Modeling the Effects of Global Change on Ecosystem Processes in a Tropical Rainforest
Abstract
:1. Introduction
- Model adaptation for the sub-biome. What insights about the ecosystem development and properties can be gained from calibrating Century for this site? We predicted that La Selva P input parameters would not need adjustment because wind patterns in this region are not likely to bring P dust inputs. We predicted that because La Selva has such high rates of N cycling [19] relative to Puerto Rican rainforests [25], N fixation parameters would need to be adjusted higher than estimates for other tropical rainforests. We expected that high rainfall at this site could limit aboveground NPP (ANPP) via limitations of light on cloudy days.
- Sensitivity to climate variables. Which aspects of climate change would have the most effect on ecosystem processes? Tropical wet forests are already experiencing measurable climate change, manifested in Central America as increased minimum air temperatures and drier dry seasons [26,27]. We predicted that higher night-time temperature would have a greater effect in this tropical wet forest, given the negative effects on aboveground ANPP found by Clark et al. [28].
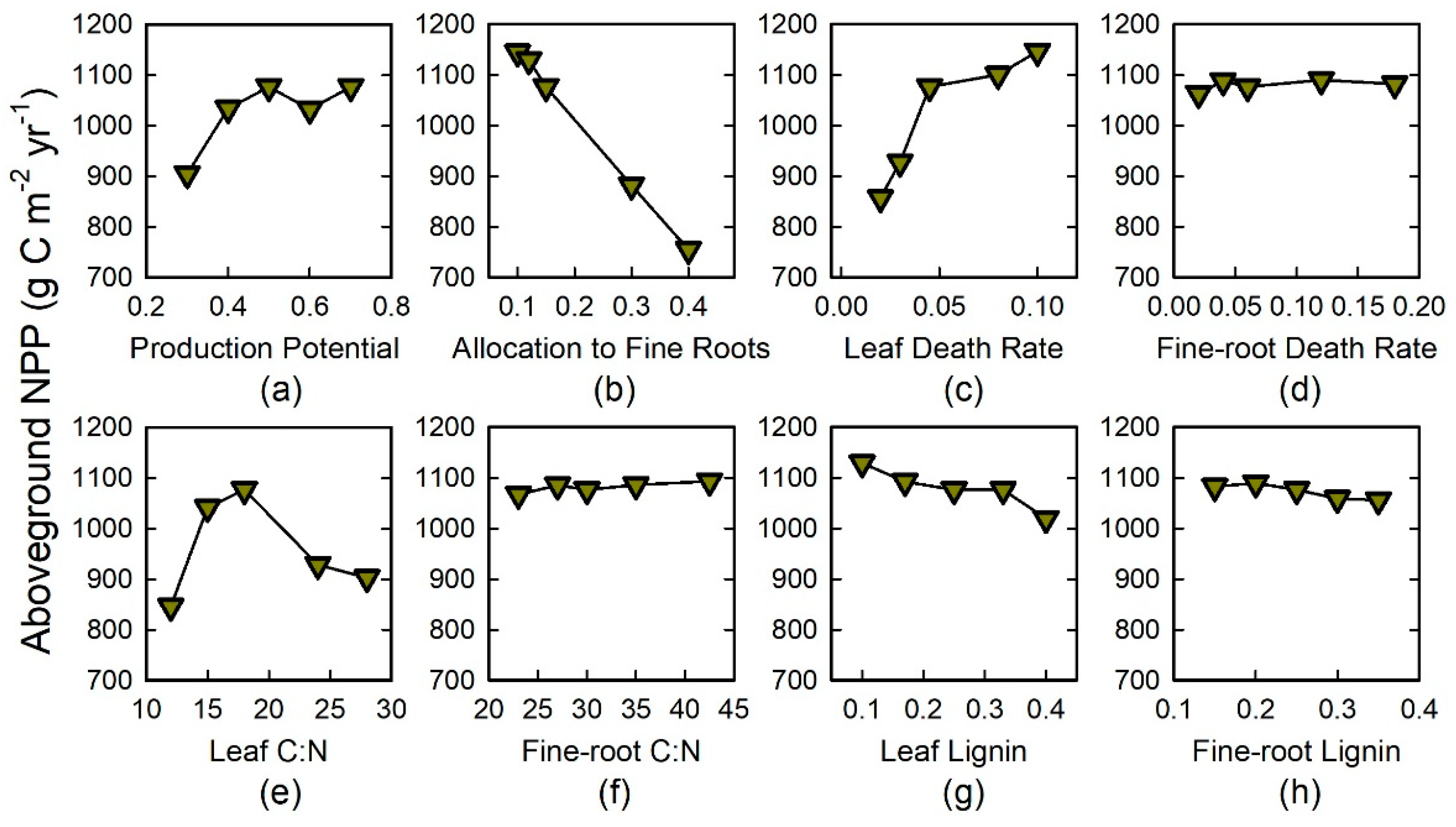
- Sensitivity to plant traits and model realism. Is this biome-based simulation model sensitive to variation in traits within a functional group of species? If so, which modeled traits have the most effect on ecosystems, and what is the extent of their effects? We were most interested in insights about the biological underpinnings of the ecosystem responses to functional plant traits (PFTs), specifically for traits associated with C and N dynamics. Previous field-based studies indicated that eight traits that are already included in Century had differed significantly among five experimental tree species in the same functional group; the species also differed in their effects on ecosystem properties and processes [18,19,29,30]. We also addressed the question of whether Century would generate realistic ecosystem properties under the five experimental tree species, and what types of model adjustments would be required to achieve this.
2. Materials and Methods
2.1. The Model
2.2. Data Available
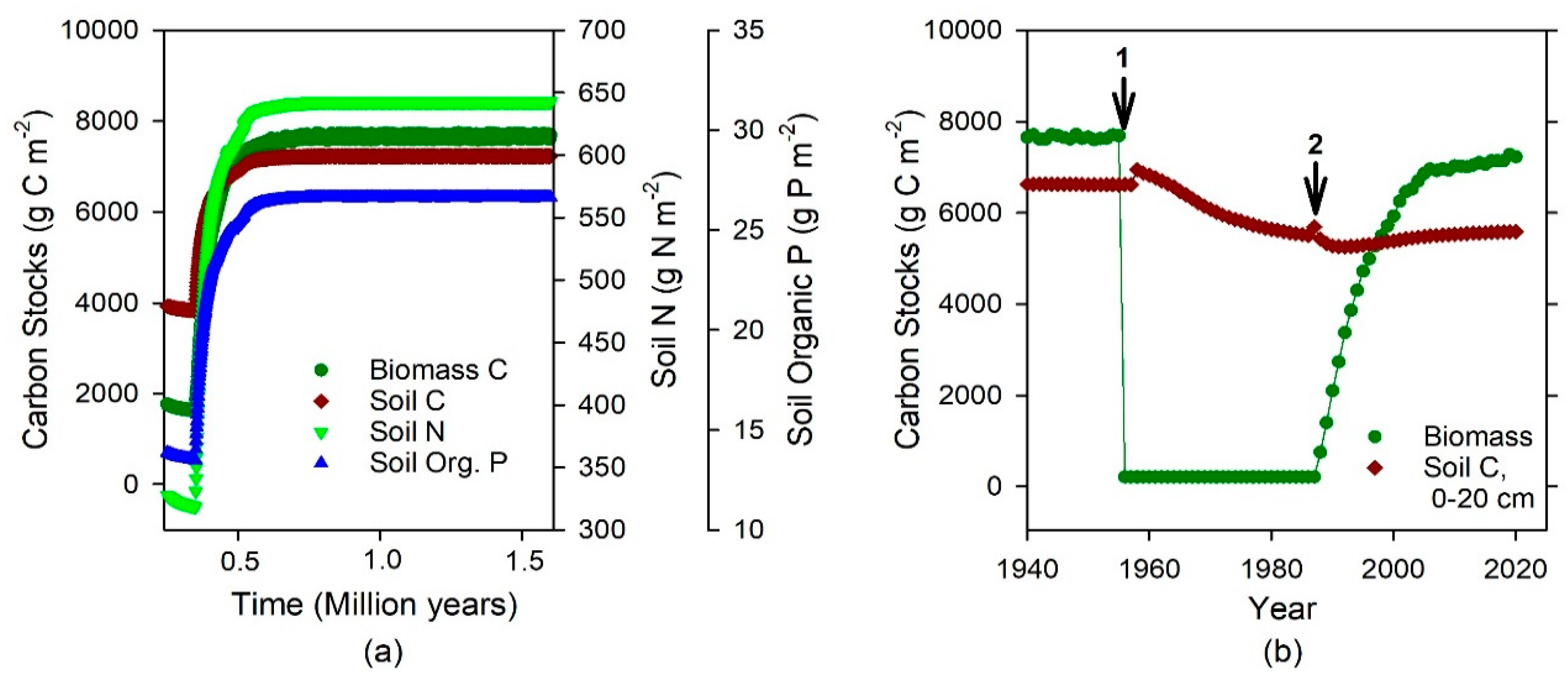
2.3. Model Parameterization and Calibration
2.4. Modeling Experiments
- (a)
- Increasing minimum air temperatures (Tmin). From the current mean Tmin of 21.47 °C at La Selva, Tmin was increased incrementally over the series of model runs to 25.77 °C (Table S5). This corresponded to a 2.1 °C change in the daily average T. The difference between Tmax and Tmin across all months of the experimental runs ranged from 7.2–8.9 °C, whereas the historical range within the year is 8.4–10.3 °C for this site (http://www.ots.ac.cr/meteoro/default.php?pestacion=2).
- (b)
- Decreasing rainfall during the drier months. Annual precipitation was decreased from 420 to 347 cm (Table S6), with all decreases occurring from January to May, the drier period at La Selva.
2.5. Evaluation
3. Results
3.1. Climate-Change Experiments
3.2. Tree-Species-Trait Experiments
3.3. Modeled Tree Species
4. Discussion
4.1. Insights from Modeling Activities
4.2. Climate Change
4.3. Tree Species Effects
4.4. Trade-Offs in Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostle, N.J.; Smith, P.; Fisher, R.; Ian Woodward, F.; Fisher, J.B.; Smith, J.U.; Galbraith, D.; Levy, P.; Meir, P.; McNamara, N.P. Integrating plant–soil interactions into global carbon cycle models. J. Ecol. 2009, 97, 851–863. [Google Scholar] [CrossRef]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M. The projected timing of climate departure from recent variability. Nature 2013, 502, 183. [Google Scholar] [CrossRef] [PubMed]
- Asner, G.P.; Rudel, T.K.; Aide, T.M.; DeFries, R.; Emerson, R. A contemporary assessment of change in humid tropical forests. Conserv. Biol. 2009, 23, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Baccini, A.; Goetz, S.; Walker, W.; Laporte, N.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.; Dubayah, R.; Friedl, M. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The role of plantations in managing the world’s forests in the Anthropocene. Front. Ecol. Environ. 2010, 8, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Herbert, D.A.; Rastetter, E.B.; Shaver, G.R.; Ågren, G.I. Effects of plant growth characteristics on biogeochemistry and community composition in a changing climate. Ecosystems 1999, 2, 367–382. [Google Scholar] [CrossRef]
- Koven, C.D.; Knox, R.G.; Fisher, R.A.; Chambers, J.; Christoffersen, B.O.; Davies, S.J.; Detto, M.; Dietze, M.C.; Faybishenko, B.; Holm, J. Benchmarking and Parameter Sensitivity of Physiological and Vegetation Dynamics using the Functionally Assembled Terrestrial Ecosystem Simulator (FATES) at Barro Colorado Island, Panama. Biogeosci. Discuss. 2019, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.; Muszala, S.; Verteinstein, M.; Lawrence, P.; Xu, C.; McDowell, N.; Knox, R.; Koven, C.; Holm, J.; Rogers, B. Taking off the training wheels: The properties of a dynamic vegetation model without climate envelopes, CLM4. 5 (ED). Geosci. Model Dev. 2015, 8, 3593–3619. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.A.; Koven, C.D.; Anderegg, W.R.; Christoffersen, B.O.; Dietze, M.C.; Farrior, C.E.; Holm, J.A.; Hurtt, G.C.; Knox, R.G.; Lawrence, P.J. Vegetation demographics in Earth System Models: A review of progress and priorities. Glob. Chang. Biol. 2018, 24, 35–54. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Uriarte, M.; González, G.; Reed, S.; Thompson, J.; Zimmerman, J.K.; Murphy, L. Improving predictions of tropical forest response to climate change through integration of field studies and ecosystem modeling. Glob. Chang. Biol. 2018, 24, e213–e232. [Google Scholar] [CrossRef] [PubMed]
- Cuddington, K.; Fortin, M.-J.; Gerber, L.; Hastings, A.; Liebhold, A.; O’connor, M.; Ray, C. Process-based models are required to manage ecological systems in a changing world. Ecosphere 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Sanford, R.L., Jr.; Parton, W.J.; Ojima, D.S.; Lodge, D.J. Hurricane effects on soil organic matter dynamics and forest production in the Luquillo Experimental Forest, Puerto Rico: Results of simulation modeling. Biotropica 1991, 23, 364–372. [Google Scholar] [CrossRef]
- Metherell, A.; Harding, L.; Cole, C.; Parton, W. CENTURY: Soil Organic Matter Model. Technical Document, Agroecosystems Version 4.0; Great Plains Systems Research Unit; US Department of Agriculture, Agricultural Research Service, Ft: Collins, CO, USA, 1993. [Google Scholar]
- Vitousek, P.M.; Turner, D.R.; Parton, W.J.; Sanford, R.L. Litter decomposition on the Mauna Loa environmental matrix, Hawai’i: Patterns, mechanisms, and models. Ecology 1994, 75, 418–429. [Google Scholar] [CrossRef]
- Slik, J.F.; Arroyo-Rodríguez, V.; Aiba, S.-I.; Alvarez-Loayza, P.; Alves, L.F.; Ashton, P.; Balvanera, P.; Bastian, M.L.; Bellingham, P.J.; Van Den Berg, E. An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. USA 2015, 112, 7472–7477. [Google Scholar] [CrossRef] [Green Version]
- Hobbie, S.E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- Russell, A.E.; Raich, J.W.; Arrieta, R.B.; Valverde-Barrantes, O.; González, E. Impacts of individual tree species on carbon dynamics in a moist tropical forest environment. Ecol. Appl. 2010, 20, 1087–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, A.E.; Raich, J.W. Rapidly growing tropical trees mobilize remarkable amounts of nitrogen, in ways that differ surprisingly among species. Proc. Natl. Acad. Sci. USA 2012, 109, 10398–10402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weintraub, S.R.; Russell, A.E.; Townsend, A.R. Native tree species regulate nitrous oxide fluxes in tropical plantations. Ecol. Appl. 2014, 24, 750–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, S.J. The future of tropical forests. Ann. N. Y. Acad. Sci. 2010, 1195, 1–27. [Google Scholar] [CrossRef]
- Friend, A.; Stevens, A.; Knox, R.; Cannell, M. A process-based, terrestrial biosphere model of ecosystem dynamics (Hybrid v3. 0). Ecol. Model. 1997, 95, 249–287. [Google Scholar] [CrossRef]
- Parton, W.J.; Neff, J.; Vitousek, P.M. Modelling Phosphorus, Carbon and Nitrogen Dynamics in Terrestrial Ecosystems. In Organic Phosphorus in the Environment; Turner, B.L., Frossard, E., Baldwin, D.S., Eds.; CAB International: Oxford, UK, 2005; pp. 325–346. [Google Scholar]
- Fisher, R.F. Amelioration of degraded rain forest soils by plantations of native trees. Soil Sci. Soc. Am. J. 1995, 59, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Chestnut, T.J.; Zarin, D.J.; Mcdowell, W.H.; Keller, M. A nitrogen budget for late-successional hillslope tabonuco forest, Puerto Rico. Biogeochemistry 1999, 46, 85–108. [Google Scholar] [CrossRef]
- Clark, D.A.; Piper, S.; Keeling, C.; Clark, D.B. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc. Natl. Acad. Sci. USA 2003, 100, 5852–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, E.P.; Adam, J.; Wood, A. Climate model based consensus on the hydrologic impacts of climate change to the Rio Lempa basin of Central America. Hydrol. Earth Syst. Sci. 2009, 13, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.A.; Clark, D.B.; Oberbauer, S.F. Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. J. Geophys. Res. Biogeosci. 2013, 118, 783–794. [Google Scholar] [CrossRef]
- Raich, J.W.; Russell, A.E.; Bedoya-Arrieta, R. Lignin and enhanced litter turnover in tree plantations of lowland Costa Rica. For. Ecol. Manag. 2007, 239, 128–135. [Google Scholar] [CrossRef]
- Russell, A.E.; Raich, J.W.; Valverde-Barrantes, O.J.; Fisher, R.F. Tree species effects on soil properties in experimental plantations in tropical moist forest. Soil Sci. Soc. Am. J. 2007, 71, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Parton, W.J.; Stewart, J.W.; Cole, C.V. Dynamics of C, N, P and S in grassland soils: A model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Parton, W. The CENTURY Model. In Evaluation of Soil Organic Matter Models; Springer: Berlin/Heidelberg, Germany, 1996; pp. 283–291. [Google Scholar]
- Parton, W.; Tappan, G.; Ojima, D.; Tschakert, P. Ecological impact of historical and future land-use patterns in Senegal. J. Arid Environ. 2004, 59, 605–623. [Google Scholar] [CrossRef]
- Parton, W.J.; Ojima, D.S.; Cole, C.V.; Schimel, D.S. A general model for soil organic matter dynamics: Sensitivity to litter chemistry, texture and management. Quant. Model. Soil Form. Process. 1994, 39, 147–167. [Google Scholar]
- Raich, J.W.; Parton, W.J.; Russell, A.E.; Sanford, R.L.; Vitousek, P.M. Analysis of factors regulating ecosystemdevelopment on Mauna Loa using the Century model. Biogeochemistry 2000, 51, 161–191. [Google Scholar] [CrossRef]
- Moorhead, D.; Currie, W.; Rastetter, E.; Parton, W.; Harmon, M. Climate and litter quality controls on decomposition: An analysis of modeling approaches. Glob. Biogeochem. Cycles 1999, 13, 575–589. [Google Scholar] [CrossRef] [Green Version]
- Probert, M.; Keating, B.; Thompson, J.; Parton, W. Modelling water, nitrogen, and crop yield for a long-term fallow management experiment. Aust. J. Exp. Agric. 1995, 35, 941–950. [Google Scholar] [CrossRef]
- Vallis, I.; Parton, W.; Keating, B.; Wood, A. Simulation of the effects of trash and N fertilizer management on soil organic matter levels and yields of sugarcane. Soil Tillage Res. 1996, 38, 115–132. [Google Scholar] [CrossRef]
- Motavalli, P.; Palm, C.; Parton, W.; Elliott, E.; Frey, S. Comparison of laboratory and modeling simulation methods for estimating soil carbon pools in tropical forest soils. Soil Biol. Biochem. 1994, 26, 935–944. [Google Scholar] [CrossRef]
- Sanford, R.; Paaby, P.; Luvall, J.C.; Phillips, E.; McDade, L.A.; Bawa, K.S.; Hespenheide, H.A.; Hartshorn, G.S. La Selva: Ecology and Natural History of a Neotropical Rain Forest; University of Chicago: Chicago, IL, USA, 1994; pp. 19–33. [Google Scholar]
- Valverde-Barrantes, O.J.; Raich, J.W.; Russell, A.E. Fine-root mass, growth and nitrogen content for six tropical tree species. Plant Soil 2007, 290, 357–370. [Google Scholar] [CrossRef]
- Alvarado, G.E. Características geológicas de la estación biológica La Selva, Costa Rica. Tecnol. Marcha 1990, 10, 11–22. [Google Scholar]
- Kleber, M.; Schwendenmann, L.; Veldkamp, E.; Rößner, J.; Jahn, R. Halloysite versus gibbsite: Silicon cycling as a pedogenetic process in two lowland neotropical rain forest soils of La Selva, Costa Rica. Geoderma 2007, 138, 1–11. [Google Scholar] [CrossRef]
- Parker, G.G. Soil fertility, nutrient acquisition, and nutrient cycling. In La Selva: Ecology and Natural History of a Neotropical Rain Forest; University of Chicago: Chicago, IL, USA, 1994; pp. 54–63. [Google Scholar]
- Clark, D.B.; Clark, D.A. Landscape-scale variation in forest structure and biomass in a tropical rain forest. For. Ecol. Manag. 2000, 137, 185–198. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A.; Brown, S.; Oberbauer, S.F.; Veldkamp, E. Stocks and flows of coarse woody debris across a tropical rain forest nutrient and topography gradient. For. Ecol. Manag. 2002, 164, 237–248. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of leaf trait relationships: A test across six biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Constantinides, M.; Fownes, J. Nitrogen mineralization from leaves and litter of tropical plants: Relationship to nitrogen, lignin and soluble polyphenol concentrations. Soil Biol. Biochem. 1994, 26, 49–55. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Cusack, D.F.; Chou, W.W.; Yang, W.H.; Harmon, M.E.; Silver, W.L.; Team, L. Controls on long-term root and leaf litter decomposition in neotropical forests. Glob. Chang. Biol. 2009, 15, 1339–1355. [Google Scholar] [CrossRef]
- Porder, S.; Clark, D.A.; Vitousek, P.M. Persistence of rock-derived nutrients in the wet tropical forests of La Selva, Costa Rica. Ecology 2006, 87, 594–602. [Google Scholar] [CrossRef]
- Yang, X.; Post, W.M. Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 2011, 8, 2907–2916. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.J.; Silver, W.L. Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Glob. Chang. Biol. 2013, 19, 2804–2813. [Google Scholar] [CrossRef]
- Hall, S.J.; McNicol, G.; Natake, T.; Silver, W. Large fluxes and rapid turnover of mineral-associated carbon across topographic gradients in a humid tropical forest: Insights from paired 14 C analysis. Biogeosciences 2015, 12, 2471–2487. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.J.; Silver, W.L. Reducing conditions, reactive metals, and their interactions can explain spatial patterns of surface soil carbon in a humid tropical forest. Biogeochemistry 2015, 125, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Sollins, P.; Robertson, G.P.; Uehara, G. Nutrient mobility in variable-and permanent-charge soils. Biogeochemistry 1988, 6, 181–199. [Google Scholar] [CrossRef]
- Russell, A.E.; Hall, S.J.; Raich, J.W. Tropical tree species traits drive soil cation dynamics via effects on pH: A proposed conceptual framework. Ecol. Monogr. 2017, 87, 685–701. [Google Scholar] [CrossRef] [Green Version]
- Van Miegroet, H.; Cole, D. The impact of nitrification on soil acidification and cation leaching in a red alder ecosystem. J. Environ. Qual. 1984, 13, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Loescher, H.W.; Gholz, H.; Jacobs, J.M.; Oberbauer, S.F. Energy dynamics and modeled evapotranspiration from a wet tropical forest in Costa Rica. J. Hydrol. 2005, 315, 274–294. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220. [Google Scholar] [CrossRef]
- Mau, A.C.; Reed, S.C.; Wood, T.E.; Cavaleri, M.A. Temperate and tropical forest canopies are already functioning beyond their thermal thresholds for photosynthesis. Forests 2018, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Slot, M.; Rey-Sánchez, C.; Gerber, S.; Lichstein, J.W.; Winter, K.; Kitajima, K. Thermal acclimation of leaf respiration of tropical trees and lianas: Response to experimental canopy warming, and consequences for tropical forest carbon balance. Glob. Chang. Biol. 2014, 20, 2915–2926. [Google Scholar] [CrossRef]
- Linacre, E.T. A simple formula for estimating evaporation rates in various climates, using temperature data alone. Agric. Meteorol. 1977, 18, 409–424. [Google Scholar] [CrossRef]
- Raich, J.W.; Rastetter, E.; Melillo, J.M.; Kicklighter, D.W.; Steudler, P.; Peterson, B.; Grace, A.; Moore, B.; Vorosmarty, C.J. Potential net primary productivity in South America: Application of a global model. Ecol. Appl. 1991, 1, 399–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.B.; Clark, D.A.; Oberbauer, S.F. Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob. Chang. Biol. 2010, 16, 747–759. [Google Scholar] [CrossRef]
- Asao, S.; Ryan, M.G. Carbohydrate regulation of photosynthesis and respiration from branch girdling in four species of wet tropical rain forest trees. Tree Physiol. 2015, 35, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Bloomfield, K.J.; Reich, P.B.; Tjoelker, M.G.; Asner, G.P.; Bonal, D.; Bönisch, G.; Bradford, M.G.; Cernusak, L.A.; Cosio, E.G. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015, 206, 614–636. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.G.; Dukes, J.S. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Glob. Chang. Biol. 2013, 19, 45–63. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Sakschewski, B.; von Bloh, W.; Boit, A.; Rammig, A.; Kattge, J.; Poorter, L.; Peñuelas, J.; Thonicke, K. Leaf and stem economics spectra drive diversity of functional plant traits in a dynamic global vegetation model. Glob. Chang. Biol. 2015, 21, 2711–2725. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Binkley, D. The Influence of Tree Species on Forest Soils: Processes and Patterns. In Proceedings of the Trees and Soil Workshop; Lincoln University Press: Lincoln, New Zealand, 1995; p. 994. [Google Scholar]
- Christenson, L.; Lovett, G.; Weathers, K.; Arthur, M. The influence of tree species, nitrogen fertilization, and soil C to N ratio on gross soil nitrogen transformations. Soil Sci. Soc. Am. J. 2009, 73, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finér, L.; Jaroszewicz, B.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef] [Green Version]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Paustian, K.; Parton, W.J.; Persson, J. Modeling soil organic matter in organic-amended and nitrogen-fertilized long-term plots. Soil Sci. Soc. Am. J. 1992, 56, 476–488. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, A.E.; Parton, W.J., Jr. Modeling the Effects of Global Change on Ecosystem Processes in a Tropical Rainforest. Forests 2020, 11, 213. https://doi.org/10.3390/f11020213
Russell AE, Parton WJ Jr. Modeling the Effects of Global Change on Ecosystem Processes in a Tropical Rainforest. Forests. 2020; 11(2):213. https://doi.org/10.3390/f11020213
Chicago/Turabian StyleRussell, Ann E., and William J. Parton, Jr. 2020. "Modeling the Effects of Global Change on Ecosystem Processes in a Tropical Rainforest" Forests 11, no. 2: 213. https://doi.org/10.3390/f11020213



