Transcriptomic Analysis Reveals Hormonal Control of Shoot Branching in Salix matsudana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Hormone Content Measurements
2.3. Sample Preparation for Transcriptome and DGE Profiling Analysis
2.4. Screening of Differentially Expressed Genes (DEGs)
3. Results
3.1. Growth and Endogenous Hormone Analysis
3.2. DGE Analysis and Mapping Reads to the Transcriptome
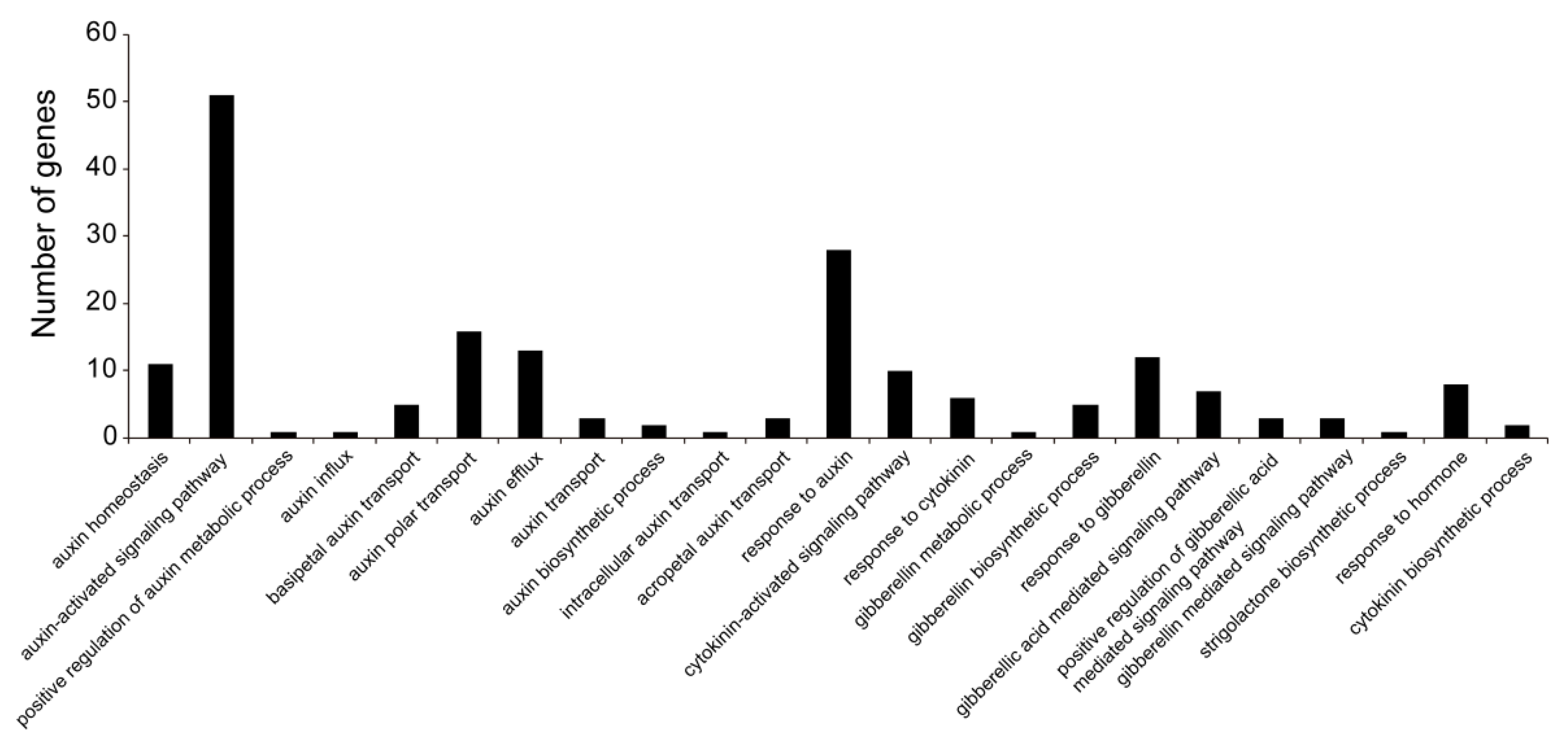
3.3. Screening of DEGs and Functional Annotation Analysis
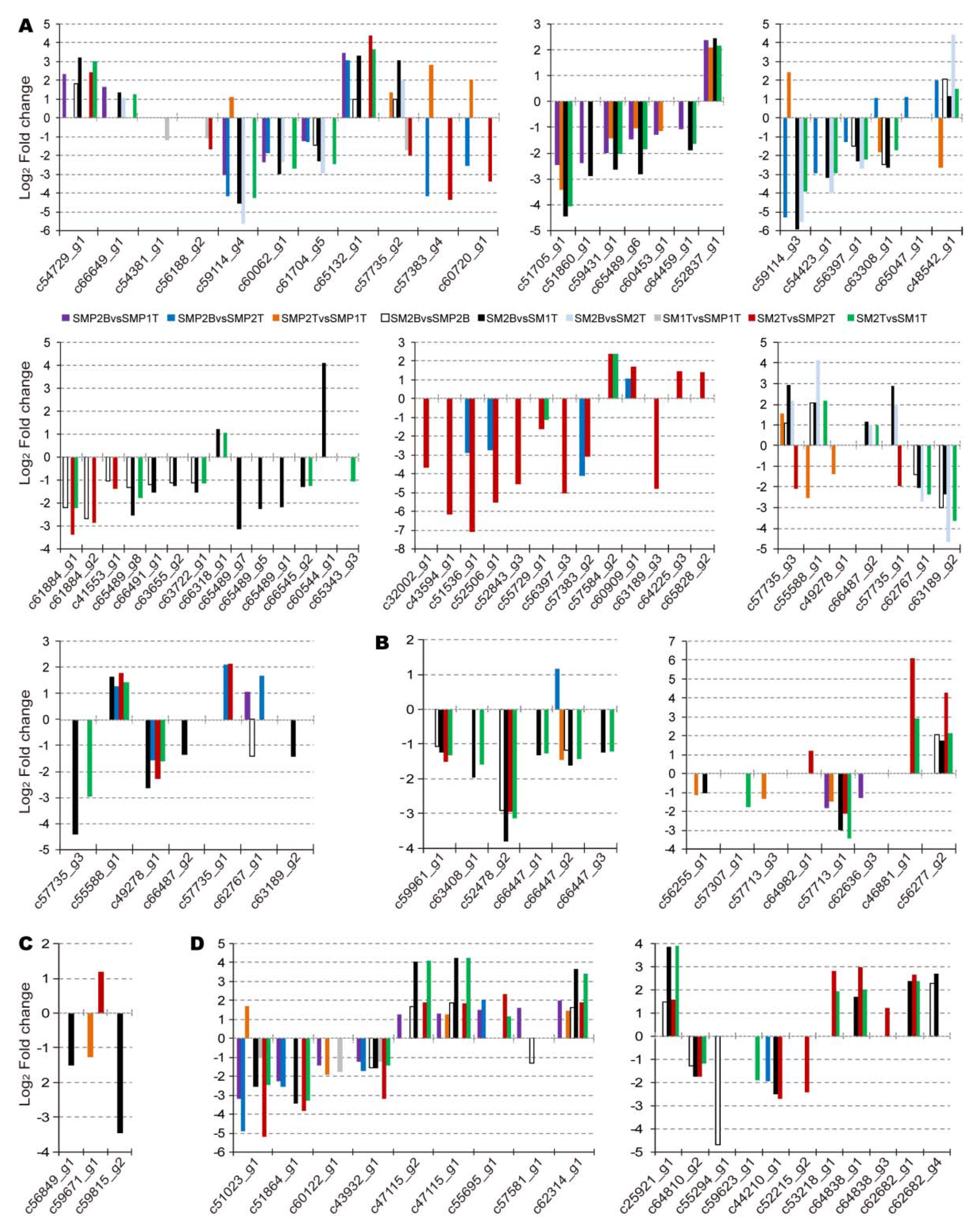
3.4. Regulatory Patterns of Hormone-Related DEGs
3.5. Transcriptional Networks Associated with Hormone Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef]
- Ward, S.P.; Salmon, J.; Hanley, S.J.; Karp, A.; Leyser, O. Using Arabidopsis to study shoot branching in biomass willow. Plant Physiol. 2013, 162, 800–811. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944. [Google Scholar] [CrossRef]
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Imran, M.; Wang, Y.; Xu, J.; Ding, Y.; Wang, S. Transcriptome analysis revealed the interaction among strigolactones, auxin, and cytokinin in controlling the shoot branching of rice. Plant Cell Rep. 2019, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Gao, C.; Chen, M.-S.; Pan, B.-Z.; Ye, K.; Xu, Z.-F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.L.H.; Paul, L.K.; Vahala, J.; Kangasjärvi, J.; van der Schoot, C. Axillary buds are dwarfed shoots that tightly regulate GA pathway and GA-inducible 1,3-β-glucanase genes during branching in hybrid aspen. J. Exp. Bot. 2016, 67, 5975–5991. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef]
- Leyser, O. The control of shoot branching: An example of plant information processing. Plant Cell Environ. 2009, 32, 694–703. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; Chatfield, S.P.; Leyser, H.M.O. AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis. Plant Physiol. 1999, 121, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.; Sieberer, T.; Willett, B.; Booker, J.; Luschnig, C.; Leyser, O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 2006, 16, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Li, S.; Qin, G.; Novák, O.; Pěnčík, A.; Ljung, K.; Aoyama, T.; Liu, J.; Murphy, A.; et al. ADP1 affects plant architecture by regulating local auxin biosynthesis. PLoS Genet. 2014, 10, e1003954. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; Brewer, P.B.; Beveridge, C.A. Strigolactones: Discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009, 14, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, S.; Åstot, C.; Sitbon, F.; Moritz, T.; Olsson, O.; Sandberg, G. Transgenic tobacco plants co-expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin- and cytokinin- overproducing phenotypes. Plant J. 2000, 23, 279–284. [Google Scholar] [CrossRef]
- Tanaka, M.; Takei, K.; Kojima, M.; Sakakibara, H.; Mori, H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006, 45, 1028–1036. [Google Scholar] [CrossRef]
- Müller, D.; Waldie, T.; Miyawaki, K.; To, J.P.C.; Melnyk, C.W.; Kieber, J.J.; Kakimoto, T.; Leyser, O. Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J. 2015, 82, 874–886. [Google Scholar] [CrossRef]
- Waldie, T.; Leyser, O. Cytokinin targets auxin transport to promote shoot branching. Plant Physiol. 2018, 177, 803–818. [Google Scholar] [CrossRef]
- Leyser, O. Regulation of shoot branching by auxin. Trends Plant Sci. 2003, 8, 541–545. [Google Scholar] [CrossRef]
- Nordström, A.; Tarkowski, P.; Tarkowska, D.; Norbaek, R.; Åstot, C.; Dolezal, K.; Sandberg, G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 2004, 101, 8039–8044. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Xue, Y.-L.; Miyakawa, T.; Hou, F.; Qin, H.-M.; Fukui, K.; Shi, X.; Ito, E.; Ito, S.; Park, S.-H.; et al. Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 2013, 4, 2613. [Google Scholar] [CrossRef]
- Waldie, T.; McCulloch, H.; Leyser, O. Strigolactones and the control of plant development: Lessons from shoot branching. Plant J. 2014, 79, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Nieminen, K.; Sánchez-Ferrero, J.C.; Rodríguez, M.L.; Chagoyen, M.; Hardtke, C.S.; Cubas, P. Strigolactone promotes degradation of DWARF14, an α/β hydrolase essential for strigolactone signaling in Arabidopsis. Plant Cell 2014, 26, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fang, J.; Xing, J.; Liu, W.; Peng, P.; Long, H.; Zhao, J.; Zhang, W.; Li, X. Identification and functional analysis of two cotton orthologs of MAX2 which control shoot lateral branching. Plant Mol. Biol. Rep. 2017, 35, 480–490. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sato, A.; Kuwata, K.; Inukai, Y.; Kinoshita, T.; Itami, K.; Tsuchiya, Y.; Hagihara, S. Discovery of shoot branching regulator targeting strigolactone receptor DWARF14. Acs Central Sci. 2018, 4, 230–234. [Google Scholar] [CrossRef]
- Arite, T.; Umehara, M.; Ishikawa, S.; Hanada, A.; Maekawa, M.; Yamaguchi, S.; Kyozuka, J. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009, 50, 1416–1424. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospíšil, T.; Zeljković, S.Ć. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, L.; Challis, R.; Leyser, O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J. Exp. Bot. 2010, 61, 3069–3078. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef]
- Bennett, T.; Liang, Y.; Seale, M.; Ward, S.; Müller, D.; Leyser, O. Strigolactone regulates shoot development through a core signalling pathway. Biol. Open 2016, 5, 1806–1820. [Google Scholar] [CrossRef]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Morris, S.E.; Parmenter, K.; Young, N.; Wang, H.; Jones, A.; Rameau, C.; Turnbull, C.G.N.; Beveridge, C.A. Feedback regulation of xylem cytokinin content is conserved in pea and Arabidopsis. Plant Physiol. 2007, 143, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Young, N.F.; Ferguson, B.J.; Antoniadi, I.; Bennett, M.H.; Beveridge, C.A.; Turnbull, C.G.N. Conditional auxin response and differential cytokinin profiles in shoot branching mutants. Plant Physiol. 2014, 165, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, M.-L.; Chen, M.-S.; Pan, B.-Z.; Tao, Y.-B.; Xu, Z.-F. Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas. Sci. Rep. 2017, 7, 11417. [Google Scholar] [CrossRef]
- Marzec, M. Strigolactones and gibberellins: A new couple in the phytohormone world? Trends Plant Sci. 2017, 22, 813–815. [Google Scholar] [CrossRef]
- Lo, S.-F.; Yang, S.-Y.; Chen, K.-T.; Hsing, Y.-I.; Zeevaart, J.A.D.; Chen, L.-J.; Yu, S.-M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2015, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.P.; Ross, J.J. Auxin regulation of the gibberellin pathway in pea. Plant Physiol. 2002, 130, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- De Saint Germain, A.; Ligerot, Y.; Dun, E.A.; Pillot, J.-P.; Ross, J.J.; Beveridge, C.A.; Rameau, C. Strigolactones stimulate internode elongation independently of gibberellins. Plant. Physiol. 2013, 163, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, Y.; Yan, P.; He, C.; Zhang, J. Transcriptional and hormonal regulation of weeping trait in Salix matsudana. Genes 2017, 8, 359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011, 12, 93–99. [Google Scholar] [CrossRef]
- Faith, J.J.; Hayete, B.; Thaden, J.T.; Mogno, I.; Wierzbowski, J.; Cottarel, G.; Kasif, S.; Collins, J.J.; Gardner, T.S. Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. Plos Biol. 2007, 5, e8. [Google Scholar] [CrossRef]
- Cao, X.-W.; Cui, H.-M.; Yao, Y.; Xiong, A.-S.; Hou, X.-L.; Li, Y. Effects of endogenous hormones on variation of shoot branching in a variety of non-heading Chinese cabbage and related gene expression. J. Plant. Biol. 2017, 60, 343–351. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, M.-S.; Wu, Y.-Q.; Gao, X.-F.; Li, X.-F.; Wang, W.-Z. The roles of auxin in regulating “shoot branching’’ of Cremastra appendiculata. J. Plant. Growth Regul. 2017, 36, 281–289. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Li, M.; Fang, S.; Shen, X.; Chu, J.; Zhang, Z. UNBRANCHED3 regulates branching by modulating cytokinin biosynthesis and signaling in maize and rice. New Phytol. 2017, 214, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hamiaux, C.; Drummond, R.S.M.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012, 22, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.S.M.; Sheehan, H.; Simons, J.L.; Martínez-Sánchez, N.M.; Turner, R.M.; Putterill, J.; Snowden, K.C. The expression of petunia strigolactone pathway genes is altered as part of the endogenous developmental program. Front. Plant. Sci. 2012, 2, 115. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.D.; Amasino, R.M. Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant. Cell 2003, 15, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Agharkar, M.; Lomba, P.; Altpeter, F.; Zhang, H.; Kenworthy, K.; Lange, T. Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant. Biotechnol. J. 2007, 5, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Elfving, D.C.; Visser, D.B.; Henry, J.L. Gibberellins stimulate lateral branch development in young sweet cherry trees in the orchard. Int. J. Fruit Sci. 2011, 11, 41–54. [Google Scholar] [CrossRef]
- Xu, J.; Zha, M.; Li, Y.; Ding, Y.; Chen, L.; Ding, C.; Wang, S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant. Cell Rep. 2015, 34, 1647–1662. [Google Scholar] [CrossRef]
- Marhavý, P.; Bielach, A.; Abas, L.; Abuzeineh, A.; Duclercq, J.; Tanaka, H.; Pařezová, M.; Petrášek, J.; Friml, J.; Kleine-Vehn, J.; et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev. Cell. 2011, 21, 796–804. [Google Scholar] [CrossRef]



| Category | S. matsudana | S. matsudana var. pseudo-matsudana | ||||
|---|---|---|---|---|---|---|
| Stages | S1 | S2 | S2 | S1 | S2 | S2 |
| Tissues | Stem apex | Stem apex | Basal branch | Stem apex | Stem apex | Basal branch |
| Sample name | SM1T | SM2T | SM2B | SMP1T | SMP2T | SMP2B |
| Clean reads | 6,630,850 | 6,382,060 | 6,728,207 | 6,490,499 | 7,159,844 | 5,637,627 |
| Q30 (%) | 95.14% | 95.21% | 95.09% | 95.25% | 95.33% | 95.11% |
| GC content (%) | 44.46% | 44.34% | 43.76% | 44.45% | 44.59% | 44.05% |
| Total mapped | 80.96% | 78.68% | 78.69% | 80.22% | 81.47% | 78.12% |
| Number of unigenes | 58,457 | 58,225 | 58,373 | 57,792 | 57,523 | 58,325 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ni, B.; Zeng, Y.; He, C.; Zhang, J. Transcriptomic Analysis Reveals Hormonal Control of Shoot Branching in Salix matsudana. Forests 2020, 11, 287. https://doi.org/10.3390/f11030287
Liu J, Ni B, Zeng Y, He C, Zhang J. Transcriptomic Analysis Reveals Hormonal Control of Shoot Branching in Salix matsudana. Forests. 2020; 11(3):287. https://doi.org/10.3390/f11030287
Chicago/Turabian StyleLiu, Juanjuan, Bingbing Ni, Yanfei Zeng, Caiyun He, and Jianguo Zhang. 2020. "Transcriptomic Analysis Reveals Hormonal Control of Shoot Branching in Salix matsudana" Forests 11, no. 3: 287. https://doi.org/10.3390/f11030287
APA StyleLiu, J., Ni, B., Zeng, Y., He, C., & Zhang, J. (2020). Transcriptomic Analysis Reveals Hormonal Control of Shoot Branching in Salix matsudana. Forests, 11(3), 287. https://doi.org/10.3390/f11030287





