25.5 | 5.1 | 3.0 | 17 |
| AU35 | 14.1 | 47.72 | 1250 | 2.4 | 9.2 | 1495 | 779 | 7.6 | 1.3 | 3.2 | 26 |
| AU36 | 14.85 | 47.53 | 1100 | 2.9 | 9.9 | 1168 | 648 | 10.4 | 1.6 | 3.1 | 32 |
| PL43 | 22.82 | 49.25 | 900 | 6.3 | 14.1 | 762 | 433 | 21.5 | 2.9 | 2.7 | 35 |
| PL67 | 18.17 | 54.33 | 250 | 5.8 | 13.2 | 633 | 336 | 24.6 | 4.3 | 2.4 | 30 |
| CZ Zbraslav | 14.37 | 49.95 | 360 | 8.25 | 15.6 | 532 | 330 | 33.5 | 5.1 | na | na |
| SK Tále | 19.03 | 48.63 | 850 | 6.58 | 14.1 | 842 | 441 | 19.1 | 2.5 | na | na |
| Factor | Df | Trait | LA | SD | PCI | mleaf | Sleaf | SLA |
|---|---|---|---|---|---|---|---|---|
| Provenance | 1 | F | 57.95 | 17.65 | 11.59 | 3.79 | 10.83 | 0.69 |
| p | *** | *** | *** | ** | *** | 0.64 | ||
| Plot | 8 | F | 1771.5 | 457.4 | 1221.25 | 31.35 | 21.58 | 9.48 |
| p | *** | *** | *** | *** | *** | ** | ||
| Provenance × Plot | 8 | F | 18.09 | 4.642 | 7.66 | 2.72 | 3.92 | 1.64 |
| p | *** | *** | *** | * | *** | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrík, P.; Petek, A.; Konôpková, A.; Bosela, M.; Fleischer, P.; Frýdl, J.; Kurjak, D. Stomatal and Leaf Morphology Response of European Beech (Fagus sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions. Forests 2020, 11, 1359. https://doi.org/10.3390/f11121359
Petrík P, Petek A, Konôpková A, Bosela M, Fleischer P, Frýdl J, Kurjak D. Stomatal and Leaf Morphology Response of European Beech (Fagus sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions. Forests. 2020; 11(12):1359. https://doi.org/10.3390/f11121359
Chicago/Turabian StylePetrík, Peter, Anja Petek, Alena Konôpková, Michal Bosela, Peter Fleischer, Josef Frýdl, and Daniel Kurjak. 2020. "Stomatal and Leaf Morphology Response of European Beech (Fagus sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions" Forests 11, no. 12: 1359. https://doi.org/10.3390/f11121359
APA StylePetrík, P., Petek, A., Konôpková, A., Bosela, M., Fleischer, P., Frýdl, J., & Kurjak, D. (2020). Stomatal and Leaf Morphology Response of European Beech (Fagus sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions. Forests, 11(12), 1359. https://doi.org/10.3390/f11121359







