Oak Resprouting Survival and Competition for 19 Years after Wildfire in the Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
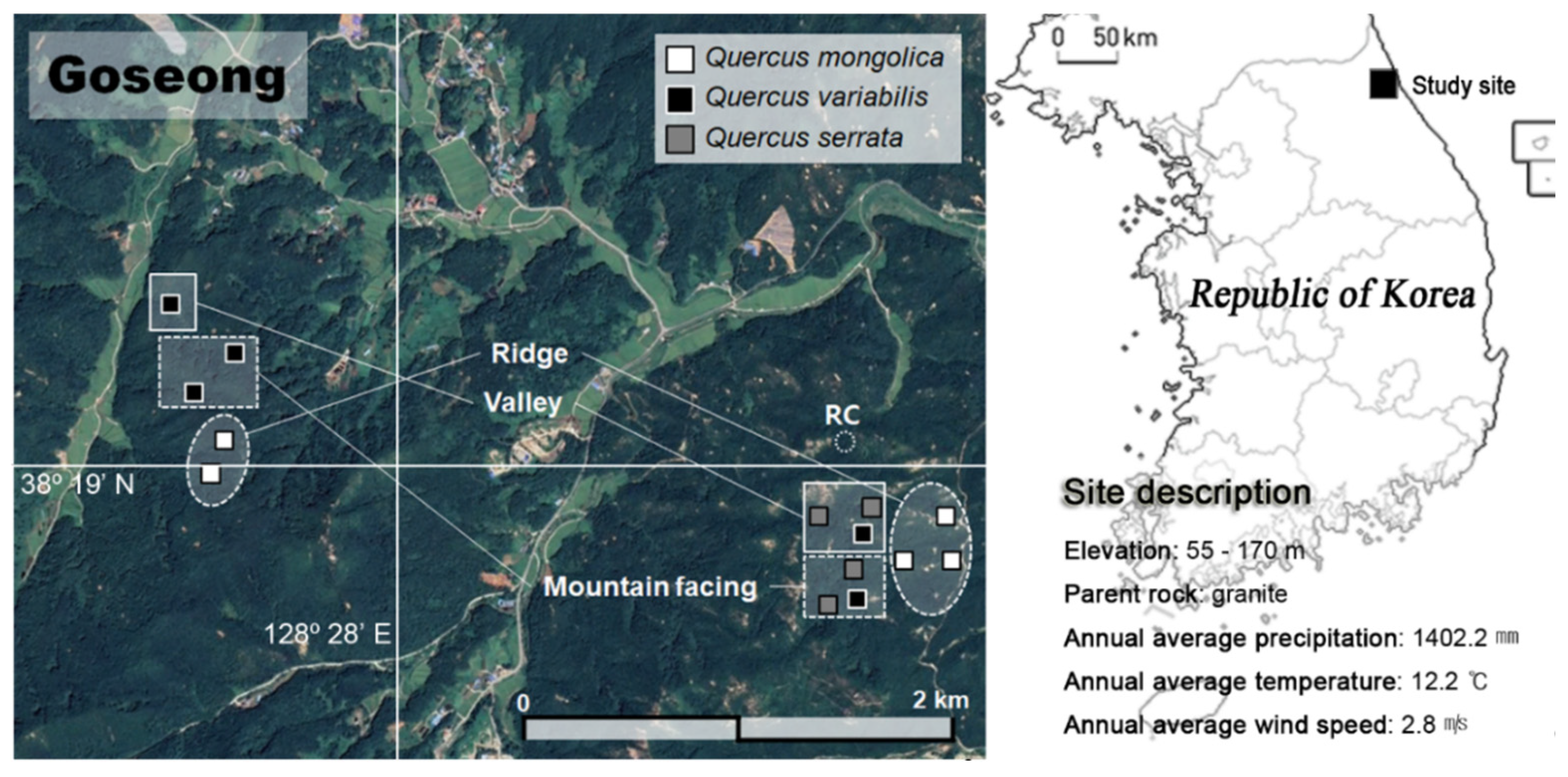
2.1. Study Site Description and Survey to Tree Growth
2.2. Analysis
3. Results
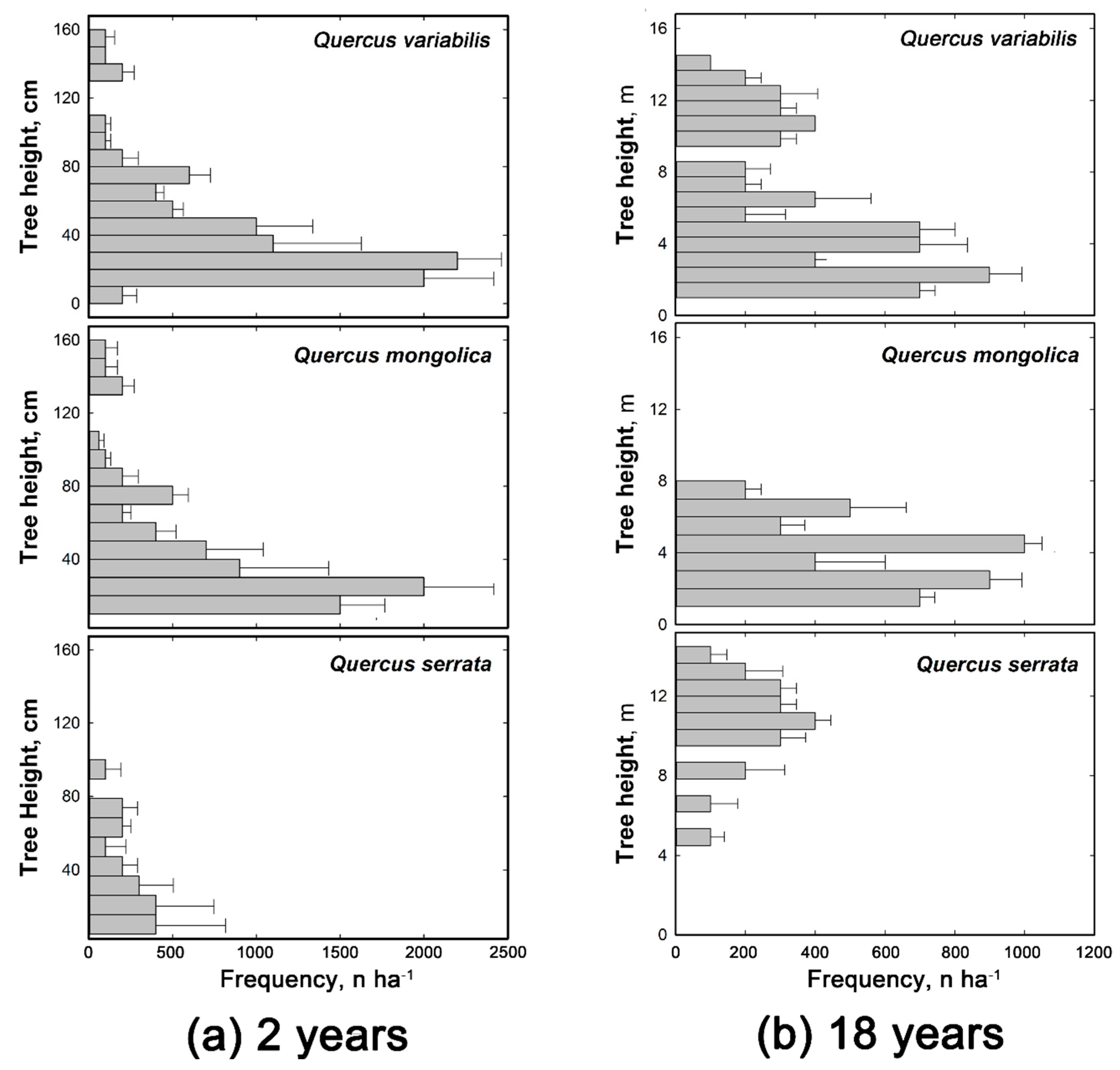
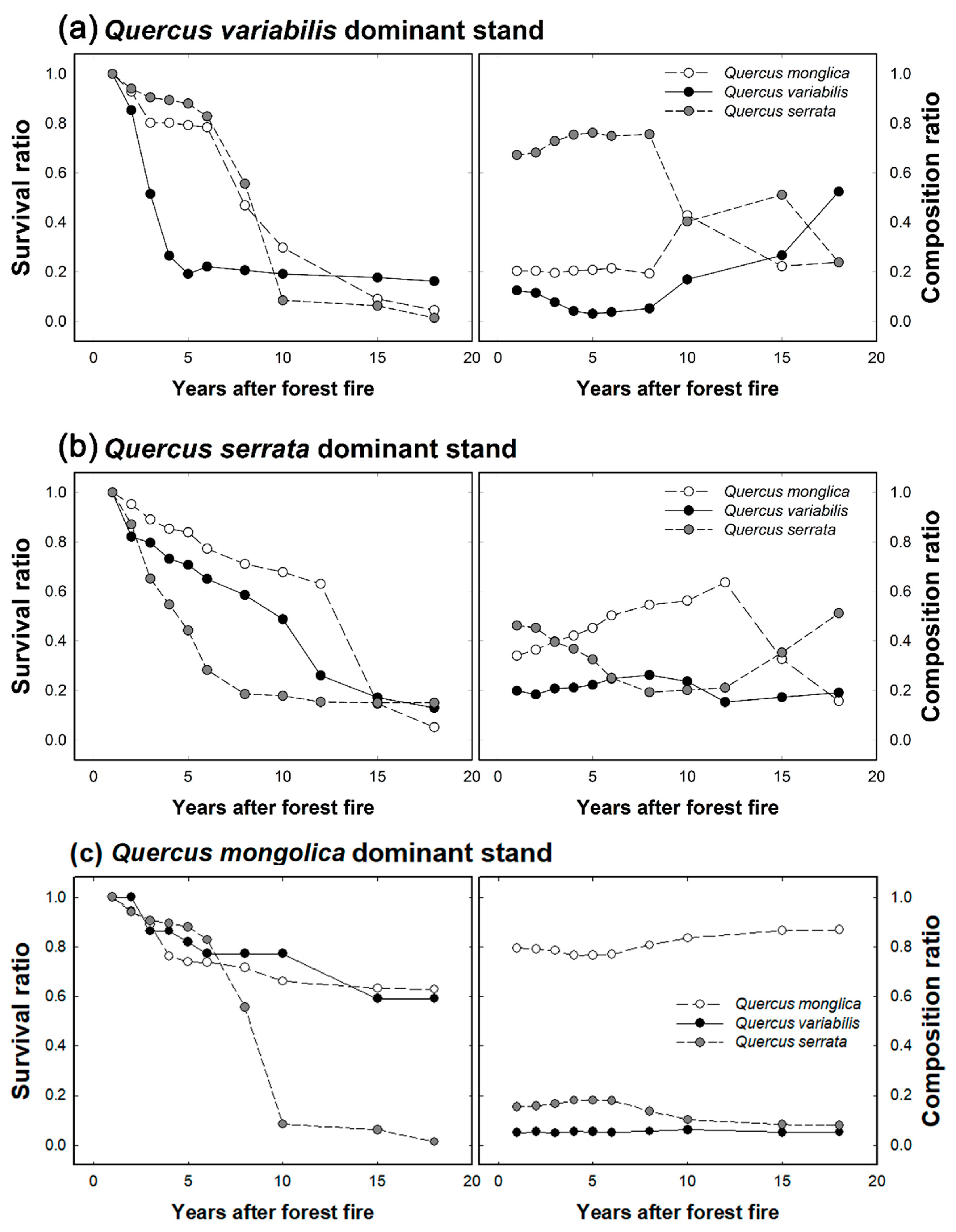
3.1. Sprout Growth and Composition over Time
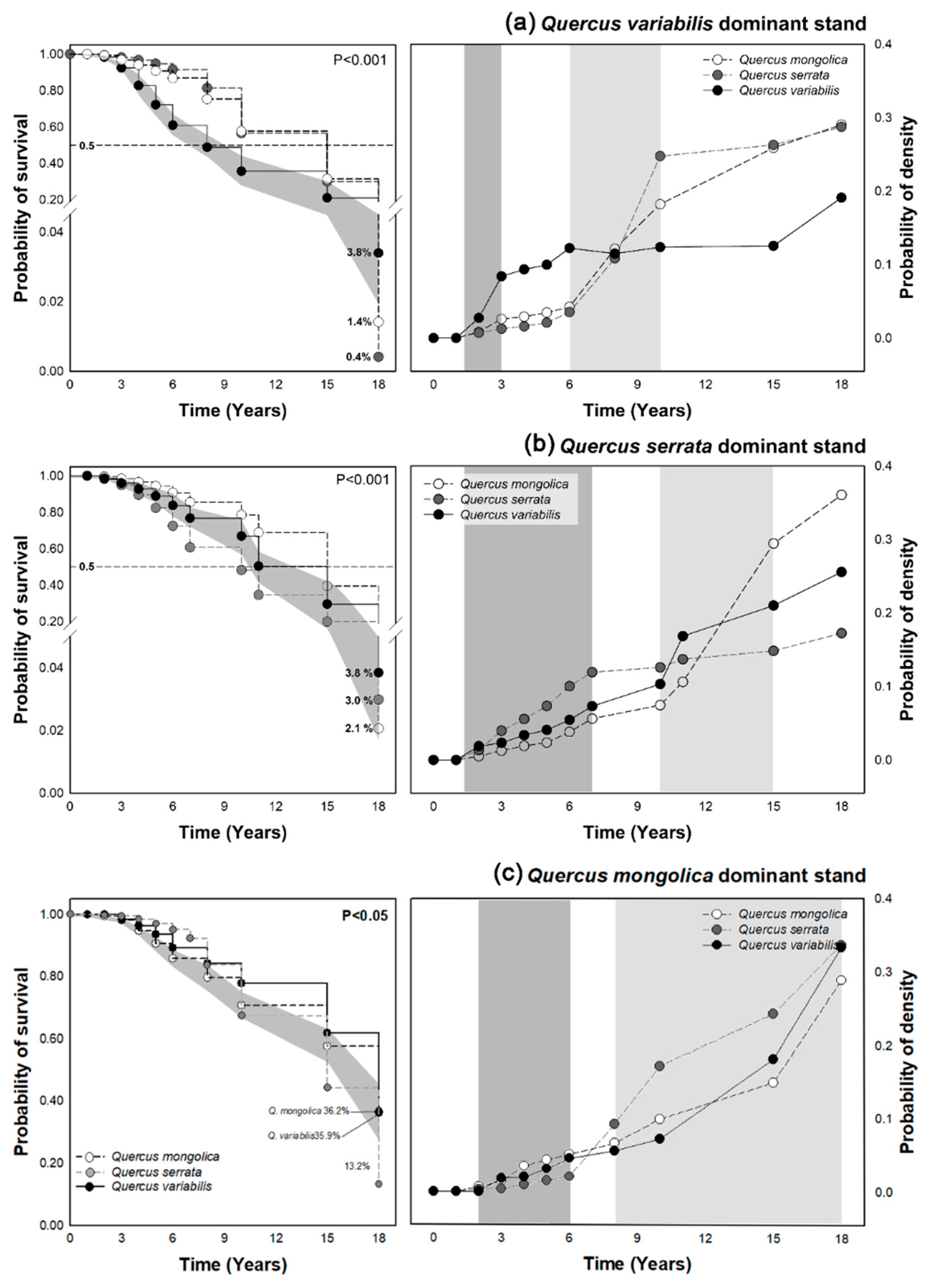
3.2. Survival Analysis of Oak Sprouts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, B.V.; Zak, D.R.; Denton, S.R.; Spurr, S.H. Forest Ecology, 4th ed.; Wiley: New York, NY, USA, 1998; p. 792. [Google Scholar]
- Korea Forest Research Institute. Restoration of Fire Damaged Area; Korea Forest Research Institute (KFRI): Seoul, Korea, 1999; p. 173. [Google Scholar]
- Fulé, P.; García-Arévalo, A.; Covington, W. Effects of an Intense Wildfire in a Mexican oak-pine forest. Forest Sci. 2000, 46, 51–61. [Google Scholar] [CrossRef]
- Choung, Y.; Lee, B.C.; Cho, J.H.; Lee, K.S.; Jang, I.S.; Kim, S.H.; Hong, S.K.; Jung, H.C.; Choung, H.L. Forest response to the large-scale east coast fires in Korea. Ecol. Res. 2004, 19, 43–54. [Google Scholar] [CrossRef]
- Korea Forest Service. Forest Fire Statistical Yearbook; Korea Forest Service (KFS): Daejeon, Korea, 2018; p. 263, (In Korean without English abstract).
- Korea Forest Service. Annual Statistics Report of Forest Fire; Semmunhwa: Seoul, Korea, 2016; p. 242, (In Korean without English abstract).
- Lee, M.; Lee, S.; Lee, J.H. Study of the characteristics of forest fire based on statistics of forest fire in Korea. J. Korean Soc. Hazaard Mitigatioin 2012, 12, 185–192, (In Korean with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Korea Forest Service. The Occurrence of Forest Fire. KFS 2020. Available online: http://forset.go.kr/newkfsweb/kfi/kfs/frfr/selectFrfrStats.do?searchCnd=2010&mn=KFS_02_02_01_05_01 (accessed on 11 February 2020).
- Kim, S.S.; Lee, M.W. Analysis of forest fire based on statistics over the past 50 years in Korea. J. KOSHAM 2013, 13, 275–280, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Lim, J. Forest fire and meteorology of Eastern Korea. Korean J. Agric. For. Meteor. 2000, 2, 62–67, (In Korean with English abstract). [Google Scholar]
- Lee, W.; Kim, S.; Bae, S.; Lee, J.; Shin, H.; Jung, M.; Moon, H.; Bae, E. Vegetation type and stand structure of Pinus densiflora forests in Kangwon northern regions in Korea. J. Agric. Life Sci. 2009, 43, 7–17, (In Korean with English abstract). [Google Scholar]
- Kim, Y.; Shin, D. Volatile components and antibacterial effects of pine needle (Pinus densiflora S. and Z.) extracts. Food Microbiol. 2005, 22, 37–45. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, N.; Shu, L. Flammability ranking of foliage species by factor analysis of physical and chemical pyric properties. Fire Mater. 2008, 32, 371–382. [Google Scholar] [CrossRef]
- Korea Forest Research Institute. Economy Species 1: Pinus Densiflora; Korea Forest Research Institute: Seoul, Korea, 2012; p. 250, (In Korean without English abstract). [Google Scholar]
- Patra, J.; Kim, S.; Hwang, H.; Choi, J.; Baek, K. Volatile compounds and antioxidant capacity of the bio-oil obtained by pyrolysis of Japanese red pine (Pinus densiflora Siebold and Zucc.). Molecules 2015, 20, 3986–4006. [Google Scholar] [CrossRef] [Green Version]
- National Science and Technology Information Service (NTIS), National R&D Project in Korea. Available online: https://www.ntis.go.kr/ThSearchProjectList.do?gubun=link&searchWord=%EC%82%B0%EB%B6%88&searchSentence=&searchViewData=&searchType=&oldSearchWord=%EC%82%B0%EB%B6%88&resultSearch=&pageNumber=4&ssoKnfSlct=&ascDesc=ASC&useYn=N&oldQuery=%EC%82%B0%EB%B6%88&oldAddQuery=%26SN04%3D2019&dbt=project&checkYn=&searchOption16=&pageYn=Y&technologyClassification=ST&directorySearchYear=&directorySearchOption1=&directorySearchOption2=&directorySearchOption3=&downloadTarget=project&startRow=&endRow=&rqstPurpCd=&infoPrctuseDes=&layerChoice=&sort=RANK%2FDESC&pageSize=20&navigation1%5B0%5D=2019 (accessed on 27 February 2020).
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.R.; Choi, H.T.; Lim, J.H.; Le, L.K.; Ahn, Y.S. Post-fire restoration plan for sustainable forest management in south Korea. Forests 2017, 8, 188–201. [Google Scholar] [CrossRef] [Green Version]
- Korea Forest Research Institute. Post-Fire Restoration to Establish a Healthy and Sustainable Forest Ecosystem, English Edition; KFRI: Seoul, Korea, 2010; p. 60. [Google Scholar]
- Denk, T.; Grimm, G.; Manos, P.; Deng, M.; Hipp, A. An Updated Infrageneric Classification of the Oaks: Review of Previous Taxonomic Schemes and Synthesis of Evolutionary Pattern. Oaks Physiological Ecology Exploring the Functional Diversity of Genus Quercus L. Tree Physiol. 2017, 13–38. [Google Scholar] [CrossRef]
- Dolezal, J.; Song, J.S.; Altman, J.; Janecek, S.; Cerny, T.; Srutek, M.; Kolbek, J. Tree growth and competition in a post-logging Quercus mongolica forest on Mt. Sobaek, South Korea. Ecol. Res. 2009, 24, 281–290. [Google Scholar] [CrossRef]
- Korea Forest Research Institute. Economic Tree Species ② Oak; KFRI: Seoul, Korea, 2012; p. 250, (In Korean without English abstract). [Google Scholar]
- Lee, D. Forest Ecological Management; Seoul University: Seoul, Korea, 2012; p. 471, (Korean only). [Google Scholar]
- Da, L.J.; Kang, M.M.; Song, K.; Shang, K.K.; Yang, Y.C.; Xia, A.M.; Qi, Y.F. Altitudinal zonation of human-disturbed vegetation on Mt. Tianmu, Eastern China. Ecol. Res. 2009, 24, 1287–1299. [Google Scholar] [CrossRef]
- Xu, N.; Guo, W.; Liu, J.; Du, N.; Wang, R. Increased nitrogen deposition alleviated the adverse effects of drought stress on Quercus variabilis and Quercus mongolica seedlings. Acta Physiol. Plant. 2015, 37, 107. [Google Scholar] [CrossRef]
- Wang, S.; Xuan, L.; Guo, C. Community ecology of poplar-birch forest in China. J. Northeast For. Univ. 1996, 7, 7–11. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, W.; Zhou, J.; Ma, C.; Ma, L. Effects of stump diameter, stump height, and cutting season on Quercus variabilis stump sprouting. Scand. J. For. Res. 2013, 28, 223–231. [Google Scholar] [CrossRef]
- Dey, D.C.; Gardiner, E.S.; Schweitzer, C.J.; Kabrick, J.M.; Jacobs, D.F. Underplanting to sustain future stocking of oak (Quercus) in temperate deciduous forests. New For. 2012, 43, 955–978. [Google Scholar] [CrossRef]
- Lee, W.K.; van Gadow, K.; Chung, D.-J.; Lee, J.L.; Shin, M.Y. DBH growth model for Pinus densiflora and Quercus variabilis mixed forests in central Korea. Ecol. Modell. 2004, 176, 187–200. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, S.; Liu, D.; Yu, M.; Tang, L.T. Influence of thinning time and density on sprout development, biomass production and energy stocks of sawtooth oak stumps. For. Ecol. Manag. 2011, 262, 299–306. [Google Scholar] [CrossRef]
- Mostacedo, B.; Putz, F.E.; Fredericksen, T.S.; Villca, A.; Palacios, T. Contribution of root and stump sprouts to natural regeneration of a logged tropical dry forest in Bolivia. For. Ecol. Manag. 2009, 258, 978–985. [Google Scholar] [CrossRef]
- Kauppi, A.; Kiviniitty, M.; Ferm, A. Growth habits and crown architecture of Betula pubescens Ehrh. of seed and sprout origin. Can. J. For. Res. 1998, 18, 1603–1613. [Google Scholar] [CrossRef]
- Pytte, P.L.; Fisher, U.F.; Suchomel, C.; Gärtner, S.M.; Bauhus, J. The effect of harvesting on stump mortality and re-sprouting in aged oak coppice forests. For. Ecol. Manag. 2013, 289, 18–27. [Google Scholar] [CrossRef]
- Canellas, I.; Del, M.; Roig, S.; Montero, G. Growth response to thinning in Quercus pyrenaica Willd. coppice stand in Spanish central mountain. Ann. For. Sci. 2004, 61, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Ji, D.; Lee, Y.G.; Lee, M. Study on the management system of oak coppice forest on forest fire site. J. Korean. For. Soc. 2009, 98, 652–658, (In Korean with English abstract). [Google Scholar]
- Swaim, J.T.; Dey, D.C.; Saunders, M.R.; Weigel, D.R.; Thornton, C.D.; Kabrick, J.M.; Jenkins, M.A. Predicting the height growth of oak species (Quercus) reproduction over a 23-year period following clearcutting. For. Ecol. Manag. 2016, 364, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Korea Meteorological Administration. Climatological Normals of Korea (1981~2010); Korea Meteorological Administration: Seoul, Korea, 2011; p. 678. [Google Scholar]
- Song, J.H.; Lim, J.H.; Kwon, J.; Yun, C.W. Comparison vegetation structure change between 2003 and 2014 in forest fire damaged are of Bihwjin basin, Samcheok in Korea. J. Korean For. Soc. 2017, 106, 150–168. [Google Scholar] [CrossRef]
- Kortet, R.; Hedrick, A. A behavioural syndrome in the field cricket Gryllus integer: Intrasexual aggression is correlated with activity in a novel environment. Biol. J. Linn Soc. Lond. 2007, 91, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Ahnn, S.; Anderson, S. Sample size determination in complex clinical trials comparing more than two groups for survival endpoints. Statist. Med. 1998, 17, 2525–2534. [Google Scholar] [CrossRef]
- Sdak, Z. Retangular confidence regions for the mean of multivariate normal distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar]
- Korea Forest Research Institute. Forest Tree Volume, Biomass and Yield Table; KFRI: Pocheon-si, Korea, 2012; p. 261, (Korean only). [Google Scholar]
- National Institute of Forest Science. Yield Table for Actual Forest; National Institute of Forest Science: Seoul, Korea, 2016; p. 54, (Korean only). [Google Scholar]
- Dinh, T.T.; Kajikawa, C.; Akaji, Y.; Yamada, K.; Matsumoto, T.K.; Makimoto, T.; Miki, N.H.; Hirobe, M.; Sakamoto, K. Stump sprout dynamics of Quercus serrata Thunb. and Q. acutissima Carruth. four years after cutting in an abandoned coppice forest in Western Japan. For. Ecol. Manag. 2019, 435, 45–56. [Google Scholar] [CrossRef]
- Wang, X. Assessment and Management of Oak Coppice Stands in Shangnam County, Southern Shaanxi Province, China. Ph.D. Thesis, München Technical University, München, Germany, 2013; p. 573. [Google Scholar]
- Staaf, H.; Stjernquist, I. Seasonal dynamics, especially autumnal retranslocation of nitrogen and phosphorus in foliage of dominant and suppressed trees of beech. Fagus Sylvatica. Scand. J. For. Res. 1986, 1, 333–342. [Google Scholar] [CrossRef]
- Peri, P.L.; Gargaglione, V.; Pastur, M. Above- and belowground nutrient storage and biomass accumulation in marginal Nothofagus antarctica forests in Southern Patagonia. For. Ecol. Manag. 2008, 255, 2502–2511. [Google Scholar] [CrossRef]
- Keim, R.F.; Chambers, J.L.; Hughes, M.S.; Dimov, L.D.; Conner, W.H.; Shaffer, G.P.; Gardiner, E.S.; Day, J.W.J. Long-term success of stump sprouts in high-graded bald cypress-water tupelo swamps in the Mississippi delta. For. Ecol. Manag. 2006, 234, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.L.; Tripathi, R.S. Effects of stump diameter, stump height and sprout density on the sprout growth of four tree species in burnt and unburnt forest plots. Acta Oecol. 1989, 10, 303–316. [Google Scholar]
- Brevik, E.C.; Fenton, T.E.; Horton, R. Effect of daily soil temperature fluctuations on soil electrical conductivity as measured with the geonics EM-38. Precis. Agric. 2004, 5, 145–152. [Google Scholar] [CrossRef]
- Ebihara, K.; Masahiro, T.; Tomoaki, I.; Kouichi, O. Development of Agricultural Soil Sterilization Using Ozone Generated by High Frequency Dielectric Barrier Discharge. J. Adv. Oxid. Technol. 2005, 33–40. [Google Scholar] [CrossRef]
- Son, S.; Kwon, K.; Jeong, T. Productive structure and net production of Quercus mongolica forest in Mt. Taehwa (Kwangju, Kyonggi-do). For. Bioenergy 2002, 21, 76–82, (In Korean with English abstract). [Google Scholar]
- Hammett, E.J.; Ritchie, M.W.; Berrill, J.P. Resilience of California black oak experiencing frequent fire: Regeneration following two large wildfires 12 years apart. Fire Ecol. 2017, 13, 91–103. [Google Scholar] [CrossRef]
- Kim, J.; Yim, Y.; Kil, B. Classification and pattern analysis of the forest vegetation in Daedunsan provincial park, Korea. Korean J. Ecol. 1988, 11, 109–122. [Google Scholar]
- Kim, J.; Yim, Y. Distribution patterns of species populations along the environmnetal gradients in Mt. Moak Provincial Park, Korea. Korean J. Ecol. 1992, 15, 365–375. [Google Scholar]
- Gardiner, E.S.; Helmig, L.M. Development of water oak stump sprouts under a partial overstory. New For. 1997, 14, 55–62. [Google Scholar] [CrossRef]
| Stand | Species | Tree Height, m | Mean DBH, cm | Tree Density, Trees ha−1 |
|---|---|---|---|---|
| Quercus variabilis | Quercus variabilis1 | 9.1 (4.3) a | 11.6 (4.3) b | 870 b |
| (oriental oak) | Quercus mongolica1 | 4.5 (2.9) | 5.4 (2.4) | 670 |
| Quercus serrata1 | 4.7 (2.9) | 4.1 (3.0) | 1370 | |
| Quercus dentate | 1.9 (0.6) | 2.2 (0.8) | 120 | |
| Quercus mongolica | Quercus mongolica1 | 6.0 (2.8) b | 5.9 (2.5) c | 2030 a |
| (Mongolian oak) | Quercus variabilis1 | 4.9 (2.0) | 7.1 (3.2) | 130 |
| Quercus serrata1 | 3.6 (3.9) | 5.0 (5.9) | 200 | |
| Quercus dentate | 2.4 (0) | 2.5 (0) | 30 | |
| Quercus serrata | Quercus serrata1 | 9.8 (3.8) a | 14.3 (4.1) a | 910 b |
| (glandbearing oak) | Quercus variabilis1 | 4.1 (3.1) | 4.9 (2.3) | 670 |
| Quercus mongolica1 | 2.9 (2.4) | 3.8 (2.9) | 330 | |
| Quercus dentate | 2.1 (1.5) | 2.3 (1.3) | 170 | |
| Quercus acutissima | 3.4 (3.3) | 3.7 (3.4) | 200 | |
| Kruskal-Wallis H-value 1 | 28.703 | 63.364 | 8.409 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lim, J.-H.; Shin, M.; Han, S.-H.; Kang, W. Oak Resprouting Survival and Competition for 19 Years after Wildfire in the Republic of Korea. Forests 2020, 11, 515. https://doi.org/10.3390/f11050515
Kim J, Lim J-H, Shin M, Han S-H, Kang W. Oak Resprouting Survival and Competition for 19 Years after Wildfire in the Republic of Korea. Forests. 2020; 11(5):515. https://doi.org/10.3390/f11050515
Chicago/Turabian StyleKim, Jeonghwan, Joo-Hoon Lim, Moonhyun Shin, Seung-Hyun Han, and Wonseok Kang. 2020. "Oak Resprouting Survival and Competition for 19 Years after Wildfire in the Republic of Korea" Forests 11, no. 5: 515. https://doi.org/10.3390/f11050515





