Tree Communities in Three-Year-Old Post-Mining Sites Under Different Forest Restoration Techniques in the Brazilian Amazon
Abstract
:1. Introduction
2. Material and Methods
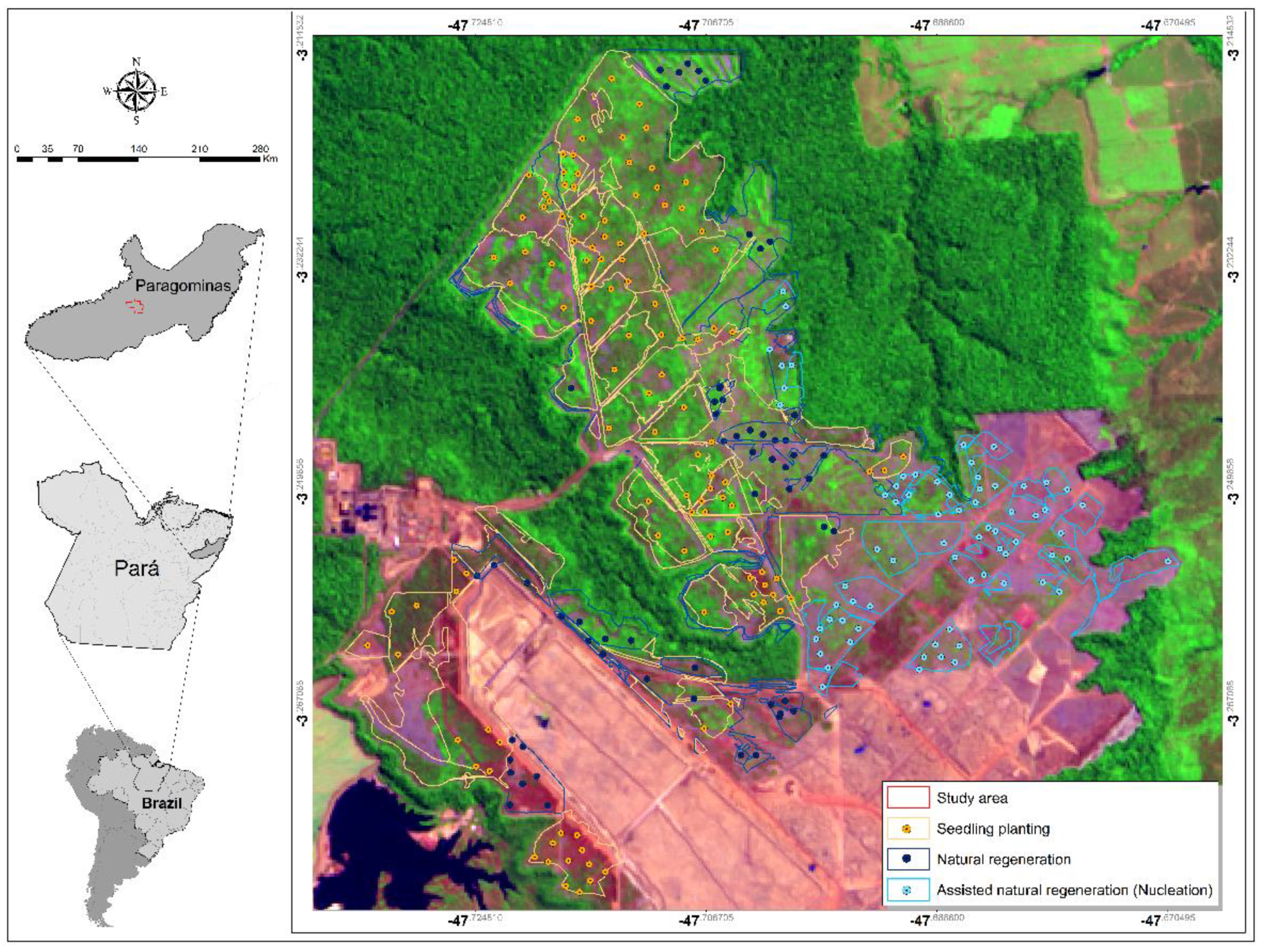
2.1. Characterization of the Study Area
2.2. Forest Restoration Techniques
2.3. Data Collection
2.4. Data Analysis
3. Results
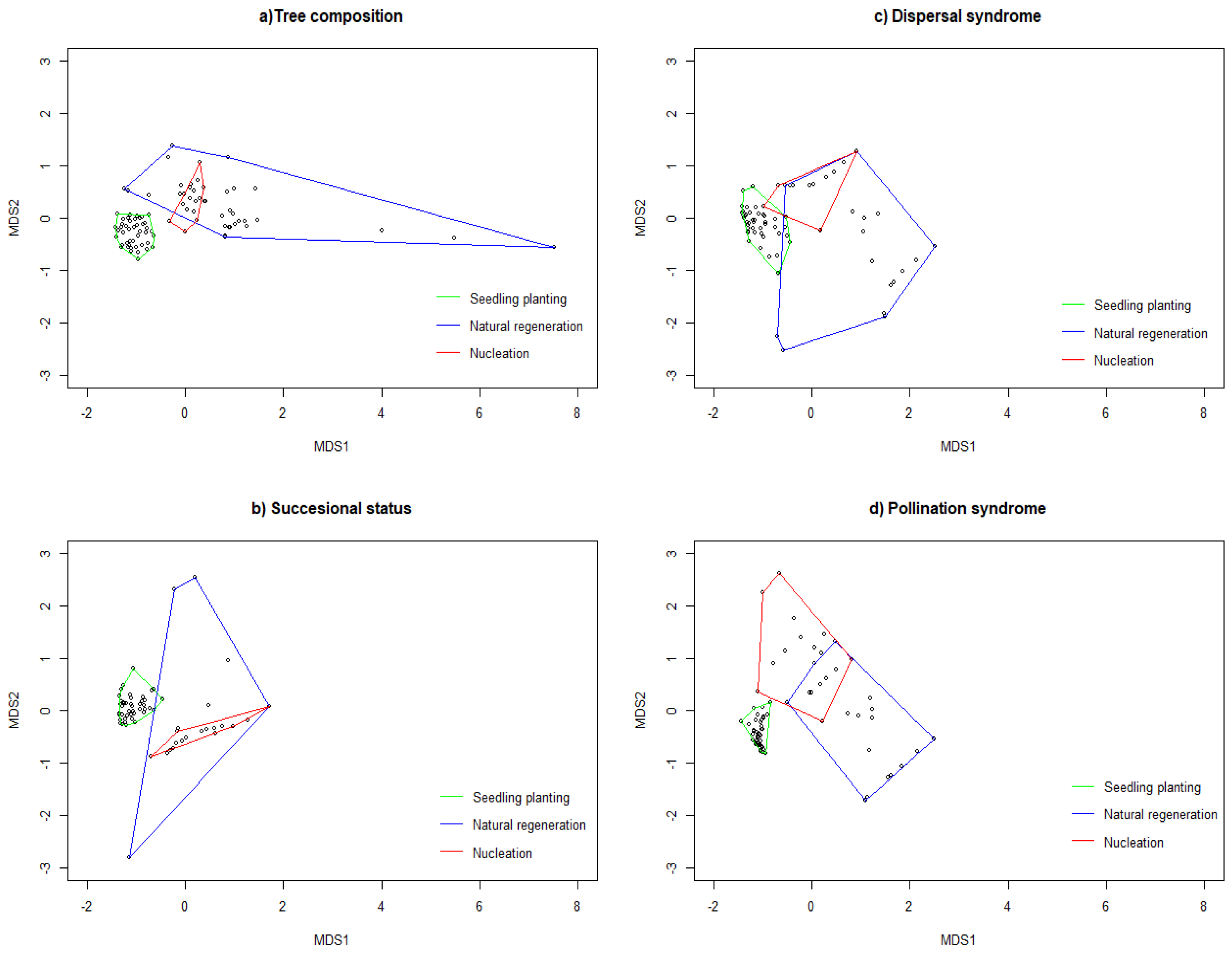
3.1. Tree Composition at the Restored Sites
3.2. Species Richness and Diversity
3.3. Functional Types
3.4. Effects of Distance to Seed Sources
3.5. Conservation Status
4. Discussion
4.1. Effects of Restoration Techniques on Tree Community
4.2. Proximity to Seed Sources Accelerates Vegetation Recovery
4.3. Relevance for Biodiversity Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.; et al. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feeley, K.J.; Silman, M.R. Extinction risks of Amazonian plant species. Proc. Natl. Acad. Sci. USA 2009, 106, 12382–12387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. B Boil. Sci. 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Hubbell, S.P. Species–area relationships always overestimate extinction rates from habitat loss. Nature 2011, 473, 368–371. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Hubbell, S. Estimating extinction from species–area relationships: Why the numbers do not add up. Ecology 2013, 94, 1905–1912. [Google Scholar] [CrossRef] [Green Version]
- Sonter, L.J.; Barrett, D.; Soares-Filho, B.S.; Moran, C. Global demand for steel drives extensive land-use change in Brazil’s Iron Quadrangle. Glob. Environ. Chang. 2014, 26, 63–72. [Google Scholar] [CrossRef]
- Sonter, L.J.; Moran, C.J.; Barrett, D.J.; Soares-Filho, B.S. Processes of land use change in mining regions. J. Clean. Prod. 2014, 84, 494–501. [Google Scholar] [CrossRef]
- Schueler, V.; Kuemmerle, T.; Schröder, H. Impacts of Surface Gold Mining on Land Use Systems in Western Ghana. Ambio 2011, 40, 528–539. [Google Scholar] [CrossRef] [Green Version]
- Mwitwa, J.; German, L.; Muimba-Kankolongo, A.; Puntodewo, A. Governance and sustainability challenges in landscapes shaped by mining: Mining-forestry linkages and impacts in the Copper Belt of Zambia and the DR Congo. For. Policy Econ. 2012, 25, 19–30. [Google Scholar] [CrossRef]
- Edwards, D.P.; Sloan, S.; Weng, L.; Dirks, P.; Sayer, J.; Laurance, S.G.W. Mining and the African Environment. Conserv. Lett. 2013, 7, 302–311. [Google Scholar] [CrossRef]
- Edwards, D.P.; Laurance, W.F. Preventing tropical mining disasters. Science 2015, 350, 1482. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, D.; Gollner, S.; Jones, D.O.B.; Kaiser, S.; Martinez-Arbizu, P.; Menzel, L.; Mestre, N.C.; Morato, T.; Pham, C.; Pradillon, F.; et al. Potential Mitigation and Restoration Actions in Ecosystems Impacted by Seabed Mining. Front. Mar. Sci. 2018, 5, 467. [Google Scholar] [CrossRef] [Green Version]
- Festin, E.S.; Tigabu, M.; Chileshe, M.N.; Syampungani, S.; Odén, P.C. Progresses in restoration of post-mining landscape in Africa. J. For. Res. 2018, 30, 381–396. [Google Scholar] [CrossRef] [Green Version]
- Ahirwal, J.; Kumar, A.; Pietrzykowski, M.; Maiti, S.K. Reclamation of coal mine spoil and its effect on Technosol quality and carbon sequestration: A case study from India. Environ. Sci. Pollut. Res. 2018, 25, 27992–28003. [Google Scholar] [CrossRef] [PubMed]
- Fiqa, A.P.; Fauziah, F.; Lestari, D.A.; Budiharta, S. The importance of in-situ conservation area in mining concession in preserving diversity, threatened and potential floras in East Kalimantan, Indonesia. Biodiversitas J. Boil. Divers. 2018, 20, 198–210. [Google Scholar] [CrossRef] [Green Version]
- INPE Instituto Nacional de Pesquisas Espaciais. Projeto Prodes Monitoramento da Floresta Amazônica Brasileira por Satélite. Available online: http://www.dpi.inpe.br/prodesdigital/prodesmunicipal.php (accessed on 21 October 2019).
- Celentano, D.; Rousseau, G.X.; Muniz, F.H.; van Deursen Varga, I.; Martinez, C.; Carneiro, M.S.; Miranda, M.V.C.; Barros, M.N.R.; Freitas, L.; Narvaes, I.d.S.; et al. Towards zero deforestation and forest restoration in the Amazon region of Maranhão state, Brazil. Land Use Policy 2017, 68, 692–698. [Google Scholar] [CrossRef]
- Moura, R. Ainda é Pouco: “Maior projeto de Reflorestamento da História” Recupera menos de 5% do Desmatamento anual na Amazônia. BBC Bras. Available online: http://www.bbc.com/portuguese/internacional-42485742 (accessed on 25 January 2019).
- Schmidt, I.B.; Urzedo, D.; Piña-Rodrigues, F.C.; Vieira, D.L.M.; Rezende, G.M.; Sampaio, A.B.; Junqueira, R.G.P.; De Urzedo, D.I.; De Rezende, G.M. Community-based native seed production for restoration in Brazil–the role of science and policy. Plant Boil. 2018, 21, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.G.; Rodrigues, S.B.; Campos-Filho, E.M.; Carmo, G.H.P.D.; Da Veiga, J.M.; Junqueira, R.G.; Vieira, D.L.M. Evaluating the success of direct seeding for tropical forest restoration over ten years. For. Ecol. Manag. 2019, 438, 224–232. [Google Scholar] [CrossRef]
- Cruz, D.C.; Benayas, J.M.R.; Ferreira, G.C.; Santos, S.R.; Schwartz, G. An overview of forest loss and restoration in the Brazilian Amazon. New For. 2020, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Brancalion, P.H.; Rodrigues, R.R.; Gandolfi, S.; Kageyama, P.Y.; Nave, A.G.; Gandara, F.B.; Barbosa, L.M.; Tabarelli, M. Instrumentos legais podem contribuir para a restauração de florestas tropicais biodiversas. Revista Árvore 2010, 34, 455–470. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Nicoleite, E.; Overbeck, G.E.; Müller, S.C. Degradation by coal mining should be priority in restoration planning. Perspect. Ecol. Conserv. 2017, 15, 202–205. [Google Scholar] [CrossRef]
- Scarano, F.; Bozelli, R.L.; Dias, A.T.C.; Assireu, A.; Capossoli, D.J.; Esteves, F.D.A.; Figueiredo-Barros, M.P.; Nunes, M.F.Q.S.; Roland, F.; Sansevero, J.B.B.; et al. Twenty-Five Years of Restoration of an Igapó Forest in Central Amazonia, Brazil. In Igapó (Black-Water Flooded Forests) of the Amazon Basin; Springer Science and Business Media LLC: Oklahoma, OK, USA, 2018; pp. 279–294. [Google Scholar]
- Knowles, O.H.; Parotta, J. Amazonian forest restoration: An innovative system for native species selection based on phenological data and field performance indices. Commonw. For. Rev. 1995, 74, 230–243. [Google Scholar]
- Freire, J.M.; Urzedo, D.I.; Piña-Rodrigues, F.C.M. A realidade das sementes nativas no Brasil: Desafios e oportunidades para a produção em larga escala. Seed News 2017, 21, 24–28. [Google Scholar]
- DeSandoli, L.; Turkington, R.; Fraser, L. Restoration of slash pile burn scars to prevent establishment and propagation of non-native plants. Can. J. For. Res. 2016, 46, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Polster, D.F. Natural Processes for the restoration of Drastically Disturbed Sites. J. Am. Soc. Min. Reclam. 2016, 2016, 77–90. [Google Scholar] [CrossRef]
- Meli, P.; Holl, K.D.; Benayas, J.M.R.; Jones, H.P.; Jones, P.C.; Montoya, D.; Mateos, D.M. A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLoS ONE 2017, 12, e0171368. [Google Scholar] [CrossRef]
- Blignaut, J.; Aronson, J.; De Groot, R. Restoration of natural capital: A key strategy on the path to sustainability. Ecol. Eng. 2014, 65, 54–61. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Oliet, J.A.; Aronson, J.; Bolte, A.; Bullock, J.M.; Donoso, P.J.; Landhäusser, S.M.; Madsen, P.; Peng, S.; Benayas, J.M.R.; et al. Restoring forests: What constitutes success in the twenty-first century? New For. 2015, 46, 601–614. [Google Scholar] [CrossRef]
- Chia, K.A.; Koch, J.M.; Sadler, R.; Turner, S.R. Re-establishing the mid-storey tree Persoonia longifolia (Proteaceae) in restored forest following bauxite mining in southern Western Australia. Ecol. Res. 2016, 31, 627–638. [Google Scholar] [CrossRef]
- Silva, K.D.A.; Martins, S.V.; Miranda Neto, A.; Demolinari, R.D.A.; Lopes, A.T. Forest restoration after bauxite mining: Assessment of planted tree species. Floresta Ambient. 2016, 23, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Barliza, J.C.; Rodríguez, O.B.; Peláez, J.D.L.; Chávez, L.F. Planted forests for open coal mine spoils rehabilitation in Colombian drylands: Contributions of fine litterfall through an age chronosequence. Ecol. Eng. 2019, 138, 180–187. [Google Scholar] [CrossRef]
- Norman, M.A.; Koch, J.M.; Grant, C.D.; Morald, T.K.; Ward, S.C. Vegetation Succession After Bauxite Mining in Western Australia. Restor. Ecol. 2006, 14, 278–288. [Google Scholar] [CrossRef]
- Chazdon, R.; Guariguata, M.R. Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Kleine, M.; Mansourian, S.; Parrotta, J.; Madsen, P.; Kant, P.; Burns, J.; Bolte, A. Implementing forest landscape restoration under the Bonn Challenge: A systematic approach. Ann. For. Sci. 2019, 76, 50. [Google Scholar] [CrossRef]
- Lembani, R.L.; Knight, J.; Adam, E. Use of Landsat multi-temporal imagery to assess secondary growth Miombo woodlands in Luanshya, Zambia. South. For. A J. For. Sci. 2018, 81, 129–140. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Shiferaw, W.; Demissew, S.; Bekele, T. Ecology of soil seed banks: Implications for conservation and restoration of natural vegetation: A review. Int. J. Biodivers. Conserv. 2018, 10, 380–393. [Google Scholar]
- Benayas, J.M.R.; Bullock, J.M.; Newton, A. Creating woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front. Ecol. Environ. 2008, 6, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Corbin, J.D.; Holl, K.D. Applied nucleation as a forest restoration strategy. For. Ecol. Manag. 2012, 265, 37–46. [Google Scholar] [CrossRef]
- Volis, S. How to conserve threatened Chinese plant species with extremely small populations? Plant Divers. 2016, 38, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, E.; Loyola, R.D.; Martinelli, G. Challenges and Perspectives for Achieving the Global Strategy for Plant Conservation Targets in Brazil. Ann. Mo. Bot. Gard. 2017, 102, 347–356. [Google Scholar] [CrossRef]
- Monks, L.; Barrett, S.; Beecham, B.; Byrne, M.; Chant, A.; Coates, D.; Cochrane, J.A.; Crawford, A.; Dillon, R.; Yates, C. Recovery of threatened plant species and their habitats in the biodiversity hotspot of the Southwest Australian Floristic Region. Plant Divers. 2018, 41, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; De Blois, S. Using functional traits to assess the role of hedgerow corridors as environmental filters for forest herbs. Boil. Conserv. 2006, 130, 592–603. [Google Scholar] [CrossRef]
- Keddy, P.A. Plant Ecology: Organs Processes, Consequences, 2nd ed.; Cambridge University Press: Cambridge UK, 2017; pp. 322–335. [Google Scholar]
- De Cáceres, M.; Franklin, S.; Hunter, J.T.; Landucci, F.; Dengler, J.; Roberts, D. Global overview of plot-based vegetation classification approaches. Phytocoenologia 2018, 48, 101–112. [Google Scholar] [CrossRef]
- MacKenzie, W.H.; Meidinger, D.V. The Biogeoclimatic Ecosystem Classification Approach: An ecological framework for vegetation classification. Phytocoenologia 2018, 48, 203–213. [Google Scholar] [CrossRef]
- Brancalion, P.H.; Holl, K. Functional composition trajectory: A resolution to the debate between Suganuma, Durigan, and Reid. Restor. Ecol. 2015, 24, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Giannini, T.C.; Harley, R.M.; Viana, P.L.; Alves, R.; Pinto, C.E.; Mota, N.F.D.O.; Caldeira, C.F.; Furtini, A.E.; Siqueira, J.O.; Giulietti, A.M.; et al. Selecting plant species for practical restoration of degraded lands using a multiple-trait approach. Austral Ecol. 2016, 42, 510–521. [Google Scholar] [CrossRef]
- Gatica-Saavedra, P.; Echeverría, C.; Nelson, C.R. Ecological indicators for assessing ecological success of forest restoration: A world review. Restor. Ecol. 2017, 25, 850–857. [Google Scholar] [CrossRef]
- Zucchi, M.I.; Sujii, P.S.; Mori, G.M.; Viana, J.P.G.; Grando, C.; Silvestre, E.D.A.; Schwarcz, K.D.; Macrini, C.M.; Bajay, M.M.; Araújo, F.L.; et al. Genetic diversity of reintroduced tree populations in restoration plantations of the Brazilian Atlantic Forest. Restor. Ecol. 2017, 26, 694–701. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.J.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Leitão, F.H.; Marques, M.C.M.; Ceccon, E. Young restored forests increase seedling recruitment in abandoned pastures in the Southern Atlantic rainforest. Rev. Biol. Trop. 2010, 58, 1271–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenmayer, D.; Thorn, S.; Banks, S. Please do not disturb ecosystems further. Nat. Ecol. Evol. 2017, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.W.R.; Carvalho, E.J.M.; Silva, L.G.T. Diagnóstico agrícola do município de Paragominas, PA. Embrapa Amaz. Orient.-Bol. Pesqui. Desenvolv. (INFOTECA-E) 2014, 91, 1–26. [Google Scholar]
- Swaine, M.D.; Whitmore, T.C. On the definition of ecological species groups in tropical rain forests. Vegetatio 1988, 75, 81–86. [Google Scholar] [CrossRef]
- Van Der Pijl, L. Principles of Dispersal in Higher Plants; Springer Science and Business Media LLC: Berlin, Germany, 1982; pp. 22–90. [Google Scholar]
- Real, L. Pollination Biology; Academic Press: Orlando, FL, USA, 1983; pp. 163–202. [Google Scholar]
- Esri, A. ArcGIS 10.1; Environmental Systems Research Institute: Redlands, CA, USA, 2012. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- R Core Team. The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org/ (accessed on 13 February 2019).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, H.M.; et al. Vegan: Community Ecology Package. R Package Version 2.5.3—2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 February 2019).
- Hill, M.O. Correspondence Analysis: A Neglected Multivariate Method. J. R. Stat. Soc. Ser. C (Appl. Stat.) 1974, 23, 340. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Package ‘Mass’. Cran R, 538. Available online: Cran.r-project.org/web/packages/MASS (accessed on 26 October 2019).
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.; Grelle, C.E.V.; Benayas, J.M.R. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 11666. [Google Scholar] [CrossRef]
- Suding, K. Toward an Era of Restoration in Ecology: Successes, Failures, and Opportunities Ahead. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 465–487. [Google Scholar] [CrossRef] [Green Version]
- Bullock, J.M.; Aronson, J.; Newton, A.; Pywell, R.; Benayas, J.M.R. Restoration of ecosystem services and biodiversity: Conflicts and opportunities. Trends Ecol. Evol. 2011, 26, 541–549. [Google Scholar] [CrossRef]
- Hutto, R.L.; Flesch, A.D.; Fylling, M.A. A bird’s-eye view of forest restoration: Do changes reflect success? For. Ecol. Manag. 2014, 327, 1–9. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Ferreira, M.S.; Chazdon, R.; Lindenmayer, D.; Sansevero, J.B.B.; Monteiro, L.; Iribarrem, A.; Latawiec, A.E.; Strassburg, B.B.N. Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci. Adv. 2017, 3, e1701345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.P.; Jones, P.C.; Barbier, E.B.; Blackburn, R.C.; Rey Benayas, J.M.; Holl, K.D.; Mateos, D.M. Restoration and repair of Earth’s damaged ecosystems. Proc. R. Soc. B Biol. Sci. 2018, 285, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.L.; Fagan, M.E.; Zahawi, R.A. Positive site selection bias in meta-analyses comparing natural regeneration to active forest restoration. Sci. Adv. 2018, 4, eaas9143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plue, J.; Cousins, S.A.O. Temporal dispersal in fragmented landscapes. Boil. Conserv. 2013, 160, 250–262. [Google Scholar] [CrossRef]
- Reid, J.L.; Holl, K.; Zahawi, R.A. Seed dispersal limitations shift over time in tropical forest restoration. Ecol. Appl. 2015, 25, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Chen, J.; Ai, X.; Li, R.; Ai, Y.; Li, W. The texture, structure and nutrient availability of artificial soil on cut slopes restored with OSSS–Influence of restoration time. J. Environ. Manag. 2017, 200, 502–510. [Google Scholar] [CrossRef]
- Carrasco-Carballido, V.; Martínez-Garza, C.; Jiménez-Hernández, H.; Márquez-Torres, F.; Campo-Alves, J. Effects of Initial Soil Properties on Three-Year Performance of Six Tree Species in Tropical Dry Forest Restoration Plantings. Forests 2019, 10, 428. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Hui, D.; Zhou, G.; Deng, Q.; Zhang, D.; et al. Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands. Forests 2019, 10, 1124. [Google Scholar] [CrossRef] [Green Version]
- Thyroff, E.; Burney, O.T.; Jacobs, D.F. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests 2019, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Schempf, W.M.; Jacobs, D.F. Hardwood Species Show Wide Variability in Response to Silviculture during Reclamation of Coal Mine Sites. Forests 2020, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.D.C.E.; Soares-Silva, L.H. Arboreal Flora of the Godoy Forest State Park, Londrina, pr. Brazil. Edinb. J. Bot. 2000, 57, 107–120. [Google Scholar] [CrossRef]
- Antiqueira, P.A.P.; Romero, G.Q. Floral asymmetry and predation risk modify pollinator behavior, but only predation risk decreases plant fitness. Oecologia 2016, 181, 475–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Flores, J.; Hernández-Esquivel, K.B.; González-Rodríguez, A.; Ibarra-Manríquez, G. Flowering phenology, growth forms, and pollination syndromes in tropical dry forest species: Influence of phylogeny and abiotic factors. Am. J. Bot. 2016, 104, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holl, K.; Reid, J.L.; Chaves-Fallas, J.M.; Oviedo-Brenes, F.; Zahawi, R.A. Local tropical forest restoration strategies affect tree recruitment more strongly than does landscape forest cover. J. Appl. Ecol. 2016, 54, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.C.; Sabogal, C.; Melendez, C.S. Restoring Degraded Forest Land with Native Tree Species: The Experience of “Bosques Amazónicos” in Ucayali, Peru. Forests 2019, 10, 851. [Google Scholar] [CrossRef] [Green Version]
- Chaves, R.B.; Durigan, G.; Brancalion, P.H.; Aronson, J. On the need of legal frameworks for assessing restoration projects success: New perspectives from São Paulo state (Brazil). Restor. Ecol. 2015, 23, 754–759. [Google Scholar] [CrossRef]
- Fiore, N.; Ferreira, C.C.; Dzedzej, M.; Massi, K.G. Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil. Forests 2019, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Dutra, V.F.; Garcia, F.C.P.; Lima, H.D.; Queiroz, L.D. Diversidade Floristica de Leguminosae Adans. Em áreas de campo rupestre. Megadiversidade 2008, 4, 117–125. [Google Scholar]
- Gama, J.R.V.; Botelho, S.A.; Bentes-Gama, M.D.M.; Scolforo, J.R.S. Estrutura e potencial futuro de utilização da regeneração natural de floresta de várzea alta no município de Afuá, estado do Pará. Ciência Florest. 2003, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Zahawi, R.A.; Holl, K.D.; Cole, R.J.; Reid, J.L. Faculty of 1000 evaluation for Testing applied nucleation as a strategy to facilitate tropical forest recovery. J. Appl. Ecol. 2013, 50, 88–96. [Google Scholar] [CrossRef]
- Massoca, P.E.S.; Jakovac, X.C.C.; Bentos, T.V.; Williamson, G.B.; Mesquita, R.C.G. Dinâmica e trajetórias da sucessão secundária na Amazônia central. Bol. Mus. Para. Emílio Goeldi Ciênc. Nat. 2012, 7, 235–250. [Google Scholar]
- Cubiña, A.; Aide, T.M. The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture 1. Biotropica 2001, 33, 260–267. [Google Scholar] [CrossRef]
- Moreno-Fernández, D.; Ledo, A.; Cañellas, I.; Montes, F. Strategies for Modeling Regeneration Density in Relation to Distance from Adult Trees. Forests 2020, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Wijedasa, L.S.; Vernimmen, R.; Page, S.E.; Mulyadi, D.; Bahri, S.; Randi, A.; Evans, T.A.; Lasmito; Priatna, D.; Jensen, R.M.; et al. Distance to forest, mammal and bird dispersal drive natural regeneration on degraded tropical peatland. For. Ecol. Manag. 2020, 461, 117868. [Google Scholar] [CrossRef]
- Durden, J.M.; Murphy, K.; Jaeckel, A.; Van Dover, C.L.; Christiansen, S.; Gjerde, K.; Ortega, A.; Jones, D.O.B. A procedural framework for robust environmental management of deep-sea mining projects using a conceptual model. Mar. Policy 2017, 84, 193–201. [Google Scholar] [CrossRef]
- Gastauer, M.; Silva, J.R.; Junior, C.F.C.; Ramos, S.J.; Filho, P.W.M.S.; Neto, A.E.F.; Siqueira, J.O. Mine land rehabilitation: Modern ecological approaches for more sustainable mining. J. Clean. Prod. 2018, 172, 1409–1422. [Google Scholar] [CrossRef]
- Bechara, F.C.; Dickens, S.J.; Farrer, E.C.; Larios, L.; Spotswood, E.N.; Mariotte, P.; Suding, K. Neotropical rainforest restoration: Comparing passive, plantation and nucleation approaches. Biodivers. Conserv. 2016, 25, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.W.; Andrade, A.; Ribeiro, J.E.L.D.S.; Giraldo, J.P.; Lovejoy, T.E.; Condit, R.; Chave, J.; Harms, K.E.; et al. Rapid decay of tree-community composition in Amazonian forest fragments. Proc. Natl. Acad. Sci. USA 2006, 103, 19010–19014. [Google Scholar] [CrossRef] [Green Version]
- Slocum, M.G.; Horvitz, C.C. Seed arrival under different genera of trees in a neotropical pasture. Plant Ecol. 2000, 149, 51–62. [Google Scholar] [CrossRef]
- Holl, K.D. Do bird perching structures elevate seed rain and seedling establishment in abandoned tropical pasture? Restor. Ecol. 1998, 6, 253–261. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leight, J.E.G.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, P.; Aguilar, S.; Valencia, P.; Villa, G.; et al. Beta diversity in tropical trees. Science 2002, 295, 666–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbell, S.P. Neutral theory and the evolution of ecological equivalence. Ecology 2006, 87, 1397–1398. [Google Scholar] [CrossRef] [Green Version]
- Santo-Silva, E.E.; Almeida, W.R.; Melo, F.P.L.; Zickel, C.S.; Tabarelli, M. The Nature of Seedling Assemblages in a Fragmented Tropical Landscape: Implications for Forest Regeneration. Biotropica 2012, 45, 386–394. [Google Scholar] [CrossRef]
- Aronson, J.; Alexander, S. Ecosystem Restoration is Now a Global Priority: Time to Roll up our Sleeves. Restor. Ecol. 2013, 21, 293–296. [Google Scholar] [CrossRef]
- Wang, W.; Fu, H.; Lee, S.Y.; Fan, H.; Wang, M. Can Strict Protection Stop the Decline of Mangrove Ecosystems in China? From Rapid Destruction to Rampant Degradation. Forests 2020, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Rankin-De-Mérona, J.M.; Prance, G.T.; Hutchings, R.W.; Da Silva, M.F.; Rodrigues, W.A.; Uehling, M.E. Preliminary Results of a Large-Scale Tree Inventory of Upland Rain Forest in the Central Amazon. Acta Amaz. 1992, 22, 493–534. [Google Scholar] [CrossRef]
- Lenka, Š.; Řehounková, K.; Prach, K. Spontaneous revegetation vs. forestry reclamation in post-mining sand pits. Environ. Sci. Pollut. Res. 2015, 23, 13598–13605. [Google Scholar] [CrossRef]
- Prach, K.; Tolvanen, A. How can we restore biodiversity and ecosystem services in mining and industrial sites? Environ. Sci. Pollut. Res. 2016, 23, 13587–13590. [Google Scholar] [CrossRef] [Green Version]
- Cross, A.T.; Young, R.; Nevill, P.; McDonald, T.; Prach, K.; Aronson, J.; Wardell-Johnson, G.W.; Dixon, K.W. Appropriate aspirations for effective post-mining restoration and rehabilitation: A response to Kaźmierczak et al. Environ. Earth Sci. 2018, 77, 256. [Google Scholar] [CrossRef]
- Sena, K.; Agouridis, C.; Miller, J.; Barton, C. Spoil Type Influences Soil Genesis and Forest Development on an Appalachian Surface Coal Mine Ten Years after Placement. Forests 2018, 9, 780. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.L.; Barton, C.; Sena, K.; Angel, P. Reforesting Appalachian Surface Mines from Seed: A Five-Year Black Walnut Pilot Study. Forests 2019, 10, 573. [Google Scholar] [CrossRef] [Green Version]
- Kaneda, S.; Angst, Š.; Frouz, J. Development of Nutrient Uptake by Understory Plant Arrhenatherum elatius and Microbial Biomass during Primary Succession of Forest Soils in Post-Mining Land. Forests 2020, 11, 247. [Google Scholar] [CrossRef] [Green Version]




| Restoration Technique | Abundance | Richness | Diversity Indices | ||
|---|---|---|---|---|---|
| Shannon | Simpson | Pielou | |||
| Seedling planting | 525 ± 2.71 a | 59.5 ± 0.36 a | 3.5 ± 0.01 a | 0.96 ± 0 a | 0.87 ± 0 a |
| Natural regeneration | 154 ± 0.6 b | 13.5 ± 0.04 b | 1.5 ± 0.01 b | 0.64 ± 0 b | 0.59 ± 0 b |
| Nucleation | 85.7 ± 0.81 b | 11.3 ± 0.15 b | 1.9 ± 0.01 c | 0.81 ± 0 b | 0.8 ± 0 b |
| (a) Tree Species Composition | ||||||
| df | SS | MS | F.Model | R2 | Pr(>F) | |
| Techniques | 2 | 8.582 | 4.2909 | 11.808 | 0.1926 | 0.001 *** |
| Residuals | 99 | 35.976 | 0.3634 | 0.8074 | ||
| Total | 101 | 44.558 | 1.0000 | |||
| (b) Successional Status | ||||||
| Df | SS | MS | F.Model | R2 | Pr(>F) | |
| Techniques | 2 | 12.851 | 6.4254 | 47.329 | 0.48879 | 0.001*** |
| Residuals | 99 | 13.440 | 0.1358 | 0.51121 | ||
| Total | 101 | 26.291 | 1.00000 | |||
| (c) Dispersal Syndrome | ||||||
| df | SS | MS | F.Model | R2 | Pr(>F) | |
| Techniques | 2 | 12.732 | 6.3658 | 32.195 | 0.39409 | 0.001 *** |
| Residuals | 99 | 19.575 | 0.1977 | 0.60591 | ||
| Total | 101 | 32.306 | 1.00000 | |||
| (d) Pollination Syndrome | ||||||
| df | SS | MS | F.Model | R2 | Pr(>F) | |
| Techniques | 2 | 16.085 | 8.0423 | 48.965 | 0.49728 | 0.001*** |
| Residuals | 99 | 16.260 | 0.1642 | 0.50272 | ||
| Total | 101 | 32.345 | 1.00000 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conrado da Cruz, D.; Rey Benayas, J.M.; Costa Ferreira, G.; Santos Ribeiro, S. Tree Communities in Three-Year-Old Post-Mining Sites Under Different Forest Restoration Techniques in the Brazilian Amazon. Forests 2020, 11, 527. https://doi.org/10.3390/f11050527
Conrado da Cruz D, Rey Benayas JM, Costa Ferreira G, Santos Ribeiro S. Tree Communities in Three-Year-Old Post-Mining Sites Under Different Forest Restoration Techniques in the Brazilian Amazon. Forests. 2020; 11(5):527. https://doi.org/10.3390/f11050527
Chicago/Turabian StyleConrado da Cruz, Denis, José María Rey Benayas, Gracialda Costa Ferreira, and Sabrina Santos Ribeiro. 2020. "Tree Communities in Three-Year-Old Post-Mining Sites Under Different Forest Restoration Techniques in the Brazilian Amazon" Forests 11, no. 5: 527. https://doi.org/10.3390/f11050527
APA StyleConrado da Cruz, D., Rey Benayas, J. M., Costa Ferreira, G., & Santos Ribeiro, S. (2020). Tree Communities in Three-Year-Old Post-Mining Sites Under Different Forest Restoration Techniques in the Brazilian Amazon. Forests, 11(5), 527. https://doi.org/10.3390/f11050527






