Homogenization of Temperate Mixed Deciduous Forests in Białowieża Forest: Similar Communities Are Becoming More Similar
Abstract
:1. Introduction
2. Materials and Methods
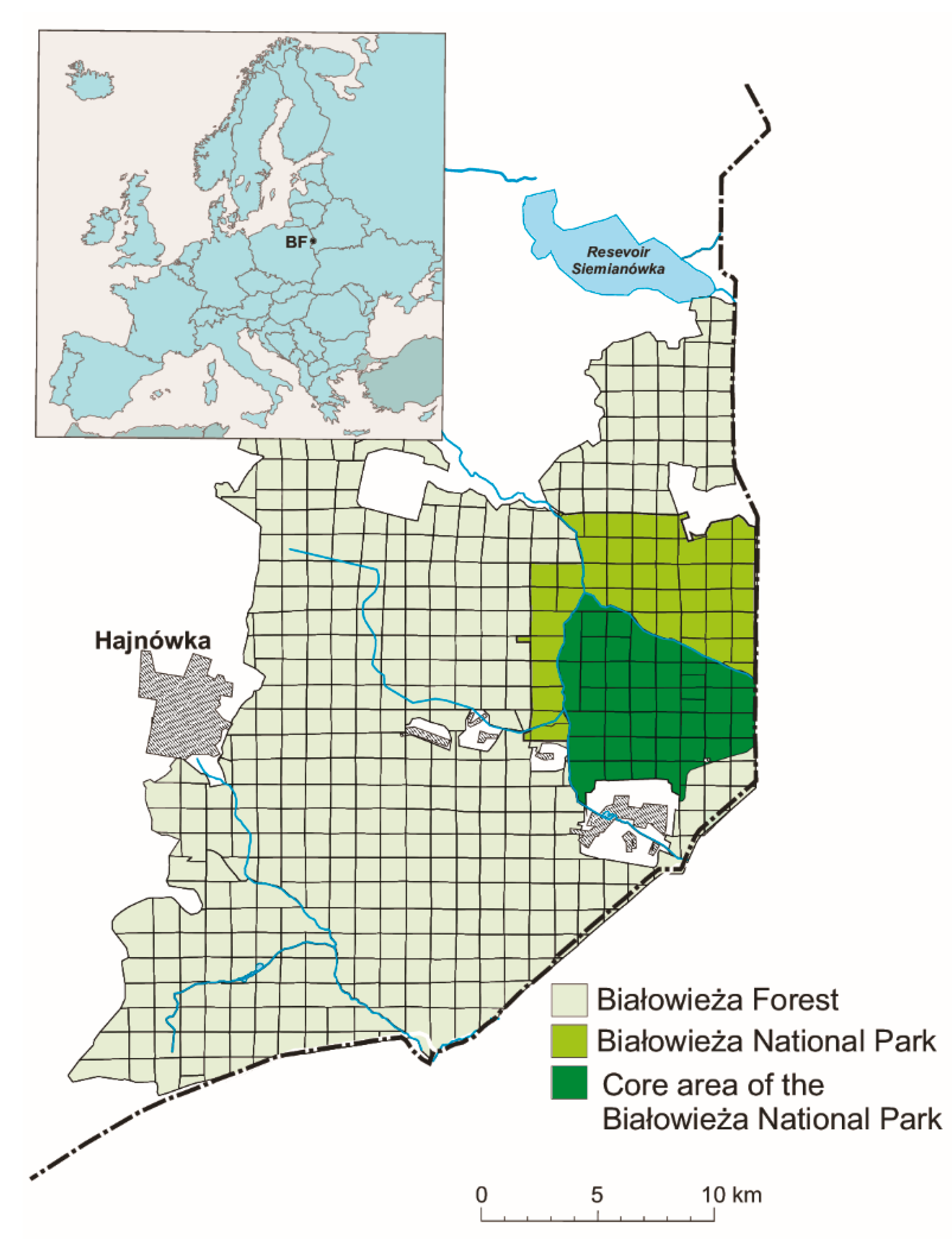
2.1. Study Area
2.2. Data Sampling
2.3. Data Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nadeau, C.P.; Urban, M.C.; Bridle, J.R. Climates Past, Present, and Yet-to-Come Shape Climate Change Vulnerabilities. TREE 2017, 32, 786–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hisano, M.; Searle, E.B.; Chen, H.Y.H. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. 2018, 93, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe Drought Effects on Mediterranean Woody Flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar]
- Vittoz, P.; Randin, C.; Dutoit, A.; Bonnet, F.; Hegg, O. Low impact of climate change on subalpine grasslands in the Swiss Northern Alps. Glob. Chang. Biol. 2009, 15, 209–220. [Google Scholar] [CrossRef]
- Becker-Scarpitta, A.; Vissault, S.; Vellend, M. Four decades of plant community change along a continental gradient of warming. Glob. Chang. Biol. 2018, 25, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, M.; Dupré, C. Acidification and eutrophication of deciduous forests in northwestern Germany demonstrated by indicator species analysis. J. Veg. Sci. 1997, 8, 855–864. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Baeten, L.; Craven, D.; De Frenne, P.; Hédl, R.; Lenoir, J.; Bert, D.; Brunet, J.; Chudomelová, M.; Decocq, G.; et al. Drivers of temporal changes in temperate forest plant diversity vary across spatial scales. Glob. Chang. Biol. 2015, 21, 3726–3737. [Google Scholar] [CrossRef]
- Becker, T.; Spanka, J.; Schröder, L.; Leuschner, C. Forty years of vegetation change in former coppice-with-standards woodlands as a result of management change and N deposition. Appl. Veg. Sci. 2017, 20, 304–313. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Slabejová, D.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecká, J.; Mikulová, K.; Šibíková, M.; Škodová, I.; Zaliberová, M.; Jarolímek, I. Comparison of the understory vegetation of native forests and adjacent Robinia pseudoacacia plantations in the Carpathian-Pannonian region. For. Ecol. Manag. 2019, 439, 28–40. [Google Scholar] [CrossRef]
- Mikulová, K.; Jarolímek, I.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecká, J.; Slabejová, D.; Šibík, J.; Škodová, I.; Zaliberová, M.; et al. The Effect of Non-Native Black Pine (Pinus nigra J. F. Arnold) Plantations on Environmental Conditions and Undergrowth Diversity. Forests 2019, 10, 548. [Google Scholar] [CrossRef] [Green Version]
- McGill, B.J.; Dornelas, M.; Gotelli, N.J.; Magurran, A.E. Fifteen forms of biodiversity trend in the Anthropocene. TREE 2015, 30, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, M.M.P.D.; Mauquoy, D.; van Geel, B.; Berendse, F. Long-term effects of climate change on vegetation and carbon dynamics in peat bogs. J. Veg. Sci. 2008, 19, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? TREE 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Ross, L.C.; Woodin, S.J.; Hester, A.J.; Thompson, D.B.A.; Birks, H.J.B. Biotic homogenization of upland vegetation: Patterns and drivers at multiple spatial scales over five decades. J. Veg. Sci. 2012, 23, 755–770. [Google Scholar] [CrossRef]
- Olden, J.D.; Comte, L.; Giam, X. The Homogocene: A research prospectus for the study of biotic homogenisation. NeoBiota 2018, 37, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeog. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- van der Plas, F.; Manning, P.; Soliveres, S.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Ampoorter, E.; Baeten, L.; et al. Biotic homogenization can decrease landscape-scale forest multifunctionality. Proc. Natl. Acad. Sci. USA 2016, 113, 3557–3562. [Google Scholar] [CrossRef] [Green Version]
- Šibíková, M.; Jarolímek, I.; Hegedüšová, K.; Májeková, J.; Mikulová, K.; Slabejová, D.; Škodová, I.; Zaliberová, M.; Medvecká, J. Effect of planting alien Robinia pseudoacacia trees on homogenization of Central European forest vegetation. Sci. Total Environ. 2019, 687, 1164–1175. [Google Scholar] [CrossRef]
- Bühler, C.; Roth, T. Spread of common species results in local-scale floristic homogenization in grassland of Switzerland. Div. Distr. 2011, 17, 1089–1098. [Google Scholar] [CrossRef]
- Qian, H.; Ricklefs, R.E. The role of exotic species in homogenizing the North American flora. Ecol. Lett. 2006, 9, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Thorne, J.H.; Viers, J.H. Biotic homogenization of the California flora in urban and urbanizing regions. Biol. Cons. 2006, 127, 282–291. [Google Scholar] [CrossRef]
- Gong, C.F.; Chen, J.; Yu, S. Biotic homogenization and differentiation of the flora in artificial and near-natural habitats across urban green spaces. Land. Urb. Plan. 2013, 120, 158–169. [Google Scholar] [CrossRef]
- Stotz, G.C.; Gianoli, E.; Cahill, J.F., Jr. Biotic homogenization within and across eight widely distributed grasslands following invasion by Bromus inermis. Ecology 2019, 100, e02717. [Google Scholar] [CrossRef] [PubMed]
- Lôbo, D.; Leão, T.; Melo, F.P.L.; Santos, A.M.M.; Tabarelli, M. Forest fragmentation drives Atlantic forest of northeastern Brazil to biotic homogenization. Div. Distr. 2011, 17, 287–296. [Google Scholar] [CrossRef]
- McCune, J.L.; Vellend, M.; Fridley, J. Gains in native species promote biotic homogenization over four decades in a human-dominated landscape. J. Ecol. 2013, 101, 1542–1551. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. TREE 2004, 19, 18–24. [Google Scholar] [CrossRef]
- Czerepko, J. Długookresowe zmiany roślinności w zespole sosnowego boru bagiennego Vaccinio uliginosi-Pinetum Kleist 1929. Leśn. Pr. Bad. 2011, 72, 21–29. [Google Scholar]
- Malzahn, E. Monitoring zagrożeń i zanieczyszczenia środowiska leśnego Puszczy Białowieskiej. Kosmos 2002, 51, 435–441. [Google Scholar]
- Boczoń, A.; Kowalska, A.; Ksepko, M.; Sokołowski, K. Climate Warming and Drought in the Bialowieza Forest from 1950–2015 and Their Impact on the Dieback of Norway Spruce Stands. Water 2018, 10, 1502. [Google Scholar] [CrossRef] [Green Version]
- Pierzgalski, E.; Boczoń, A.; Tyszka, J. Zmienność opadów i położenia wód gruntowych w Białowieskim Parku Narodowym. Kosmos 2002, 51, 415–425. [Google Scholar]
- Adamowski, W. The flora of vascular plants. In Białowieża National Park. Know It—Understand It—Protect It; Okołów, C., Karaś, M., Bołbot, A., Eds.; Białowieski Park Narodowy: Białowieża, Poland, 2009; pp. 59–72. [Google Scholar]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.M.; Zimny, M.; Samojlik, T.; Latałowa, M. Białowieża Forest—A Relic of the High Naturalness of European Forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]
- Latałowa, M.; Zimny, M.; Pędziszewska, A.; Kupryjanowicz, M. Postglacjalna historia puszczy Białowieskiej—Roślinność, klimat i działalność człowieka. Parki Nar. Rez. Przyr. 2016, 35, 3–49. [Google Scholar]
- Sabatini, F.; Sabatini, M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; et al. Where are Europe’s last primary forests? Div. Distr. 2018, 24, 1426–1439. [Google Scholar] [CrossRef] [Green Version]
- Sokołowski, A.W. Lasy Puszczy Białowieskiej; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2004. [Google Scholar]
- Wesołowski, T.; Gutowski, J.M.; Jaroszewicz, B.; Kowalczyk, R.; Niedziałkowski, K.; Rok, J.; Wójcik, J.M. Park Narodowy Puszczy Białowieskiej—Ochrona Przyrody i Rozwój Lokalnych Społeczności. Article 2. 2018, pp. 1–28. Available online: www.forestbiology.org (accessed on 7 April 2020).
- Więcko, E. Puszcza Białowieska [The Białowieża Forest]; PWN: Warszawa, Poland, 1984. [Google Scholar]
- Faliński, J.B. Vegetation Dynamics in Temperate Lowland Primeval Forests: Ecological Studies in Białowieża Forest; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Prusinkiewicz, Z.; Michalczuk, C. Gleby Białowieskiego Parku Narodowego (z mapą 1:20 000). Phytocoenosis 1998, 10 (Suppl. 10), 1–40. [Google Scholar]
- Świtoniak, M.; Kabała, C.; Charzyński, P. Proposal of English equivalents for the soil taxa names in the Polish Soils Classification. Soil Sci. Ann. 2016, 67, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Matuszkiewicz, W. Zespoły leśne Białowieskiego Parku Narodowego. Die Waldassoziationen von Białowieża-Nationalpark. Ann. UMCS 1952, (Suppl. 6), 1–218. [Google Scholar]
- Kapfer, J.; Hédl, R.; Jurasinski, G.; Kopecký, M.; Schei, F.H.; Grytnes, J.-A. Resurveying historical vegetation data—Opportunities and challenges. Appl. Veg. Sci. 2017, 20, 164–171. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie; Biologische Studienbücher: Berlin, Germany, 1928. [Google Scholar]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, F.J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package, R Package Version 2.5-1; Available online: https://www.researchgate.net/publication/324693493_vegan_Community_Ecology_Package_Ordination_methods_diversity_analysis_and_other_functions_for_community_and_vegetation_ecologists_Version_25-1_URL_httpsCRANR-projectorgpackagevegan (accessed on 12 May 2020).
- De Caceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wal, R.; Pearce, I.; Brooker, R.; Scott, D.; Welch, D.; Woodin, S. Interplay between nitrogen deposition and grazing causes habitat degradation. Ecol. Lett. 2003, 6, 141–146. [Google Scholar] [CrossRef]
- Jacobs, B.F.; Kingston, J.D.; Jacobs, L.L. The Origin of Grass-Dominated Ecosystems. Ann. Miss. Bot. Gard. 1999, 86, 590–643. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S. Leaf dry matter content and lateral spread predict response to land use change for six subalpine grassland species. J. Veg. Sci. 2007, 18, 289–300. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Pirożnikow, E.; Sondej, I. Endozoochory by the guild of ungulates in Europe’s primeval forest. Forest Eco. Manag. 2013, 305, 21–28. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Pirożnikow, E. Dung longevity influences the fate of endozoochorically dispersed seeds in forest ecosystems. Botany 2011, 89, 779–785. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Fan, Z.; Zhao, Q.; Wang, M.; Ran, J.; Huang, H.; Niklas, K.J. Global Data Analysis Shows That Soil Nutrient Levels Dominate Foliar Nutrient Resorption Efficiency in Herbaceous Species. Front. Plant. Sci. 2018, 9, 1431. [Google Scholar] [CrossRef]
- Xing, A.; Xu, L.; Shen, H.; Du, E.; Liu, X.; Fang, Y. Long term effect of nitrogen addition on understory community in a Chinese boreal forest. Sci. Tot. Env. 2019, 646, 989–995. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Jędrzejewski, W.; Bunevich, A.N.; Miłkowski, L.; Krasiński, Z.A. Factors shaping population densities and increase rates of ungulates in Białowieża Primeval Forest (Poland and Belarus) in the 19th and 20th centuries. Acta Theriol. 1997, 42, 399–451. [Google Scholar] [CrossRef] [Green Version]
- Holland, E.A.; Braswell, B.H.; Sulzman, J.; Lamarque, J.-F. Nitrogen deposition onto the United States and Western Europe: Synthesis of observations and models. Ecol. Appl. 2005, 15, 38–57. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef] [Green Version]
- Keith, S.A.; Newton, A.C.; Morecroft, M.D.; Bealey, C.E.; Bullock, J.M. Taxonomic homogenization of woodland plant communities over 70 years. Proc. R. Soc. B 2009, 276, 3539–3544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łubek, A.; Kukwa, M.; Jaroszewicz, B.; Czortek, P. Changes in the epiphytic lichen biota of Białowieża Primeval Forest are not explained by climate warming. Sci. Tot. Env. 2018, 643, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Landuyt, D.; Maes, S.; Depauw, L.; Ampoorter, E.; Blondeel, H.; Perring, M.; Brūmelis, G.; Brunet, J.; Decocq, G.; van Ouden, J.; et al. Drivers of aboveground understorey biomass and nutrient stocks in temperate deciduous forests. J. Ecol. 2019. [Google Scholar] [CrossRef]
- Maes, S.L.; Blondeel, H.; Perring, M.P.; Depauw, L.; Brümelis, G.; Brunet, J.; Decocq, G.; den Ouden, J.; Härdtle, W.; Hédl, R.; et al. Litter quality, land-use history, and nitrogen deposition effects on topsoil conditions across European temperate forests. Forest Ecol. Manag. 2019, 433, 405–418. [Google Scholar] [CrossRef] [Green Version]
- Toreti, A.; Belward, A.; Perez-Dominguez, I.; Naumann, G.; Luterbacher, J.; Cronie, O.; Seguini, L.; Manfron, G.; Lopez-Lozano, R.; Baruth, B.; et al. The exceptional 2018 European water seesaw calls for action on adaptation. Earth’s Future 2019, 7, 652–663. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Morrison, L.W. Observer error in vegetation surveys: A review. J. Plant. Ecol. 2016, 9, 367–379. [Google Scholar] [CrossRef]
- Sparks, T.H.; Jaroszewicz, B.; Krawczyk, M.; Tryjanowski, P. Advancing phenology in Europe’s last lowland primeval forest: Non-linear temperature response. Clim. Res. 2009, 39, 221–226. [Google Scholar] [CrossRef] [Green Version]
- 69. Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. (Eds.) Climate Change and Land: An IPCC special report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. IPCC. 2019. Available online: https://www.ipcc.ch/report/SRCCL/ (accessed on 7 April 2020).
- Zellweger, F.; Coomes, D.; Lenoir, J.; Depauw, L.; Maes, S.L.; Wulf, M.; Kirby, K.J.; Brunet, J.; Kopecký, M.; Máliš, F.; et al. Seasonal drivers of understorey temperature buffering in temperate deciduous forests across Europe. Glob. Ecol. Biogeog. 2019, 28, 1774–1786. [Google Scholar] [CrossRef] [Green Version]
- Matuszkiewicz, J.M.; Kozłowska, A.B. Przegląd fitosocjologiczny zbiorowisk leśnych Polski—Ciepłolubne dąbrowy. Fragm. Flor. Geobot. 1991, 36, 203–256. [Google Scholar]
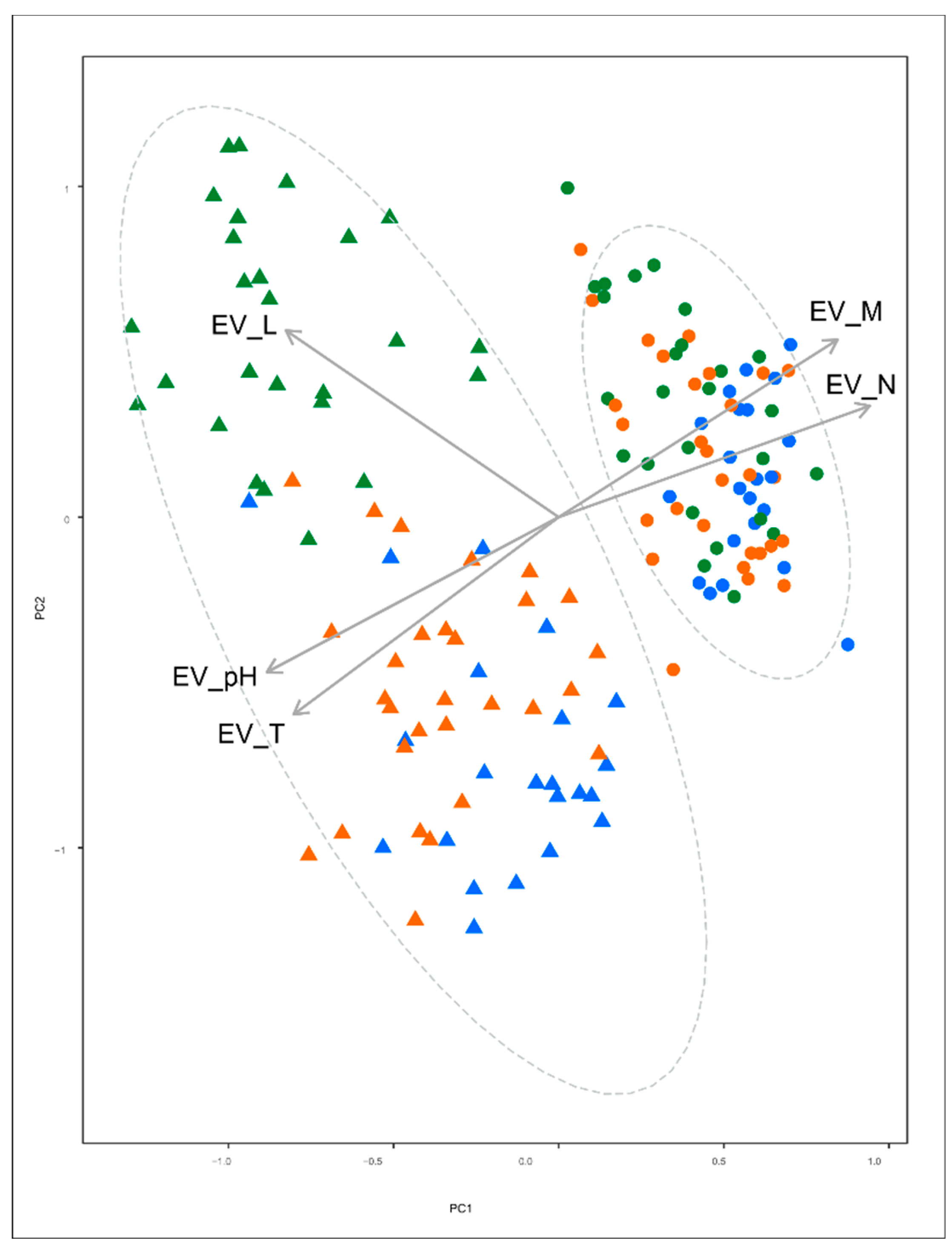
| Factor | Querceto-Carpinetum CaricetosumPilosae | Querceto-Carpinetum Typicum | Querceto-Carpinetum Stachyetosum Silvaticae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Diff | SD | t | p-Value | Mean Diff | SD | t | p-Value | Mean Diff | SD | t | p-Value | |
| Tree_cov (%) | 11.6 | 13.44 | 4.32 | 0.0002 | 13.896 | 19.19 | 4.8455 | <0.0001 | 11.13 | 13.84 | 2.728 | 0.013 |
| Shrub_cov (%) | 19.52 | 31.39 | 3.11 | 0.005 | 26.6 | 24.71 | 5.7981 | <0.0001 | 22.09 | 31.05 | 3.3369 | 0.0031 |
| Understorey_cov (%) | 11.8 | 24.32 | 2.43 | 0.02 | 11.37 | 22.32 | 2.7459 | 0.01042 | 19.95 | 22.66 | 4.13 | 0.0004 |
| EV_L | 0.26 | 0.43 | 3.05 | 0.0055 | −0.0760 | 0.45 | −0.9033 | 0.374 | −0.03 | 0.42 | −0.33 | 0.743 |
| EV_T | 0.184 | 0.23 | 3.93 | 0.0006 | 0.0837 | 0.23 | 1.9360 | 0.063 | 0.081 | 0.18 | 2.0855 | 0.049 |
| EV_M | −0.3147 | 0.41 | −3.817 | 0.00083 | −0.1787 | 0.33 | −2.9322 | 0.006638 | −0.1521 | 0.33 | 2.1392 | 0.0443 |
| EV_N | −0.46 | 0.49 | −4.75 | <0.0001 | −0.3551 | 0.66 | −2.8777 | 0.00758 | −0.2236 | 0.44 | −2.3918 | 0.0262 |
| EV_pH | 0.16 | 0.50 | 1.59 | 0.1243 | 0.2413 | 0.55 | 2.3677 | 0.02504 | 0.2975 | 0.44 | 3.1779 | 0.0045 |
| SCAI | −0.301 | 0.42 | −3.59 | 0.00146 | −0.4829 | 0.403 | −6.45 | <0.0001 | −0.5139 | 0.76 | −3.1849 | 0.0044 |
| Species richness | 0.96 | 10.26 | 0.47 | 0.644 | 1.5517 | 8.65 | 0.96601 | 0.3423 | 5.364 | 10.93 | 2.3025 | 0.035 |
| Shannon–Wiener Index | 0.49 | 0.30 | 8.22 | <0.0001 | 0.656 | 0.29 | 12.204 | <0.0001 | 0.6685 | 0.32 | 9.83 | <0.0001 |
| 1949 | 2018 | ||||
|---|---|---|---|---|---|
| Querceto-Carpinetum Stachyetosum Silvaticae | |||||
| Indicator Species | Stat-Value | p-Value | Indicator Species | Stat-Value | p-Value |
| Stellaria nemorum | 0.728 | 0.001 | Geranium robertianum | 0.608 | 0.035 |
| Impatiens noli tangere | 0.701 | 0.001 | Fraxinus excelsior | 0.566 | 0.039 |
| Urtica dioica | 0.689 | 0.001 | Equisetum sylvaticum | 0.520 | 0.004 |
| Stachys sylvatica | 0.685 | 0.001 | Lathyrus vernus | 0.489 | 0.029 |
| Circea lutetiana | 0.658 | 0.001 | Rumex obtusifolius | 0.428 | 0.021 |
| Fraxinus excelsior | 0.641 | 0.001 | Euonymus europaea | 0.386 | 0.035 |
| Dryopteris carthusiana | 0.636 | 0.006 | Crepis paludosa | 0.369 | 0.023 |
| Geranium robertianum | 0.625 | 0.007 | - | - | - |
| Chrysosplenium alternifolium | 0.622 | 0.001 | - | - | - |
| Asarum europaeum | 0.616 | 0.003 | - | - | - |
| Geum urbanum | 0.615 | 0.003 | - | - | - |
| Festuca gigantea | 0.599 | 0.006 | - | - | - |
| Glechoma hederacea | 0.564 | 0.002 | - | - | - |
| Carex remota | 0.542 | 0.001 | - | - | - |
| Brachypodium sylvaticum | 0.527 | 0.002 | - | - | - |
| Circea alpina | 0.502 | 0.002 | - | - | - |
| Lapsana communis | 0.500 | 0.003 | - | - | - |
| Deschampsia caespitosa | 0.495 | 0.014 | - | - | - |
| Ranunculus repens | 0.477 | 0.004 | - | - | - |
| Equisetum sylvaticum | 0.433 | 0.024 | - | - | - |
| Mercurialis perennis | 0.426 | 0.010 | - | - | - |
| Ranunculus cassubicus | 0.389 | 0.043 | - | - | - |
| Crepis paludosa | 0.369 | 0.019 | - | - | - |
| Elymus europaeus | 0.369 | 0.029 | - | - | - |
| Padus avium | 0.369 | 0.015 | - | - | - |
| Querceto-Carpinetum caricetosum pilosae | |||||
| Species | Stat-Value | p-Value | Species | Stat-Value | p-Value |
| Carex pilosa | 0.948 | 0.001 | Carex pilosa | 0.715 | 0.002 |
| Anemone nemorosa | 0.654 | 0.019 | Polygonatum multiflorum | 0.563 | 0.032 |
| Maiantheum bifolium | 0.618 | 0.004 | - | - | - |
| Sorbus aucuparia | 0.545 | 0.014 | - | - | - |
| Carex digitata | 0.518 | 0.048 | - | - | - |
| Calamagrostis arundinacea | 0.506 | 0.003 | - | - | - |
| Phegopteris connectilis | 0.489 | 0.006 | - | - | - |
| Luzula pilosa | 0.435 | 0.010 | - | - | - |
| Plathantera bifolia | 0.346 | 0.038 | - | - | - |
| Querceto-Carpinetum typicum | |||||
| Species | Stat-Value | p-Value | Species | Stat-Value | p-Value |
| Galium odoratum | 0.738 | 0.019 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewińska, O.; Adamowski, W.; Jaroszewicz, B. Homogenization of Temperate Mixed Deciduous Forests in Białowieża Forest: Similar Communities Are Becoming More Similar. Forests 2020, 11, 545. https://doi.org/10.3390/f11050545
Cholewińska O, Adamowski W, Jaroszewicz B. Homogenization of Temperate Mixed Deciduous Forests in Białowieża Forest: Similar Communities Are Becoming More Similar. Forests. 2020; 11(5):545. https://doi.org/10.3390/f11050545
Chicago/Turabian StyleCholewińska, Olga, Wojciech Adamowski, and Bogdan Jaroszewicz. 2020. "Homogenization of Temperate Mixed Deciduous Forests in Białowieża Forest: Similar Communities Are Becoming More Similar" Forests 11, no. 5: 545. https://doi.org/10.3390/f11050545
APA StyleCholewińska, O., Adamowski, W., & Jaroszewicz, B. (2020). Homogenization of Temperate Mixed Deciduous Forests in Białowieża Forest: Similar Communities Are Becoming More Similar. Forests, 11(5), 545. https://doi.org/10.3390/f11050545







