Potential Impacts of Insect-Induced Harvests in the Mixed Forests of New England
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Spatial Data
2.2. Determining Harvest Response to Emerald Ash Borer
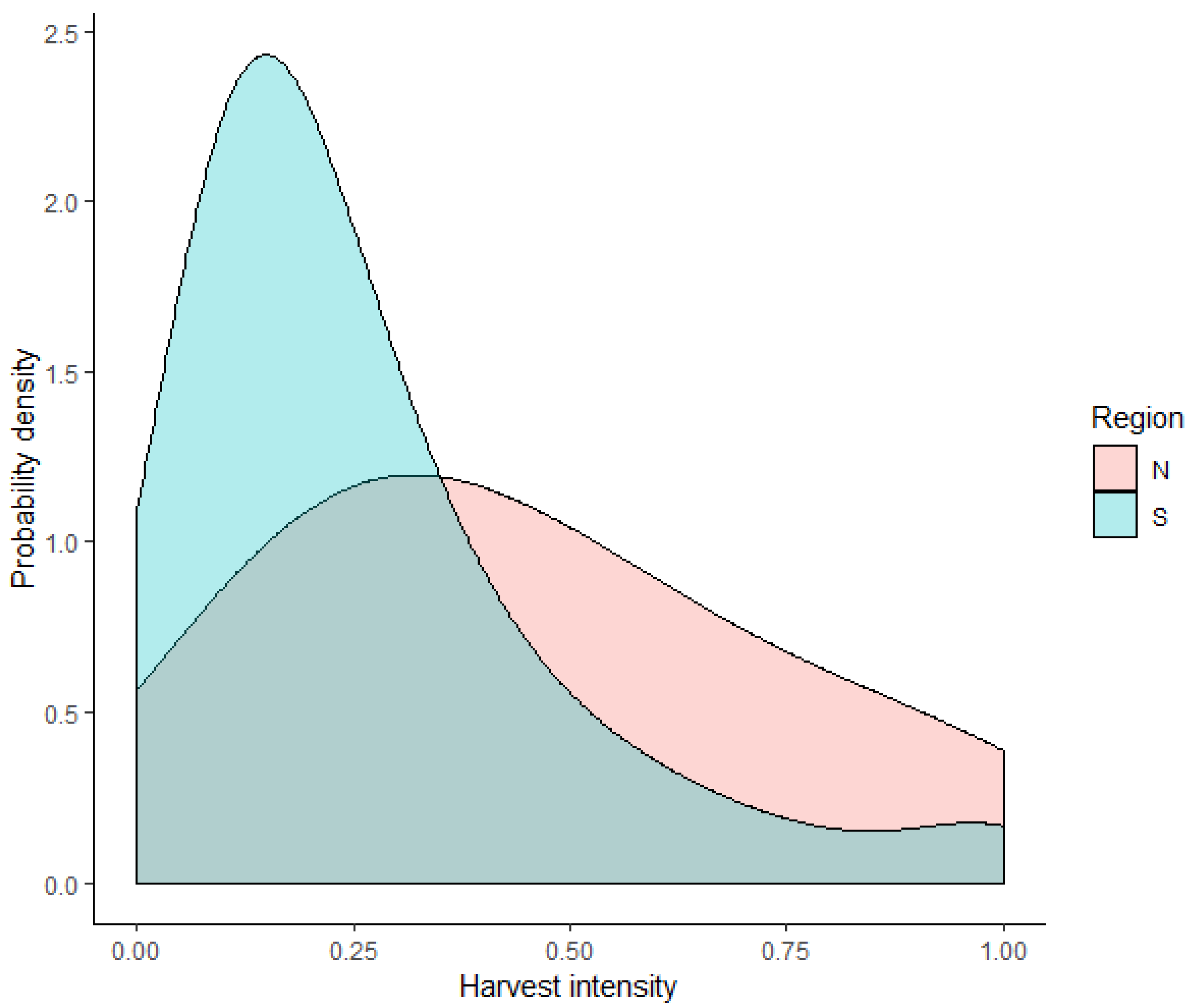
2.3. Modeling Harvest Intensities
2.4. Quantifying Cumulative Impacts of EAB
2.4.1. Scenario A: Host Removal Only; No Additional Harvesting
2.4.2. Scenario B: Typical Harvest Intensities and No Species Preferences
2.4.3. Scenario C: Typical Harvest Intensities and Value-Driven Species Preferences
2.4.4. Scenario D: Atypical High-Grading
3. Results
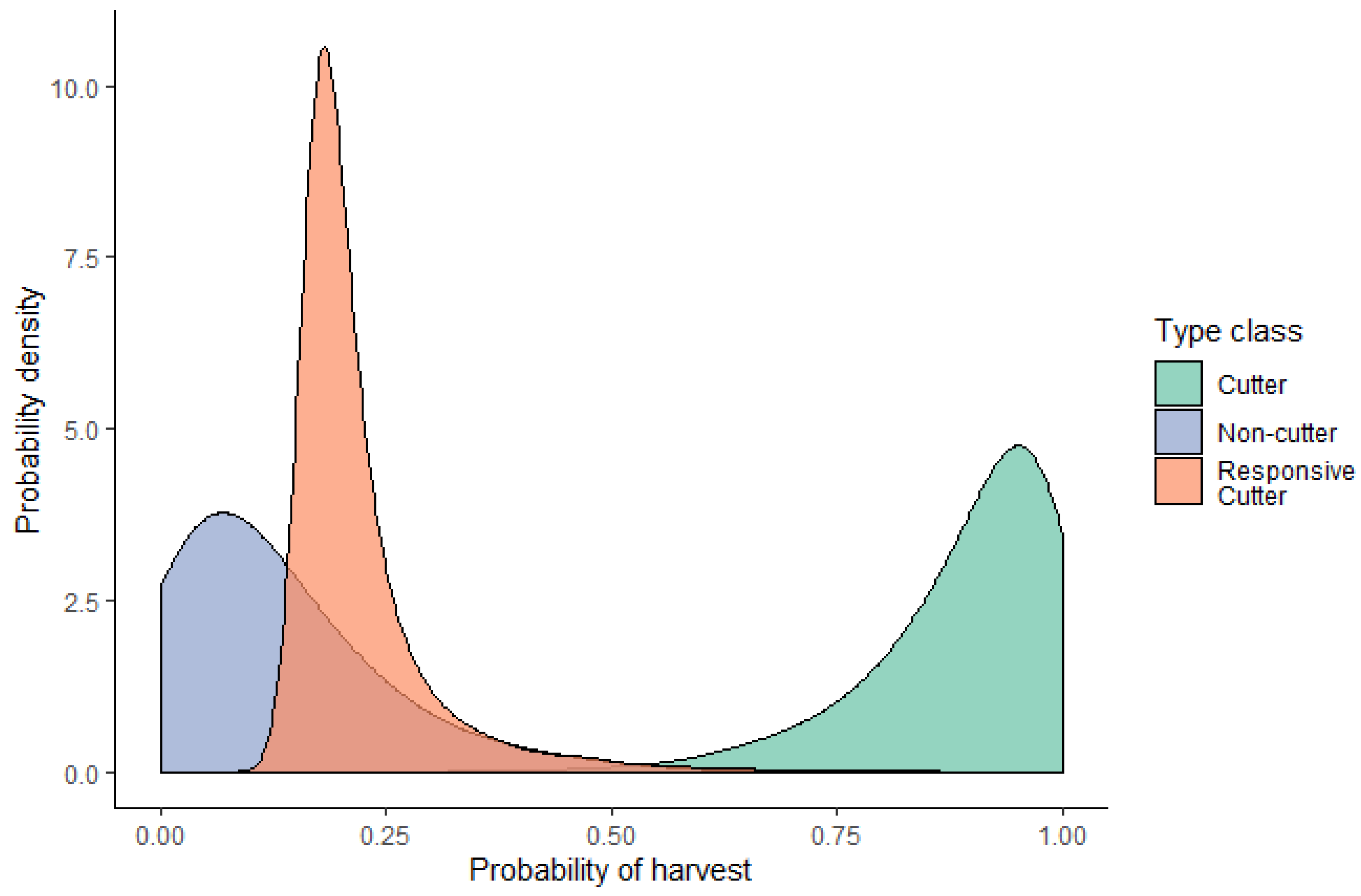
3.1. Estimating the Range of Responses to EAB
3.2. Quantifying the Impact of Insect-Induced Harvest on Regional Forest Biomass
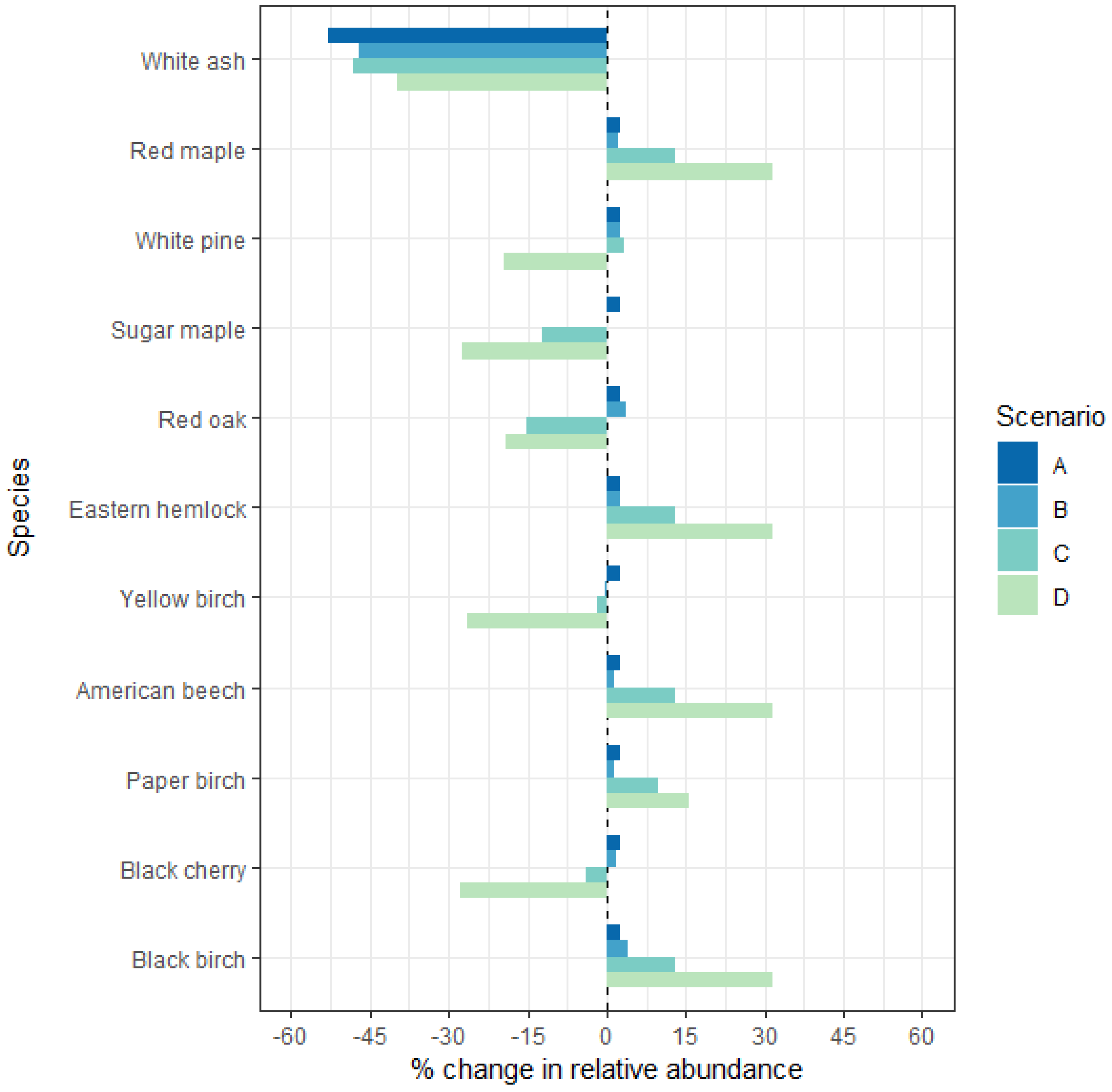
3.3. Quantifying the Impact of Insect-Induced Harvest on Regional Forest Composition
4. Discussion
5. Conclusions
Limitations and Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Family Forest Owner (FFO) Survey for Intent to Harvest

Appendix B
Defining and Characterizing Agent Functional Types
Appendix C
High Value Species in Our Harvest Regions.
| North (Northern NH and VT) | South |
|---|---|
| Sugar maple (Acer saccharum) ($315/Mbf) | Red oak (Quercus rubra) ($333/Mbf) |
| Red oak (Quercus rubra) ($310/Mbf) | Sugar maple (Acer saccharum) ($284/Mbf) |
| Black cherry (Prunus serotina) ($212/Mbf) | Black cherry (Prunus serotina) ($231/Mbf) |
| White oak (Quercus alba) ($170/Mbf) | White oak (Quercus alba) ($170/Mbf) |
| Yellow birch (Betula alleghaniensis) ($158/Mbf) | Yellow birch (Betula alleghaniensis) ($177/Mbf) |
| Red spruce (Picea rubens) ($120/Mbf) | White pine (Pinus strobus) ($105/Mbf) |
| White pine (Pinus strobus) ($112/Mbf) | |
| Paper birch (Betula papyrifera) ($99/Mbf) |
References
- Hicke, J.A.; Allen, C.D.; Desai, A.R.; Dietze, M.C.; Hall, R.J.; Ted Hogg, E.H.; Kashian, D.M.; Moore, D.; Raffa, K.F.; Sturrock, R.N.; et al. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Glob. Chang. Biol. 2012, 18, 7–34. [Google Scholar] [CrossRef]
- Williams, C.A.; Gu, H.; MacLean, R.; Masek, J.G.; Collatz, G.J. Disturbance and the carbon balance of US forests: A quantitative review of impacts from harvests, fires, insects, and droughts. Glob. Planet. Chang. 2016, 143, 66–80. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Burton, P.J.; Franklin, J.F. Salvage Logging and Its Ecological Consequences; Island Press: Washington, DC, USA, 2008; ISBN 9788578110796. [Google Scholar]
- Duveneck, M.J.; Thompson, J.R. Social and biophysical determinants of future forest conditions in New England: Effects of a modern land-use regime. Glob. Environ. Chang. 2019, 55, 115–129. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Markowski-Lindsay, M.; Borsuk, M.E.; Butler, B.J.; Duveneck, M.J.; Holt, J.; Kittredge, D.B.; Laflower, D.; MacLean, M.G.; Orwig, D.; Thompson, J.R. Compounding the Disturbance: Family Forest Owner Reactions to Invasive Forest Insects. Ecol. Econ. 2020, 167, 106461. [Google Scholar] [CrossRef]
- Brown, M.L.; Canham, C.D.; Murphy, L.; Donovan, T.M. Timber harvest as the predominant disturbance regime in northeastern U.S. forests: Effects of harvest intensification. Ecosphere 2018, 9, e02062. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Rey Benayas, J.M.; Castro, J.; Boucher, D.; Brewer, S.; Collins, B.M.; Donato, D.; Fraver, S.; Kishchuk, B.E.; Lee, E.-J.; et al. Salvage logging effects on regulating and supporting ecosystem services—A systematic map. Can. J. For. Res. 2018, 48, 1–18. [Google Scholar] [CrossRef]
- Waring, K.M.; O’Hara, K.L. Silvicultural strategies in forest ecosystems affected by introduced pests. For. Ecol. Manag. 2005, 209, 27–41. [Google Scholar] [CrossRef]
- Irland, L.C.; Dimon, J.B.; Baum, E.; Falk, J.; Stone, J.L. The Spruce Budworm Outbreak in Maine in The 1970’s —Assessment and Directions for the Future; Maine Agricultural Experiment Station: Orono, ME, USA, 1988. [Google Scholar]
- Kizlinski, M.L.; Orwig, D.A.; Cobb, R.C.; Foster, D.R. Direct and indirect ecosystem consequences of an invasive pest on forests dominated by eastern hemlock. J. Biogeogr. 2002, 29, 1489–1503. [Google Scholar] [CrossRef]
- Butler, B.J.; Hewes, J.H.; Dickinson, B.J.; Andrejczyk, K.; Butler, S.M.; Markowski-Lindsay, M. Family Forest Ownerships of the United States, 2013: Findings from the USDA Forest Service’s National Woodland Owner Survey. J. For. 2016, 114, 638–647. [Google Scholar] [CrossRef]
- Belair, E.P.; Ducey, M.J. Patterns in Forest Harvesting in New England and New York: Using FIA Data to Evaluate Silvicultural Outcomes. J. For. 2018, 116, 273–282. [Google Scholar] [CrossRef]
- Thompson, J.R.; Canham, C.D.; Morreale, L.; Kittredge, D.B.; Butler, B. Social and biophysical variation in regional timber harvest regimes. Ecol. Appl. 2017, 27, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Kittredge, D.B.; Thompson, J.R. Timber Harvesting Behaviour in Massachusetts, USA: Does Price Matter to Private Landowners? Small-Scale For. 2016, 15, 93–108. [Google Scholar] [CrossRef]
- Holt, J.R.; Borsuk, M.E.; Butler, B.J.; Kittredge, D.B.; Laflower, D.; MacLean, M.G.; Markowski-Lindsay, M.; Orwig, D.A.; Thompson, J.R. Landowner Functional Types to Characterize Responses to Forest Insects. People Nat. 2019, 2, 204–216. [Google Scholar]
- Herms, D.A.; McCullough, D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [Green Version]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic Impacts of Non-Native Forest Insects in the Continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Klooster, W.; Gandhi, K.; Long, L.; Perry, K.; Rice, K.; Herms, D.; Klooster, W.S.; Gandhi, K.J.K.; Long, L.C.; Perry, K.I.; et al. Ecological Impacts of Emerald Ash Borer in Forests at the Epicenter of the Invasion in North America. Forests 2018, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Robinett, M.A.; McCullough, D.G. White ash (Fraxinus americana) survival in the core of the emerald ash borer (Agrilus planipennis) invasion. Can. J. For. Res. 2019, 49, 510–520. [Google Scholar] [CrossRef]
- Morin, R.S.; Liebhold, A.M.; Pugh, S.A.; Crocker, S.J. Regional assessment of emerald ash borer, Agrilus planipennis, impacts in forests of the Eastern United States. Biol. Invasions 2017, 19, 703–711. [Google Scholar] [CrossRef]
- Fei, S.; Morin, R.S.; Oswalt, C.M.; Liebhold, A.M. Biomass losses resulting from insect and disease invasions in US forests. PNAS 2019, 116, 17371–17376. [Google Scholar] [CrossRef] [Green Version]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Klooster, W.S.; Herms, D.A.; Knight, K.S.; Herms, C.P.; McCullough, D.G.; Smith, A.; Gandhi, K.J.K.; Cardina, J. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions 2014, 16, 859–873. [Google Scholar] [CrossRef]
- Evans, A.M. The speed of invasion: Rates of spread for thirteen exotic forest insects and diseases. Forests 2016, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Siegert, N.W.; Mercader, R.J.; McCullough, D.G. Spread and dispersal of emerald ash borer (Coleoptera: Buprestidae): Estimating the spatial dynamics of a difficult-to-detect invasive forest pest. Can. Entomol. 2015, 147, 338–348. [Google Scholar] [CrossRef]
- Forest Health Vermont Executive Summary Ash Management Guidance for Forest Managers. Available online: http://anrmaps.vermont.gov/websites/anra5/ (accessed on 12 September 2019).
- Mercader, R.J.; McCullough, D.G.; Storer, A.J.; Bedford, J.M.; Heyd, R.; Poland, T.M.; Katovich, S. Evaluation of the potential use of a systemic insecticide and girdled trees in area wide management of the emerald ash borer. For. Ecol. Manag. 2015, 350, 70–80. [Google Scholar] [CrossRef]
- Sadof, C.S.; Hughes, G.P.; Witte, A.R.; Peterson, D.J.; Ginzel, M.D. Tools for staging and managing the emerald ash borer in an urban forest. Arboric. Urban For. 2017, 43, 15–26. [Google Scholar]
- Bechtold, W.A.; Patterson, P.L. The Enhanced Forest Inventory and Analysis Program-National Sampling Design and Estimation Procedures; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005.
- Duveneck, M.J.; Thompson, J.R.; Wilson, B.T. An imputed forest composition map for New England screened by species range boundaries. For. Ecol. Manag. 2015, 347, 107–115. [Google Scholar] [CrossRef]
- MacCallum, R.C.; Zhang, S.; Preacher, K.J.; Rucker, D.D. On the practice of dichotomization of quantiative variables. Psych. Methods 2002, 7, 19–40. [Google Scholar] [CrossRef]
- Allik, J. A mixed-binomial model for Likert-type personality measures. Front. Psychol. 2014, 5, 371. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, B.F., Eds.; Academiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Leung, B.; Springborn, M.R.; Turner, J.A.; Brockerhoff, E.G. Pathway-level risk analysis: The net present value of an invasive species policy in the US. Front. Ecol. Environ. 2014, 12, 273–279. [Google Scholar] [CrossRef]
- Homer, C.G.; Dewitz, J.A.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.D.; Wickham, J.D.; Megown, K. Completion of the 2011 National Land Cover Database for the conterminous United States-Representing a decade of land cover change information. Photogramm. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Manson, S.; Schroeder, J.; Van Riper, D.; Ruggles, S. IPUMS National Historical Geographic Information System: Version 12.0; University of Minnesota: Minneapolis, MN, USA, 2017; p. 50. [Google Scholar] [CrossRef]


| Species | Total Merchantable Timber AGB (Tg) | % of Total AGB Co-Occurring with Ash |
|---|---|---|
| Black cherry (Prunus serotina Ehrhart, c. 1780) | 4.4 | 84% |
| American basswood (Tilia americana Linnaeus, c. 1758) | 1.7 × 10−1 | 83% |
| Sugar maple (Acer saccharum Marshall, c. 1785) | 26.8 | 83% |
| American beech (Fagus grandifolia Ehrhart, c. 1780) | 7.2 | 82% |
| Yellow birch (Betula alleghaniensis Britton, c. 1896) | 8.4 | 81% |
| Paper birch (Betula papyrifera Marshall, c. 1785) | 4.6 | 80% |
| Bigtooth aspen (Populus grandidentata Michaux, c. 1803) | 8.0 × 10−1 | 80% |
| Gray birch (Betula populifolia Marshall, c. 1785) | 2.6 × 10−3 | 78% |
| Quaking aspen (Populus tremuloides Michaux, c. 1803) | 1.7 | 78% |
| Red maple (Acer rubrum Linnaeus, c. 1753) | 28.0 | 77% |
| Black birch (Betula lenta Linnaeus, c. 1758) | 4.1 | 76% |
| Eastern hemlock (Tsuga canadensis Carriere, c. 1862) | 20.3 | 76% |
| Red spruce (Picea rubens Sargent, c. 1880) | 3.6 | 75% |
| Total AGB (Tg) | % of Total AGB Harvested | ||||
|---|---|---|---|---|---|
| Species | Scenario A: Host (Ash) Only Removal | Scenario B: Typical Intensity, No Species Preference | Scenario C: Typical Antensity, Valuable Species Preference | Scenario D: Any Intensity, All Valuable Species | |
| *White ash (Fraxinus americana) | 8.3 | 54% ± 2.4% | 54% ± 2.4% | 54% ± 2.4% | 54% ± 2.4% |
| Red maple (Acer rubrum) | 27.0 | 0% | 11% ± 0.9% | 0% ± 0% | 0% ± 0% |
| White pine (Pinus strobus) | 26.4 | 0% | 11% ± 1.2% | 9% ± 1.4% | 39% ± 3.1% |
| Sugar maple (Acer saccharum) | 26.3 | 0% | 13% ± 0.8% | 22% ± 2.4% | 45% ± 1.8% |
| Red oak (Quercus rubra) | 22.7 | 0% | 10% ± 1.4% | 25% ± 2.6% | 39% ± 3.5% |
| Eastern hemlock (Tsuga canadensis) | 19.8 | 0% | 11% ± 1.2% | 0% ± 0% | 0% ± 0% |
| Yellow birch (Betula alleghaniensis) | 8.3 | 0% | 13% ± 1.0% | 13% ± 1.9% | 44% ± 2.0% |
| American beech (Fagus grandifolia) | 7.0 | 0% | 12% ± 0.9% | 0% ± 0% | 0% ± 0% |
| Paper birch (Betula papyrifera) | 4.5 | 0% | 12% ± 1.1% | 3% ± 0.4% | 12% ± 1.1% |
| Black cherry (Prunus serotina) | 4.3 | 0% | 12% ± 0.9% | 15% ± 1.7% | 45% ± 2.7% |
| Black birch (Betula lenta) | 4.0 | 0% | 10% ± 1.3% | 0% ± 0% | 0% ± 0% |
| Total | 180.1 | 2.4% ± 0.1% | 13% ± 1.0% | 12% ± 1.1% | 24% ± 1.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLean, M.G.; Holt, J.; Borsuk, M.; Markowski-Lindsay, M.; Butler, B.J.; Kittredge, D.B.; Duveneck, M.J.; Laflower, D.; Orwig, D.A.; Foster, D.R.; et al. Potential Impacts of Insect-Induced Harvests in the Mixed Forests of New England. Forests 2020, 11, 498. https://doi.org/10.3390/f11050498
MacLean MG, Holt J, Borsuk M, Markowski-Lindsay M, Butler BJ, Kittredge DB, Duveneck MJ, Laflower D, Orwig DA, Foster DR, et al. Potential Impacts of Insect-Induced Harvests in the Mixed Forests of New England. Forests. 2020; 11(5):498. https://doi.org/10.3390/f11050498
Chicago/Turabian StyleMacLean, Meghan Graham, Jonathan Holt, Mark Borsuk, Marla Markowski-Lindsay, Brett J. Butler, David B. Kittredge, Matthew J. Duveneck, Danelle Laflower, David A. Orwig, David R. Foster, and et al. 2020. "Potential Impacts of Insect-Induced Harvests in the Mixed Forests of New England" Forests 11, no. 5: 498. https://doi.org/10.3390/f11050498





