Analyses of Genetic Diversity, Differentiation and Geographic Origin of Natural Provenances and Land Races of Casuarina equisetifolia Based on EST-SSR Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Sources
2.2. DNA Extraction and SSR Markers Screening
2.3. PCR Amplification and Genotyping
2.4. Data Analyses
3. Results
3.1. Microsatellite Loci Diversity and Polymorphism
3.2. Provenance Diversity and Variation
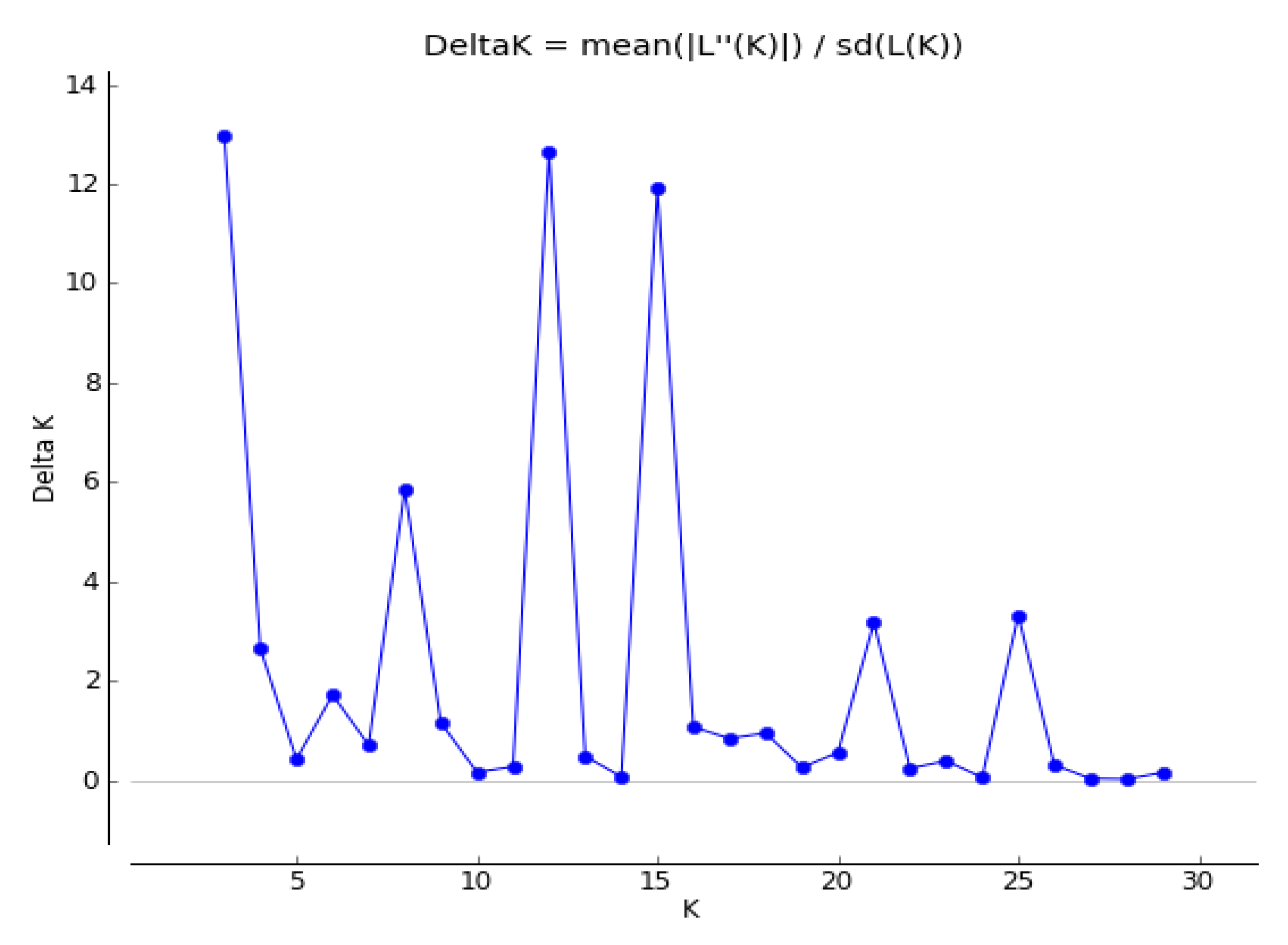
3.3. Genetic Structure of 27 Seed Sources
3.4. Genetic Relationship of 27 Seed Sources
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinyopusarerk, K.; House, A.P.N. Casuarina: An Annotated Bibliography of C. equisetifolia, C. Junghuhniana and C. oligodon; International Centre for Research in Agroforestry: Nairobi, Kenya, 1993; pp. 1–5. [Google Scholar]
- Zhong, C.L.; Zhang, Y.; Chen, Y.; Jiang, Q.B.; Chen, Z.; Wu, C.; Pinyopusarerk, K.; Franche, C.; Bogusz, D. Casuarina research and development in China. Improving Smallholder Livelihoods Through Improved Casuarina Productivity. In Proceedings of the 4th Internation Casuarina Workshop, Haikou, China, 21–25 March 2010; Chinese Forestry Publishing House: Beijing, China, 2011; pp. 5–9. [Google Scholar]
- Fall, P.L. Pollen evidence for plant introduction in a Polynesian tropical island ecosystem, Kingdom of Tonga. In Altered Ecologies; Haberle, S.H., Stevenson, J., Prebble, M., Eds.; ANU Press: Canberra, Australia, 2010; pp. 253–272. [Google Scholar]
- Pinyopusarerk, K.; Kalinganire, A.; Williams, E.R.; Aken, K.M. Evaluation of International Provenance Trials of Casuarina Equisetifolia; CSIRO Forestry and Forest Products: Canberra, Australia, 2004; pp. 25–30.
- Zhong, C.L.; Zhang, Y.; Jiang, Q.B.; Chen, Y.; Chen, Z.; Ma, N.; Hu, P.; Liu, F.; Pinyopusarerk, K.; Bogusz, D.; et al. Constraints in Casuarina cultivation in southern coastal regions of China. Casuarina Improvement for Securing Rural Livelihoods. In Proceedings of the 5th International Casuarina Workshop, Chennai, India, 3–7 February 2014; Nicodemus, A., Pinyopusarerk, K., Zhong, C.L., Franche, C., Eds.; Institute of Forest Genetics and Tree Breeding: Coimbatore, India, 2014; pp. 10–13. [Google Scholar]
- Yu, W.; Zhang, Y.; Xu, X.Y.; Zhong, C.L.; Wei, Y.C.; Meng, J.X.; Chen, Y.; Li, Z.; Bush, D.J. Molecular markers reveal low genetic diversity in Casuarina equisetifolia clonal plantations in South China. New For. 2019. [Google Scholar] [CrossRef]
- Sun, S.; Shu, C.W.; Chen, J.L.; Wang, J.; Zhou, E. Screening for resistant clones ofCasuarina equisetifoliato bacterial wilt and the analysis of AFLP markers in resistant clones. For. Pathol. 2014, 44, 276–281. [Google Scholar] [CrossRef]
- Chen, Y.Z. The main diseases and insect pests of coastal Casuarina protection forests and their control countermeasures. J. Fujian For. Sci. Technol. 1995, 3, 24–28. [Google Scholar]
- Hu, P.; Zhong, C.; Zhang, Y.; Jiang, Q.; Chen, Y.; Chen, Z.; Pinyopusarerk, K.; Bush, D. Geographic variation in seedling morphology of Casuarina equisetifolia subsp. equisetifolia (Casuarinaceae). Aust. J. Bot. 2016, 64, 160–170. [Google Scholar] [CrossRef]
- Booy, G.; Hendriks, R.J.J.; Smulders, M.J.; Groenendael, J.M.; Vosman, B. Genetic Diversity and the Survival of Populations. Plant Boil. 2000, 2, 379–395. [Google Scholar] [CrossRef]
- Sathyanarayana, N.; Pittala, R.K.; Tripathi, P.K.; Chopra, R.; Singh, H.R.; Belamkar, V.; Slatkin, M.; Barton, N.H. A comparison of three indirect methods for estimating average levels of gene flow. Evolution 1989, 43, 1349–1368. [Google Scholar]
- Schaberg, P.G.; DeHayes, D.H.; Hawley, G.J.; Nijensohn, S.E. Anthropogenic alterations of genetic diversity within tree populations: Implications for forest ecosystem resilience. For. Ecol. Manag. 2008, 256, 855–862. [Google Scholar] [CrossRef]
- Luangviriyasaeng, V. Eucalypt planting in? Thailand. In Eucalypts in Asia; Turnbull, J.W., Ed.; Australian Centre for International Agricultural Research: Zhangian, China, 2003. [Google Scholar]
- Parasurama, J.; Naik, S. Reaction of Acacia hybrid clones against pink disease caused by Corticium salmonicolor. Plant Pathol. Newsl. 2003, 21, 28–30. [Google Scholar]
- Mei, W.; Xie, S. Intensification of landfalling typhoons over the northwest Pacific since the late 1970s. Nat. Geosci. 2016, 9, 753–757. [Google Scholar] [CrossRef]
- Chakraborth, S. Potential impact of climate change on plant-pathogen interactions. Australas. Plant Pathol. 2005, 34, 443–448. [Google Scholar] [CrossRef]
- Linnakoski, R.; Kasanen, R.; Dounavi, A.; Forbes, K.M. Editorial: Forest Health under Climate Change: Effects on Tree Resilience, and Pest and Pathogen Dynamics. Front. Plant Sci. 2019, 10, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, N.; Moran, G. Microgeographical Patterns of Allozyme Variation in Casuarina cunninghamiana Miq Within and Between the Murrumbidgee and Coastal Drainage Systems. Aust. J. Bot. 1989, 37, 181. [Google Scholar] [CrossRef]
- Ho, K.Y.; Ou, C.H.; Yang, J.C.; Hsiao, J.Y. An assessment of DNA polymorphisms and genetic relationships of Casuarina equisetifolia using RAPD markers. Bot. Bull. Acad. Sin. 2002, 43, 93–98. [Google Scholar]
- Huang, G.; Zhong, C.L.; Su, X.H.; Zhang, Y.; Pinyopusarerk, K.; Franche, C.; Bogusz, D. Genetic Variation and Structure of native and introduced Casuarina equisetifolia (L. Johnson) Provenances. Silvae Genet. 2009, 58, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Kullan, A.R.K.; Kulkarni, A.V.; Kumar, R.S.; Rajkumar, R. Development of microsatellite markers and their use in genetic diversity and population structure analysis in Casuarina. Tree Genet. Genomes 2016, 12, 49–60. [Google Scholar] [CrossRef]
- Gan, S.; Shi, J.; Li, M.; Wu, K.; Bai, J. Moderate density molecular maps of Eucalyptus urophylla S.T. Blake and E. tereticornis Smith genomes based on RAPD markers. Genetic 2003, 118, 59–67. [Google Scholar] [CrossRef]
- Kalinowski, S.T. hp-rare 1.0: A computer program for performing rarefaction on measures of allelic richness. Mol. Ecol. Notes 2005, 5, 187–189. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (version 2.9.4), a Program (for Windows 95 and Above) to Estimate and Population Genetics Parameters. Lausanne University, Switzerland, 2003. Available online: https://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 10 July 2019).
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2007, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Takezaki, N.; Nei, M.; Tamura, K. POPTREE2: Software for Constructing Population Trees from Allele Frequency Data and Computing Other Population Statistics with Windows Interface. Mol. Boil. Evol. 2009, 27, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Bohonak, A.J. IBD (isolation by distance): A program for analyses of isolation by distance. J. Hered. 2002, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetic 2000, 155, 945–959. [Google Scholar]
- Earl, D.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Leinonen, T.; O’Hara, B.; Cano, J.M.; Merilä, J. Comparative studies of quantitative trait and neutral marker divergence: A meta-analysis. J. Evol. Boil. 2007, 21, 1–17. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene Flow and Local Adaptation in Trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Slatkin, M. Estimating Levels of Gene Flow in Natural Populations. Genetic 1981, 99, 323–335. [Google Scholar]
- Pinyopusarerk, K.; Williams, E.R. Variations in growth and morphological characteristics of Casuarina junghuhniana provenance growth in Thailand. J. Trop. For. Sci. 2005, 17, 574–587. [Google Scholar]
- Johnson, L.A.S. Notes on Casuarinaceae Ⅱ. J. Adel. Bot. Gard. 1982, 6, 73–82. [Google Scholar]
- Dorajraj, S.; Wilson, J. Effect of sex on growth vigour in Casuarina equisetifolia. In National Seminars on Tree Improvement; Tamil Nadu: Coimbatore, India, 1981; pp. 72–78. [Google Scholar]
- Luechanimitchit, P.; Luangviriyasaeng, V. Study of sex ratio and relationship between growth and sex in Casuarina equisetifolia in Thailand. Recent Casuarina research and development. In Proceedings of the 3th International Casuarina Workshop, Da Nang, Vietnam, 4–7 March 1996; Pinyopusarerk, K., Turnbull, J.W., Midgley, S.J., Eds.; CSIRO Forestry and Forest Products: Melbourne, Australia, 1996; pp. 30–32. [Google Scholar]
- Zhang, Y. Studies on genetic improvement of three Casuarina species. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2013. [Google Scholar]
- Schlub, R.; Mersha, Z.; Aime, M.; Badilles, A.; Cannon, P.; Marx, B.; McConnell, R.; Moore, A.; Nandwani, D.; Nelson, S.; et al. Guam ironwood (Casuarina equisetifolia) tree decline conference and follow-up. Improving smallholder livelihoods through improved casuarina productivity. In Proceedings of the 4th International Casuarina Workshop, Haikou, China, 21–25 March 2010; Zhong, C.L., Pinyopusarerk, K., Kalinganire, A., Franche, C., Eds.; Chinese Forestry Publishing House: Beijing, China, 2011; pp. 239–246. [Google Scholar]
- Zhang, Y.; Zhong, C.; Han, Q.; Jiang, Q.; Chen, Y.; Chen, Z.; Pinyopusarerk, K.; Bush, D. And Reproductive biology and breeding system in Casuarina equisetifolia (Casuarinaceae)—Implication for genetic improvement. Aust. J. Bot. 2016, 64, 120. [Google Scholar] [CrossRef]
- Ayin, C.M.; Schlub, R.L.; Yasuhara-Bell, J.; Alvarez, A.M. Identification and characterization of bacteria associated with decline of ironwood (Casuarina equisetifolia) in Guam. Australas. Plant Pathol. 2014, 44, 225–234. [Google Scholar] [CrossRef]
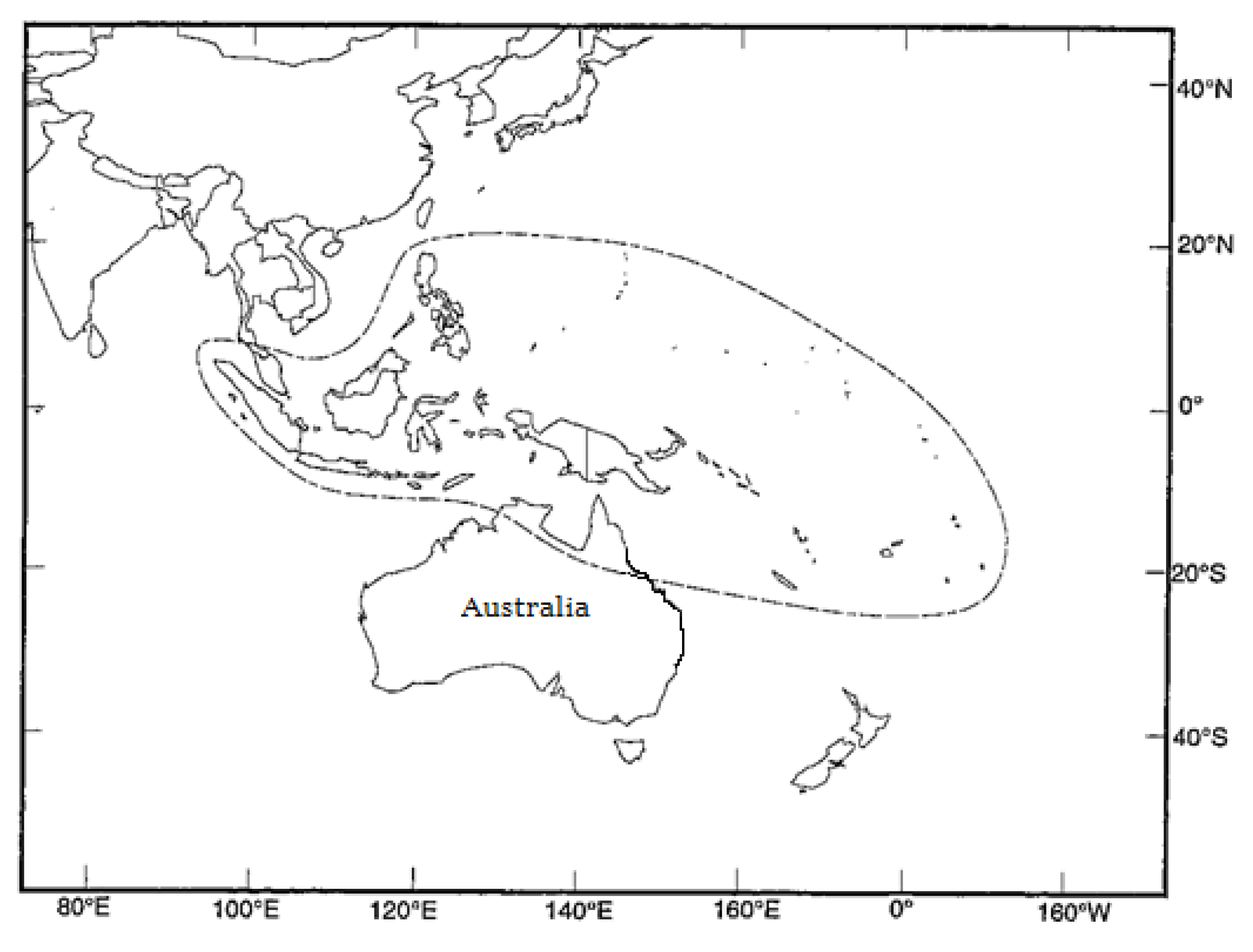



| CSIRO Seedlot | Code | Seed Source Location | Country | Latitude | Longitude | Altitude (m) | Rainfall (mm) | No. of Parents |
|---|---|---|---|---|---|---|---|---|
| Oceania Natural | ||||||||
| 17862 | AU1 | Wagait, Northern Territory | Australia | 12 25S | 130 44E | 3 | 1740 | 6 |
| 18345 | AU2 | Chili Beach, Queensland | Australia | 12 39S | 143 25E | 1 | 1600 | 5 |
| 21311 | GU | Inarajan Beach | Guam | 13 15N | 144 44E | 3 | 2100 | 9 |
| 20586 | PNG | Horno Is. Manus | Papua New Guinea | 02 19S | 147 49E | 1 | 1800 | Bulk |
| 18402 | SB | Kolombangara | Solomon Islands | 08 07S | 157 08E | 2 | 3500 | 10 |
| 18040 | TO | Navutoka, Tongatapu | Tonga | 21 04S | 175 04E | 1 | 1800 | 10 |
| 18312 | VU | Efate | Vanuatu | 17 45S | 168 18E | 30 | 2400 | Bulk |
| Asia Natural | ||||||||
| 18244 | MY1 | Bako, Sarawak | Malaysia | 01 44N | 110 30E | 50 | 4000 | 4 |
| 18376 | MY2 | Tangjong Balau, Johor | Malaysia | 01 36N | 104 16E | 15 | 2600 | 4 |
| 18357 | PH1 | Narra, Palawan | Philippines | 09 19N | 118 29E | 10 | 2500 | 6 |
| 18117 | PH2 | San Jose, Mindoro | Philippines | 12 25N | 121 03E | 20 | 2000 | Bulk |
| 18154 | PH3 | Aklan, Panay Island | Philippines | 11 55N | 121 23E | 30 | 2100 | 5 |
| 18297 | TH1 | Ban Kamphuam, Ranong | Thailand | 09 21N | 98 16E | 10 | 3000 | 8 |
| 18298 | TH2 | Had Chaomai, Trang | Thailand | 07 33N | 100 37E | 2 | 1600 | 9 |
| Asia Introduced | ||||||||
| 18267 | CN1 | Yanjiang, Guangdong | China | 23 00N | 113 03E | 4 | 1500 | 10 |
| 18268 | CN2 | Daodong, Hainan | China | 19 58N | 110 59E | 10 | 1700 | 14 |
| 18013 | IN1 | Cuttack, Orissa | India | 20 12N | 86 38E | 7 | 1400 | 6 |
| 18015 | IN2 | Balasore, Orissa | India | 21 30N | 84 53E | 2 | 1600 | 4 |
| 18119 | IN3 | Rameswaram, Tamil Nadu | India | 09 15N | 79 20E | 5 | 900 | 8 |
| 18287 | LK | Hambantota | Sri Lanka | 06 08N | 81 07E | 16 | 1000 | Bulk |
| 18128 | VN | Hai Thinh, Ha NamNinh | Vietnam | 20 22N | 106 21E | 2 | 2000 | 7 |
| 21331 | BD | Parki Beach, Chittagong | Bangladesh | 21 11N | 91 48E | 5 | 1700 | 5 |
| Africa Introduced | ||||||||
| 18355 | BJ | Cotonou | Benin | 06 24N | 02 31E | 8 | 1300 | 8 |
| 18122 | EG | Montazah | Egypt | 31 16N | 30 05E | 13 | 200 | 8 |
| 18135 | KE1 | Malindi | Kenya | 03 15S | 40 09E | 7 | 900 | 10 |
| 18142 | KE2 | Kilifi | Kenya | 03 38S | 39 95E | 20 | 1000 | 5 |
| 18565 | MU | Isle D’Ambre | Mauritius | 20 03S | 57 39E | 2 | 1700 | 4 |
| Provenance | N | Na | Ne | AR | Ho | He | FIS |
|---|---|---|---|---|---|---|---|
| AU1 | 31 | 6.00 | 3.32 | 1.64 g | 0.74 | 0.63 g | −0.17 |
| AU2 | 25 | 4.08 | 1.76 | 1.39 bcdef | 0.39 | 0.39 cdefg | 0.00 |
| GU | 57 | 3.92 | 1.67 | 1.33 bcde | 0.09 | 0.33 bcdefg | 0.73 |
| PNG | 30 | 2.23 | 1.17 | 1.13 a | 0.11 | 0.13 a | 0.13 |
| SB | 30 | 5.77 | 2.58 | 1.53 fg | 0.49 | 0.52 fg | 0.06 |
| TO | 30 | 3.54 | 1.71 | 1.29 abcd | 0.21 | 0.27 abcd | 0.22 |
| VU | 28 | 4.31 | 1.92 | 1.43 bcdef | 0.41 | 0.42 bcdef | 0.02 |
| ON | 231 | 13.85 | 2.71 | 1.39 | 0.42 | 0.58 | 0.28 |
| MY1 | 29 | 6.54 | 2.66 | 1.59 g | 0.46 | 0.57 fg | 0.19 |
| MY2 | 30 | 5.54 | 2.85 | 1.34 bcde | 0.24 | 0.33 bcde | 0.27 |
| PH1 | 15 | 6.39 | 2.41 | 1.11 a | 0.04 | 0.10 a | 0.60 |
| PH2 | 16 | 1.92 | 1.22 | 1.52 defg | 0.30 | 0.49 defg | 0.39 |
| PH3 | 41 | 4.46 | 2.07 | 1.56 g | 0.41 | 0.56 fg | 0.27 |
| TH1 | 15 | 6.00 | 2.69 | 1.16 ab | 0.12 | 0.14 ab | 0.14 |
| TH2 | 32 | 4.23 | 1.67 | 1.59 g | 0.65 | 0.58 fg | −0.12 |
| AN | 201 | 13.62 | 3.63 | 1.41 | 0.35 | 0.65 | 0.46 |
| BD | 13 | 1.77 | 1.28 | 1.22 abc | 0.17 | 0.22 abc | 0.23 |
| CN1 | 30 | 5.00 | 2.51 | 1.53 efg | 0.45 | 0.51 ef | 0.12 |
| CN2 | 30 | 5.31 | 2.64 | 1.51 defg | 0.45 | 0.49 def | 0.08 |
| IN1 | 39 | 5.54 | 2.51 | 1.51 defg | 0.50 | 0.50 defg | 0.00 |
| IN2 | 46 | 5.54 | 2.56 | 1.52 fg | 0.32 | 0.52 fg | 0.38 |
| IN3 | 30 | 5.00 | 1.93 | 1.43 cdefg | 0.38 | 0.42 cdef | 0.10 |
| LK | 15 | 3.00 | 2.18 | 1.52 fg | 0.61 | 0.47 cdef | −0.30 |
| VN | 30 | 4.54 | 1.89 | 1.41 cdef | 0.39 | 0.40 cdef | 0.03 |
| AI | 233 | 11.31 | 3.08 | 1.46 | 0.40 | 0.40 | 0.00 |
| BJ | 65 | 5.85 | 2.06 | 1.45 cdef | 0.36 | 0.44 cdef | 0.18 |
| EG | 30 | 4.31 | 1.87 | 1.38 bcdef | 0.30 | 0.37 bcdef | 0.19 |
| KE1 | 30 | 4.00 | 1.69 | 1.36 bcdef | 0.29 | 0.35 bcdef | 0.17 |
| KE2 | 30 | 6.15 | 3.11 | 1.66 g | 0.87 | 0.65 g | −0.34 |
| MU | 30 | 5.00 | 2.45 | 1.55 fg | 0.57 | 0.54 fg | −0.06 |
| AFI | 185 | 11.62 | 3.24 | 1.48 | 0.34 | 0.66 | 0.48 |
| Overall/mean | 840 | 4.66 | 2.16 | 1.44 | 0.38 | 0.41 | 0.07 |
| Primer | Accession No. | SSR Type | Primer Sequence (5′-3′) | Annealing Tm (°C) | Mg2+Concen. (mM) |
|---|---|---|---|---|---|
| P3 | FQ324509 | (AGA)6 | F: TGCAGCATCATCACTACT R: ACTCCAACCAACTCTATTC | 54 | 1.5 |
| P15 | FQ326101 | (CTTCT)5 | F: TTTGTCTTCCCTACTCCG R:AACCCTTTTCCACTTTCTTA | 52 | 1.5 |
| P19 | FQ327279 | (CTT)6 | F: TTCAAAACCCTAGCATCT R: CATACCATTAACCAAAGC | 50 | 1.5 |
| P24 | FQ327965 | (CT)14 | F: GCTGGAGGTGGTGGTGTT R: TATGGAATAGACGAGAAGTGAG | 56 | 1.5 |
| P26 | FQ328032 | (TCGCAC)3 | F: CATCTGAACTTTTGAAACCCTA R:GGCATGGCTTCGTCTTGG | 56 | 1.5 |
| P36 | FQ363031 | (CAACGACAA)3 | F: CCTCAAACCAAGACCACC R: CCGACTTCCATGCTCAAT | 52 | 2.0 |
| P48 | FQ363175 | (TAG)6 | F: GCCGAGTTATGGGGACGA R: GGTGTTTGTGACGACGCT | 58 | 2.5 |
| P52 | FQ365340 | (CGT)6 | F: GCACGGTCGTCTTATTCT R:TCGCTTCCCATACAAATC | 52 | 2.0 |
| P56 | FQ365696 | (TG)9 | F: TGCCGCTGAACAAAATGA R:ATGGTCTCGCCTGGAATG | 56 | 2.0 |
| P79 | FQ374531 | (CATCTT)3 | F: ATGGGACATTTTGGTGAT R:CTTTGCTTTAGGCGTTTT | 50 | 1.5 |
| P80 | FQ374771 | (GAC)12 | F: GCTTTGTCCTACCGTTTC R:ATCACCACCATCCTCGTC | 56 | 1.5 |
| P81 | FQ374894 | (TC)9 | F: CCCTGCTTCTGGTCATTC R: GATCTGTGGCTTTGCTTG | 50 | 1.5 |
| P93 | FQ376339 | (TC)9 | F: ACACGCCCTGTGATAGTT R: GAGGAATTGAGCTTGCTG | 54 | 1.5 |
| Locus | Na | Ne | I | PIC | Ho | He | FIS | F(null) |
|---|---|---|---|---|---|---|---|---|
| P3 | 14 | 7.03 | 2.14 | 0.83 | 0.38 | 0.85 | 0.55*** | 0.12 |
| P15 | 21 | 2.42 | 1.29 | 0.52 | 0.27 | 0.57 | 0.53*** | 0.12 |
| P19 | 10 | 4.46 | 1.66 | 0.71 | 0.30 | 0.75 | 0.60*** | 0.18 |
| P24 | 25 | 2.90 | 1.58 | 0.58 | 0.24 | 0.63 | 0.62*** | 0.21 |
| P26 | 13 | 2.02 | 0.94 | 0.42 | 0.64 | 0.49 | −0.31*** | 0.06 |
| P36 | 46 | 3.20 | 1.97 | 0.62 | 0.34 | 0.64 | 0.47*** | 0.09 |
| P48 | 24 | 4.50 | 1.85 | 0.72 | 0.48 | 0.76 | 0.37*** | 0.08 |
| P52 | 9 | 1.53 | 0.69 | 0.36 | 0.31 | 0.40 | 0.23*** | 0.04 |
| P56 | 25 | 3.52 | 1.76 | 0.67 | 0.32 | 0.70 | 0.54*** | 0.11 |
| P79 | 18 | 1.65 | 0.89 | 0.33 | 0.34 | 0.35 | 0.03*** | 0.05 |
| P80 | 32 | 3.71 | 1.95 | 0.70 | 0.36 | 0.72 | 0.50*** | 0.08 |
| P81 | 18 | 2.32 | 1.34 | 0.54 | 0.46 | 0.57 | 0.19*** | 0.03 |
| P93 | 24 | 4.75 | 1.95 | 0.77 | 0.57 | 0.79 | 0.28*** | 0.07 |
| mean | 21.46 | 3.39 | 1.54 | 0.60 | 0.39 | 0.63 | 0.39 | 0.10 |
| Source of Variation | SS | VC | V% | F-Statistics |
|---|---|---|---|---|
| Regions | 398.557 | 0.067 | 1.57 | FST = 0.299 |
| Seedlots | 1606.684 | 1.206 | 28.31 | FSC = 0.288 |
| Individuals | 4445.605 | 2.987 | 70.12 | FCT = −0.016 |
| Total | 6450.845 | 4.259 |
| Natural Provenances | Land Races | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AU1 | AU2 | GU | PNG | SB | TO | VU | MY1 | MY2 | PH1 | PH2 | PH3 | TH1 | TH2 | BD | CN1 | CN2 | IN1 | IN2 | IN3 | LK | VN | BJ | EG | KE1 | KE2 | MU | |
| AU1 | 0 | AU1 | AU1 | ||||||||||||||||||||||||
| AU2 | 0.162 | 0 | |||||||||||||||||||||||||
| GU | 0.241 | 0.170 | 0 | ||||||||||||||||||||||||
| PNG | 0.320 | 0.198 | 0.124 | 0 | |||||||||||||||||||||||
| SB | 0.159 | 0.099 | 0.186 | 0.206 | 0 | SB | |||||||||||||||||||||
| TO | 0.240 | 0.305 | 0.359 | 0.465 | 0.328 | 0 | TO | TO | TO | ||||||||||||||||||
| VU | 0.173 | 0.055 | 0.127 | 0.149 | 0.077 | 0.303 | 0 | VU | |||||||||||||||||||
| MY1 | 0.121 | 0.135 | 0.173 | 0.237 | 0.103 | 0.221 | 0.105 | 0 | MY1 | MY1 | MY1 | ||||||||||||||||
| MY2 | 0.157 | 0.138 | 0.197 | 0.234 | 0.109 | 0.295 | 0.109 | 0.114 | 0 | ||||||||||||||||||
| PH1 | 0.331 | 0.198 | 0.127 | 0.053 | 0.218 | 0.502 | 0.146 | 0.250 | 0.261 | 0 | PH1 | ||||||||||||||||
| PH2 | 0.134 | 0.106 | 0.105 | 0.109 | 0.099 | 0.268 | 0.064 | 0.093 | 0.128 | 0.129 | 0 | ||||||||||||||||
| PH3 | 0.144 | 0.104 | 0.128 | 0.180 | 0.086 | 0.243 | 0.077 | 0.111 | 0.082 | 0.196 | 0.085 | 0 | |||||||||||||||
| TH1 | 0.119 | 0.124 | 0.189 | 0.225 | 0.110 | 0.237 | 0.099 | 0.067 | 0.067 | 0.242 | 0.102 | 0.094 | 0 | TH1 | |||||||||||||
| TH2 | 0.131 | 0.144 | 0.192 | 0.231 | 0.070 | 0.271 | 0.122 | 0.099 | 0.059 | 0.242 | 0.121 | 0.086 | 0.057 | 0 | TH2 | ||||||||||||
| BD | 0.334 | 0.321 | 0.149 | 0.321 | 0.347 | 0.499 | 0.271 | 0.299 | 0.311 | 0.042 | 0.260 | 0.244 | 0.284 | 0.288 | 0 | ||||||||||||
| CN1 | 0.136 | 0.126 | 0.200 | 0.246 | 0.094 | 0.212 | 0.099 | 0.036 | 0.130 | 0.251 | 0.093 | 0.135 | 0.079 | 0.115 | 0.342 | 0 | |||||||||||
| CN2 | 0.143 | 0.123 | 0.203 | 0.240 | 0.102 | 0.215 | 0.105 | 0.050 | 0.137 | 0.248 | 0.110 | 0.131 | 0.081 | 0.119 | 0.338 | 0.024 | 0 | ||||||||||
| IN1 | 0.168 | 0.132 | 0.183 | 0.218 | 0.030 | 0.312 | 0.077 | 0.089 | 0.096 | 0.222 | 0.107 | 0.093 | 0.098 | 0.065 | 0.323 | 0.084 | 0.094 | 0 | |||||||||
| IN2 | 0.112 | 0.116 | 0.174 | 0.229 | 0.140 | 0.110 | 0.121 | 0.089 | 0.131 | 0.241 | 0.100 | 0.084 | 0.103 | 0.122 | 0.274 | 0.097 | 0.090 | 0.140 | 0 | ||||||||
| IN3 | 0.158 | 0.186 | 0.254 | 0.337 | 0.224 | 0.077 | 0.209 | 0.131 | 0.193 | 0.349 | 0.178 | 0.159 | 0.149 | 0.189 | 0.346 | 0.123 | 0.134 | 0.219 | 0.051 | 0 | |||||||
| LK | 0.169 | 0.186 | 0.271 | 0.301 | 0.140 | 0.292 | 0.162 | 0.084 | 0.134 | 0.340 | 0.153 | 0.157 | 0.069 | 0.099 | 0.392 | 0.077 | 0.074 | 0.127 | 0.141 | 0.179 | 0 | ||||||
| VN | 0.166 | 0.155 | 0.251 | 0.303 | 0.205 | 0.119 | 0.187 | 0.124 | 0.180 | 0.320 | 0.162 | 0.151 | 0.132 | 0.172 | 0.354 | 0.105 | 0.105 | 0.216 | 0.054 | 0.037 | 0.147 | 0 | |||||
| BJ | 0.176 | 0.102 | 0.088 | 0.091 | 0.089 | 0.280 | 0.049 | 0.103 | 0.088 | 0.096 | 0.053 | 0.066 | 0.088 | 0.085 | 0.226 | 0.112 | 0.117 | 0.073 | 0.102 | 0.190 | 0.147 | 0.164 | 0 | ||||
| EG | 0.145 | 0.216 | 0.321 | 0.400 | 0.270 | 0.149 | 0.261 | 0.180 | 0.247 | 0.445 | 0.206 | 0.204 | 0.181 | 0.210 | 0.452 | 0.171 | 0.172 | 0.286 | 0.086 | 0.075 | 0.214 | 0.061 | 0.238 | 0 | |||
| KE1 | 0.179 | 0.235 | 0.333 | 0.414 | 0.258 | 0.159 | 0.274 | 0.185 | 0.264 | 0.450 | 0.213 | 0.210 | 0.200 | 0.216 | 0.464 | 0.157 | 0.179 | 0.274 | 0.116 | 0.076 | 0.243 | 0.076 | 0.251 | 0.101 | 0 | ||
| KE2 | 0.080 | 0.193 | 0.247 | 0.335 | 0.173 | 0.216 | 0.188 | 0.107 | 0.171 | 0.353 | 0.135 | 0.156 | 0.139 | 0.156 | 0.333 | 0.121 | 0.137 | 0.170 | 0.113 | 0.137 | 0.166 | 0.144 | 0.183 | 0.160 | 0.139 | 0 | |
| MU | 0.113 | 0.120 | 0.179 | 0.235 | 0.071 | 0.324 | 0.107 | 0.099 | 0.109 | 0.234 | 0.110 | 0.101 | 0.104 | 0.068 | 0.315 | 0.110 | 0.117 | 0.071 | 0.124 | 0.208 | 0.150 | 0.184 | 0.091 | 0.204 | 0.243 | 0.139 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, P.; Zhong, C.; Wei, Y.; Meng, J.; Li, Z.; Pinyopusarerk, K.; Bush, D. Analyses of Genetic Diversity, Differentiation and Geographic Origin of Natural Provenances and Land Races of Casuarina equisetifolia Based on EST-SSR Markers. Forests 2020, 11, 432. https://doi.org/10.3390/f11040432
Zhang Y, Hu P, Zhong C, Wei Y, Meng J, Li Z, Pinyopusarerk K, Bush D. Analyses of Genetic Diversity, Differentiation and Geographic Origin of Natural Provenances and Land Races of Casuarina equisetifolia Based on EST-SSR Markers. Forests. 2020; 11(4):432. https://doi.org/10.3390/f11040432
Chicago/Turabian StyleZhang, Yong, Pan Hu, Chonglu Zhong, Yongcheng Wei, Jingxiang Meng, Zhen Li, Khongsak Pinyopusarerk, and David Bush. 2020. "Analyses of Genetic Diversity, Differentiation and Geographic Origin of Natural Provenances and Land Races of Casuarina equisetifolia Based on EST-SSR Markers" Forests 11, no. 4: 432. https://doi.org/10.3390/f11040432






