Abstract
Roots have high plasticity with the ability to adapt to heterogeneous nutrient distribution, but little is known about the effects of phosphorus (P) supply methods and levels on Rosa multiflora Thunb. ex Murr. root growth and nutrient accumulation. A pot study was conducted with two P supply methods (mixed and localized application) and three levels (P-deficient, P-moderate and P-adequate). The results showed that with localized application, P-deficient and P-moderate treatments significantly improved total root length, total surface area, total length of fine roots, shoot DW and total P accumulation in Rosa multiflora compared with their respective mixed application at 45 days after being transplanted (DAT) and 92 DAT; for P-adequate supply, the same trends were observed at 45 DAT, but not at 92 DAT. At 92 DAT, with localized application, when P levels increased from P-deficient to P-moderate, total P accumulation increased by 43.3%; but when P levels increased from P-moderate to P-adequate, no effect was observed. Furthermore, higher P accumulation in leaves was observed in localized P-moderate condition; decreased P uptake per root dry weight and greater root/shoot ratio were observed in localized P-adequate at 92 DAT. Total P accumulation was positively correlated with total root length and root surface area (R2: 0.68~0.94). There was a significant interaction effect among treatment days, P supply methods and levels (p ≤ 0.05) on shoot DW, root DW, root/shoot ratio and total P accumulation. These findings indicated that localized and moderate P supply appear efficient for improving R. multiflora growth and P accumulation via efficient root system development.
1. Introduction
Phosphorus (P) is an important essential macronutrient for plant growth [1,2]. It is required as a structural component of RNA, DNA and phospholipids and is a regulator in signal transduction cascades [3]. However, plant-available P is the least mobile and least available to plants in most soil conditions compared to other macronutrients [2]. Only 10%–25% of applied P fertilizer is absorbed by plants in the first growing season, and subsequent use of the residual P rarely exceeds 50% [4]. Moreover, world P reserves extracted from mines are expected to become exhausted with the next 50–200 years, with a decline in the reserve quality and an increase in the cost of production [5]. Therefore, improved P management has become an important issue in sustainable agriculture and forestry [6,7,8,9].
A large number of studies have shown that P supply is critical for early plant growth, maximum root development and optimum plant yield [10,11]. Early season limitations in P availability can lead to an irreversible response that impairs later growth and limits crop yield, even if the plant receives adequate nutrients later [12]. In addition, P fixation may occur through precipitation of PO43− as sparingly soluble Ca-phosphates in calcareous soils with alkaline pH (up to approximately 8) or as Fe- or Al-phosphates in acidic soils (pH below 5.5) [13,14]. The diffusion rate of P in soils is very low, with the diffusion coefficient of 10−12 to 10−15 m2 s−1 [15]. Localized application has proven to be an effective way to reduce the contact of P with the soil and should result in less fixation and resulting in the formation of patches of high nutrient concentrations [16]. Placing P in a band at the root zone may maximize the efficiency of plant roots to mobilize and acquire P from the soil [7,17].
To optimize P availability to plants, especially in early growth stages, P fertilizer can be applied by localized placement, with moderate amounts close to seeds or plant roots, as opposed to conventional fertilizer application by homogenous broadcast [18,19]. Plant roots respond to localized soil nutrient patches by root proliferation (morphological plasticity), and/or by increasing nutrient uptake rates (physiological plasticity) in nutrient-rich patches [20,21]. Localized P supply (as composed to broadcast) affected plant growth, nutrient uptake and P use efficiency [22,23], which was associated with altered root traits and spatial distribution [24,25]. Lateral root proliferation and root system establishment play important roles in the acquisition of resources such as water and nutrients [20], thus strongly influencing plant growth and nutrient use efficiency. Hence, localized P supply may be an efficient strategy to improve plant growth by matching the spatial root growth to soil P availability.
In recent decades, conflicting results have been reported in the literature about the influence of localized P supply on plant performance when compared to broadcast fertilizer applications [26]. Some studies reported that localized P supply (compared to broadcast) did not significantly increase P uptake, shoot biomass or grain yield [27,28]. Other studies reported that localized nutrient application can improve root growth at the early stage, but the positive effect disappeared afterwards [29,30]. One important parameter to consider is the availability of P in nutrient-rich patches, which can be affected by nutrient supply methods and levels [27,31]. Therefore, in the present study, we hypothesized that different P supply methods and levels would change the root growth and spatial distribution of Rosa multiflora, then affecting the shoot biomass and nutrient accumulation.
The aims of the present study were (i) to quantify Rosa multiflora root morphological traits and spatial distribution with different P supply methods and levels, (ii) to investigate the P accumulation in different organs of Rosa multiflora across the various P treatments, and (iii) to analyze correlations of P availability, resulting from variable P supply, in the root zone with P accumulation and root growth.
2. Materials and Methods
2.1. Plant Material and Experimental Design
The experiment was conducted in a naturally lit glasshouse at the Experimental Centre of Forestry in North China, Chinese Academy of Forestry, Beijing. The soil used in the experiment was collected from the soil layer (0–40 cm) of a mixed forest of the Jiulongshan Nature Reserve, Beijing, China (39.55° N, 116.06° E). The mixed forest, including pine (Pinus tabuliformis Carr.) and oak (Quercus variabilis Bl.) trees, was a typical forest type in North China.
The soil was air-dried, then sieved to 2 mm and thoroughly mixed. Initial soil properties were as follows: pH 8.40 (1:2.5, soil:water), Olsen-P 3.25 mg kg−1, organic carbon 8.56 g kg−1, total N 0.76 g kg−1, and exchangeable K 80.25 mg kg−1. To ensure that the supply was adequate for plant growth, the soil was also fertilized with basal nutrients at the following rates (mg kg−1 soil) and forms: 50 Ca (as CaCl2), 28 Mg (as MgSO4), 4 Zn (as ZnSO4), 0.5 Cu (as CuSO4), 1 Fe (as EDTA-Fe), 2 Mn (as MnSO4), 0.15 B (as H3BO3), and 0.01 Mo (as (NH4)6 Mo7O24). In all treatments, the soil K was adjusted to 100 mg kg−1 soil using K2SO4.
There were six treatment combinations of three soil P supply levels (deficient, moderate and adequate) and two supply methods (mixed or localized application). The pot size was 26 cm in upper diameter × 22 cm in bottom diameter × 24 cm in height. All pots were filled with 10 kg of air-dried soil. The soil was compacted to a bulk density of 1.45 g cm−3. P was applied as Ca(H2PO4)2·H2O using two different methods: 1) mixed with the whole soil volume or 2) concentrated in a narrow zone approximately 5 cm below the soil surface (50% of the half-pot soil volume), using the following specific method: 5 kg of initial soil filled one half of the pot, then soil (1.25 kg) was placed at the bottom of the other half of the pot; next, 2.5 kg soil containing P fertilizer was placed above the first layer; finally, 1.25 kg soil was added above the P-rich layer.
We choose the soil P supply levels based on a preliminary two-year experiment with five P supply rates (0, 40, 80, 160 and 320 mg P kg−1 soil, supplied as superphosphate). The results showed that the application rate of 80 mg P kg−1 soil was appropriate for root growth and trunk expansion of Rosa multiflora. In the present study, the amount of P was the same for each supply level but varied in supply methods. The same amounts of P as in the mixing treatment were applied in the localized treatment, which made concentration in that zone higher than the concentration in the mixing treatment. (1) P-deficient treatments: 40 (mixed application) and 160 mg P kg−1 soil in the P-rich zone (localized application,), (2) P-moderate treatments: 80 (mixed application) and 320 mg P kg−1 soil in the P-rich zone (localized application), (3) P-adequate treatments: 250 (mixed application) and 1000 mg P kg−1 soil in the P-rich zone (localized application). P-deficient condition with 40 mg P kg−1 soil conventionally mixed application served as the control. There were six treatments in the study, with eight replicates per treatment.
Thornless rose (Rosa multiflora Thunb. ex Murr.), as one of the important rootstock species for the rose industry, was used as the subject of our study [32,33]. Semi-hardwood cuttings of Rosa multiflora Thunb. were collected from mother stock plants at the research base of Zhonglin Yipu Company in Fangshan District, Beijing, China. The cuttings were 50 cm long and 1.5 cm in diameter made from mature, quiescent, lignified shoots after the leaves had abscised. The basal ends of the cuttings were dipped in 2000 mg L−1 indole-3-butyric acid (IBA) for five seconds and then stuck in non-woven fabric bags filled with a sterilized mixed medium of humus, pearlite, garden soil and sand (at a weight ratio of 40:20:20:20). The bags were placed randomly in a plastic greenhouse under natural light.
Uniformly rooted cuttings were transplanted into pots on May 25, 2018. Plants were watered with deionized water to maintain 75% field capacity. The pots were arranged randomly in a glasshouse with natural conditions and were repositioned every week. Glasshouse temperatures were maintained at 17–29 ℃ during the day and 12-19℃ at night. Plants were harvested for the first time at 45 days after being transplanted (DAT) on July 9, 2018, and for the second time at 92 DAT on August 30, 2018 (four replicates each time).
2.2. Sample Work
Each plant was cut at the ground level and divided into leaves, branches and trunk. After sampling shoots, each soil was divided into two equal parts, one containing a P-rich zone and the other without a P-rich zone. Each sample was sieved through a 2-mm mesh to collect the roots, which were washed in deionized water and then scanned with an EPSON root scanner at 300 dots-per-inch resolution (Epson Expression 1600 pro, Model EU-35, Tokyo, Japan). Root images were analyzed with the software Win-Rhizo (Regent Instruments Inc., Quebec, Canada) to calculate the root length and surface area.
The concentration of soil mineral N (Nmin) (NH4-N + NO3-N) was analyzed using a continuous-flow technique (Ran and Luebbe, Norderstedt, Germany) after extracting 6 g of fresh soil with 150 mL of 0.01 M CaCl2 by shaking (180 rpm) for 1 h at 25 °C. Soil P availability (Olsen-P) was determined according to the molybdo-vanadophosphate method, the air-dried soil being extracted with 0.5 M NaHCO3 at pH 8.5 [34].
Plant samples (0.5000 g) were weighted, placed in a digestion tube, added 8 ml of concentrated sulfuric acid (H2SO4) and left to stand at room temperature for about 12 h. Then, 4 mL of 30% v/v hydrogen peroxide (H2O2, guaranteed reagent) was added, shaken gently and a small funnel was placed in the mouth of the tube. The sample was digested at 250℃ for 30 min, then raised temperature to 370 ℃ and digested for 10 min. The tubes were taken out, cooled down, and added 2 mL H2O2 before the tubes were digested again. These were repeated 2-3 times until the solution was colorless. After cooling, diluted to a constant volume. In the digests, P was analyzed by the molybdo-vanadophosphate method at 440 nm [35]. IPE883 straw (Wageningen University, Wageningen, The Netherlands) was used as a reference material to assess the reliability of the analyses. In this study, shoot refers to the above-ground part of the plant, including the annual leaves and annual branches. Nutrient accumulation was calculated as mass x concentration by the annual roots and shoots.
2.3. Statistical Analysis
One-way analysis of variance was performed using SAS statistical software (SAS 8.1, USA), and significant differences among means were assessed using Duncan’s multiple range analysis test at 5% probability (p ≤ 0.05). Three-way analysis of variance (ANOVA) was performed to test the effect of treatment days, P supply methods and levels and their interaction on growth and nutrient accumulation. Linear models and empirical quadratic equations were used in SigmaPlot (SigmaPlot 10.0, USA) to analyze the relationship between root length and total shoot P accumulation or soil Olsen-P or Nmin.
3. Results
3.1. Interactive Effects of Treatment Days, P Fertilizer Supply Methods and Levels
The three-way ANOVA showed a significant interaction among treatment days, P fertilizer supply methods and levels (p ≤ 0.05) for shoot DW, root DW, root/shoot ratio and total P accumulation, but not for total root length and root surface area (Table 1). The interaction effect between days and P supply levels was significant (p ≤ 0.05) for all the variables, except shoot DW. The interaction effect between P supply methods and levels was significant (p ≤ 0.05) for total root surface area and total P accumulation. P supply methods and days had a significant interaction (p ≤ 0.05) for root DW, root/shoot ratio and total root surface area. In contrast, the main effects of days, P supply methods and levels were significant (p ≤ 0.05) for shoot DW, root DW and root/shoot ratio, respectively, but not for the other variables.

Table 1.
Three-way analysis of variance (ANOVA) on the effects of sampling time, P supply methods and levels and their interaction on growth and nutrient accumulation parameters in Rosa multiflora.
3.2. Plant Growth
The effect of P fertilizer on plant growth depended on the P supply method and level (Table 2). Plant growth was greater when the P fertilizer was localized than when it was mixed in the total volume of the soil at both harvests, except root dry weight (DW) at 45 DAT. With localized application, shoot DWs in P-deficient, -moderate and -adequate conditions increased by 83.61%, 31.33% and 28.02%, respectively, compared with the mixed treatment at 45 DAT. Shoot DW was also significantly greater in localized P-deficient and P-moderate conditions compared with respective mixing applications, but a difference was not found in the localized P-adequate condition at 92 DAT.

Table 2.
Effects of different P supply on shoot and root dry weight and root/shoot ratio in Rosa multiflora.
With the mixed application, shoot DW was highest in the P-adequate condition, followed by P-moderate and P-deficient. With the mixed method, shoot DW increased by 64.01% in the P-moderate condition and 191.04% in P-adequate at 45 DAT and increased by 37.05% in P-moderate and 83.07% in P-adequate at 92 DAT when compared to the P-deficient treatment. In localized application, shoot DW was highest in the P-adequate condition at 45 DAT and tallest in the P-moderate condition at 92 DAT. Root growth showed a trend similar to that of shoot growth, except in the localized P-adequate condition at 92 DAT. No difference was observed in the root/shoot ratios among the six treatments at 45 DAT, but there was a significant increase in root/shoot ratio in the localized P-adequate treatment compared to the other treatments, and a greater root/shoot mean ratio with localized application that mixed application at 92 DAT.
3.3. Effects on Root Length, Root Surface Area, Fine Root and Root Distribution
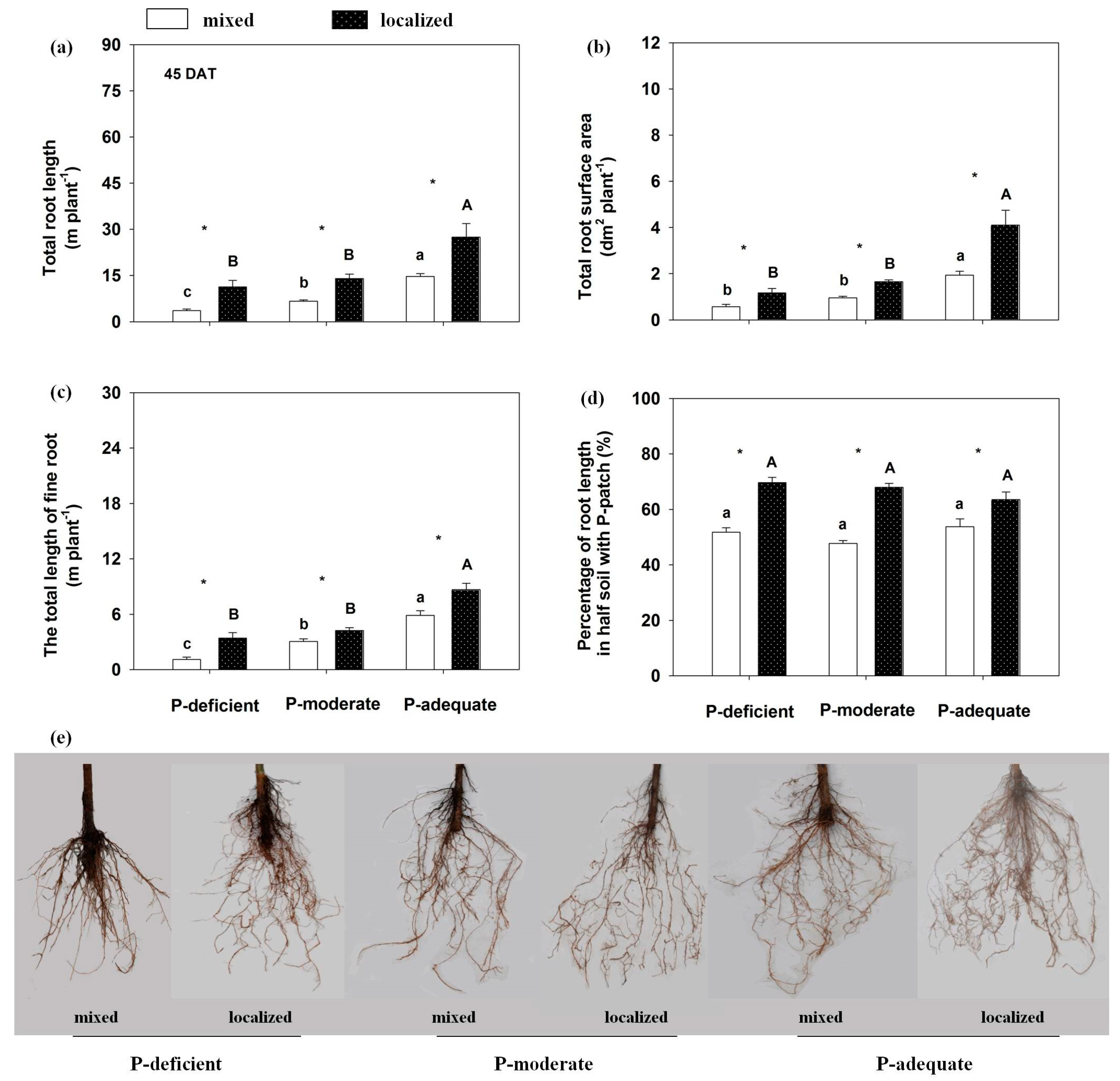
Root growth and its spatial distribution were altered by the P supply methods and levels (Figure 1 and Figure 2). At 45 DAT, localized P application significantly increased total root length in the three P levels compared with respective mixed applications. Total root length in the localized P-deficient, -moderate and -adequate treatments increased by 211.18%, 111.78% and 87.46%, respectively, compared with the mixed treatments. The total root length increased with an increase in P supply level, which was highest in the P-adequate condition, followed by P-moderate and P-deficient in either supply method. Total root surface area and total length of fine root (with a diameter < 0.2 mm) showed trends similar to total root length. Compared with mixed applications, localized P applications significantly improved root proliferation in the nutrient-rich zone, leading to a higher percentage of the root length in the P-rich zone. Furthermore, the percentages of P-rich zone root length in the whole plant were similar among the three P levels in either supply method at 45 DAT (p = 0.11–0.17).
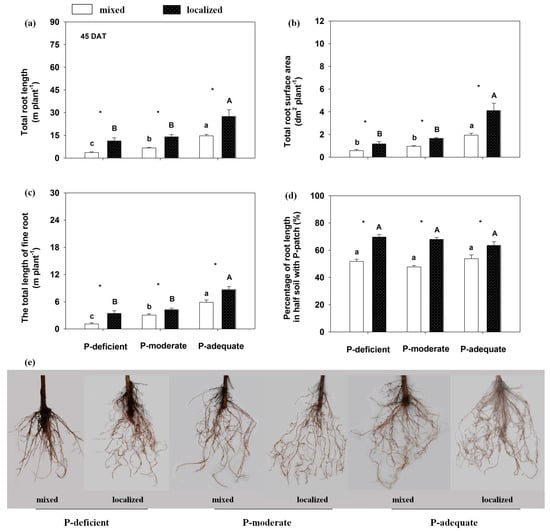
Figure 1.
Rosa multiflora root morphological traits in response to different P supply methods and levels at 45 DAT. (a) total root length, (b) total root surface area, (c) the total length of fine roots (diameter 0–0.2 mm), (d) percentage of root length in half soil containing P-rich patches and (e) root images. Mean values + SE are presented. Different lower-case letters denote significant differences (p ≤ 0.05) among treatments at three P levels with mixed application, and capital letters denote significant differences among treatments at three P levels with localized application. * indicates significant differences at p ≤ 0.05 between mixed and localized application for a particular P level.
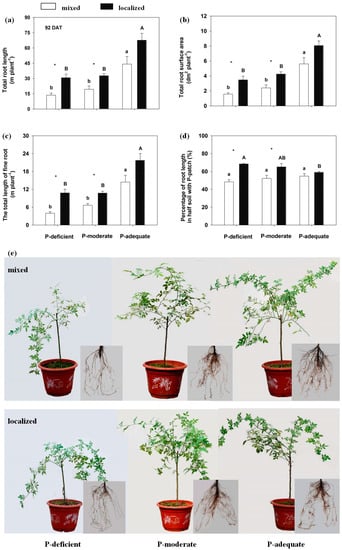
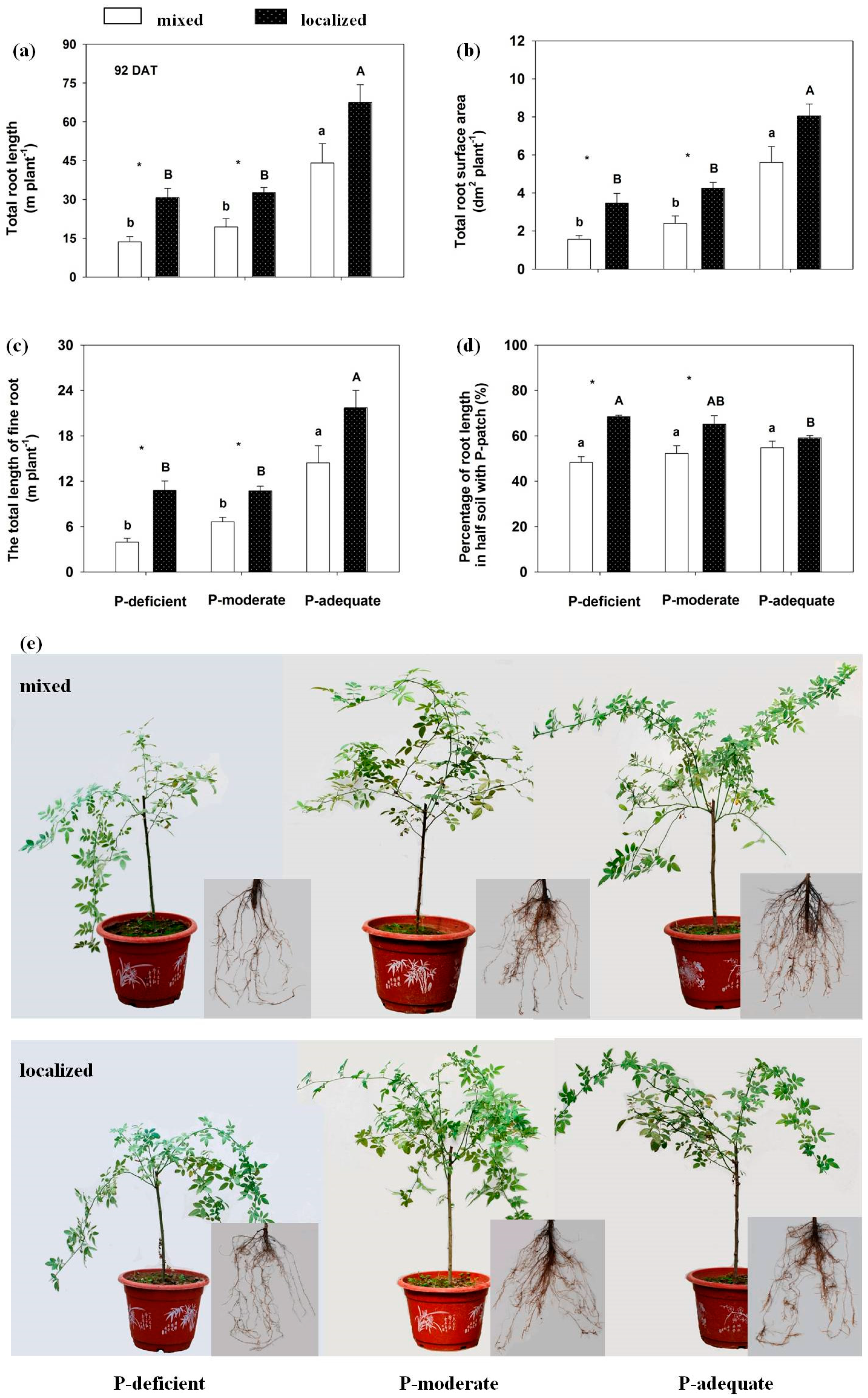
Figure 2.
Rosa multiflora root morphological traits in response to different P supply methods and levels at 92 DAT. For an explanation of subfigures and the symbols, see Figure 1.
At 92 DAT, compared with mixed application, total root length increased by 125.58% and 68.87%, respectively, in the localized P-deficient and P-moderate conditions, but not in localized P-adequate. Total root length was significantly higher in P-adequate treatment than in the other treatments in either supply method. Total root surface area and total length of fine root showed the same trends as total root length among the six treatments. In contrast, the percentage of P-rich zone root length in the whole plant in the localized P-adequate treatment was similar to its mixed treatment and lower than that of the other two localized treatments.
3.4. Effects on P Accumulation in Annual Leaves, Branches, Roots and the Whole Plant and P Uptake Rate
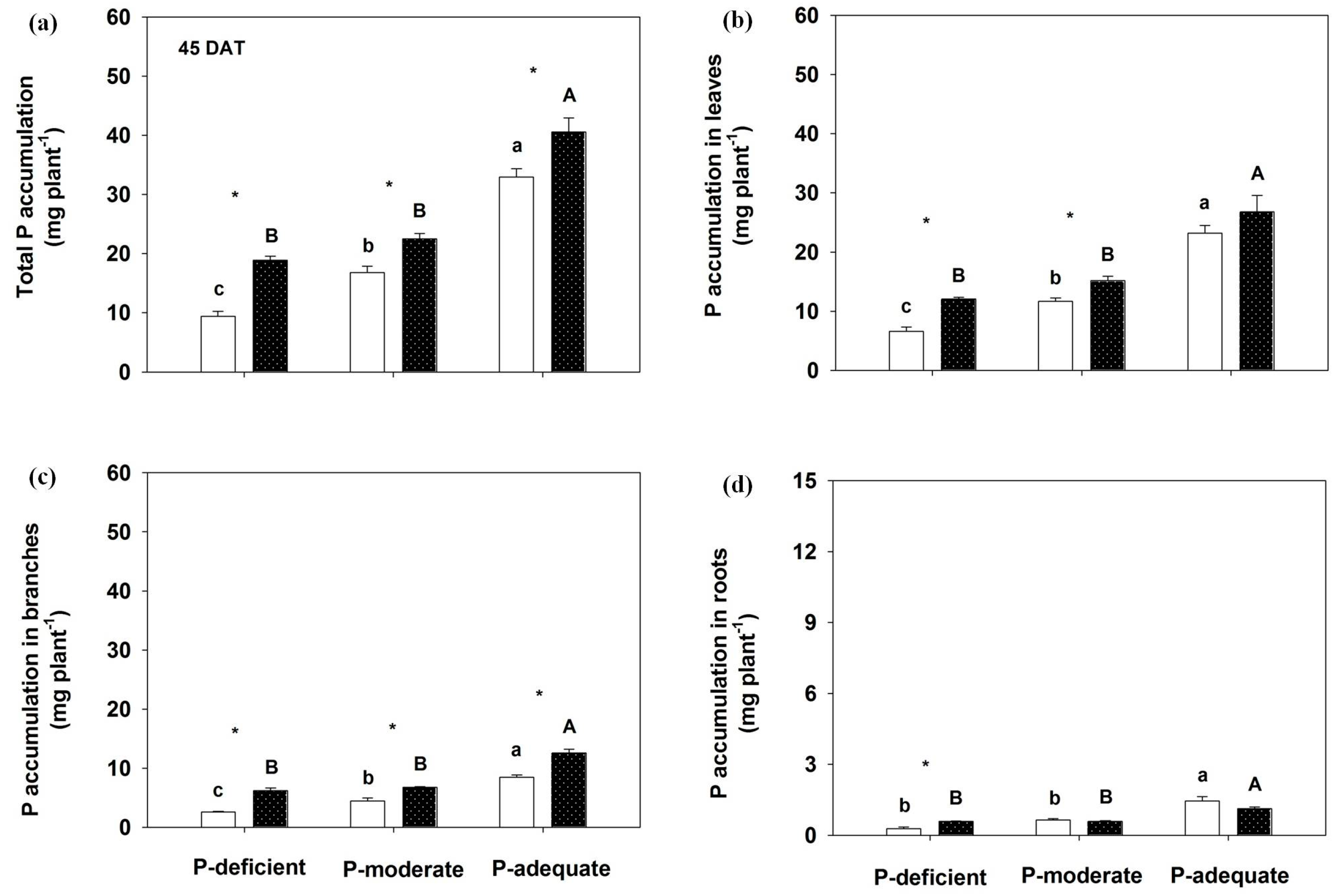
Both P supply methods and levels markedly influenced shoot and root P accumulation (Figure 3 and Figure 4). At 45 DAT, total P accumulation increased by 99.87%, 34.08% and 23.12%, respectively, in localized P-deficient, -moderate and -adequate treatments compared to its mixed treatment. P accumulation levels in leaves, branches and roots were greater in the localized P-deficient condition than in its mixed application. Greater P accumulation in branches was found in localized P-moderate and P-adequate conditions than in the respective mixed applications. With mixed application, P accumulation levels in annual leaves, branches, roots and the whole plant were highest in the P-adequate treatment, followed by P-moderate and P-deficient. With localized application, P accumulation in the organs was greater in P-adequate than in the other two levels.
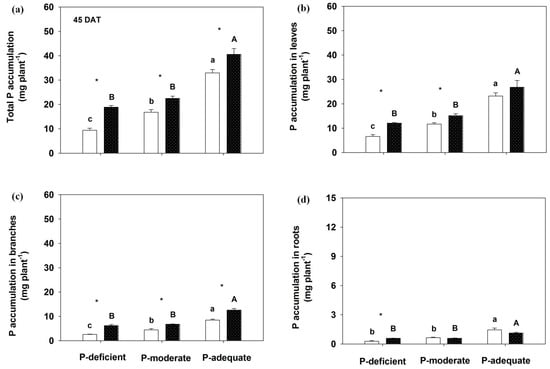
Figure 3.
Effects on total P accumulation (a) and annual leaves (b), branches (c) and roots P accumulation (d) by Rosa multiflora at 45 DAT. Mean values + SE are presented. For an explanation of the symbols, see Figure 1.
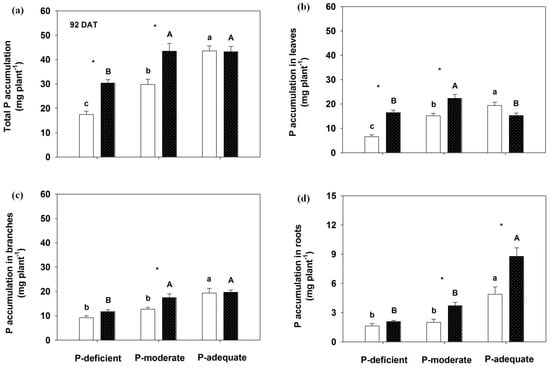
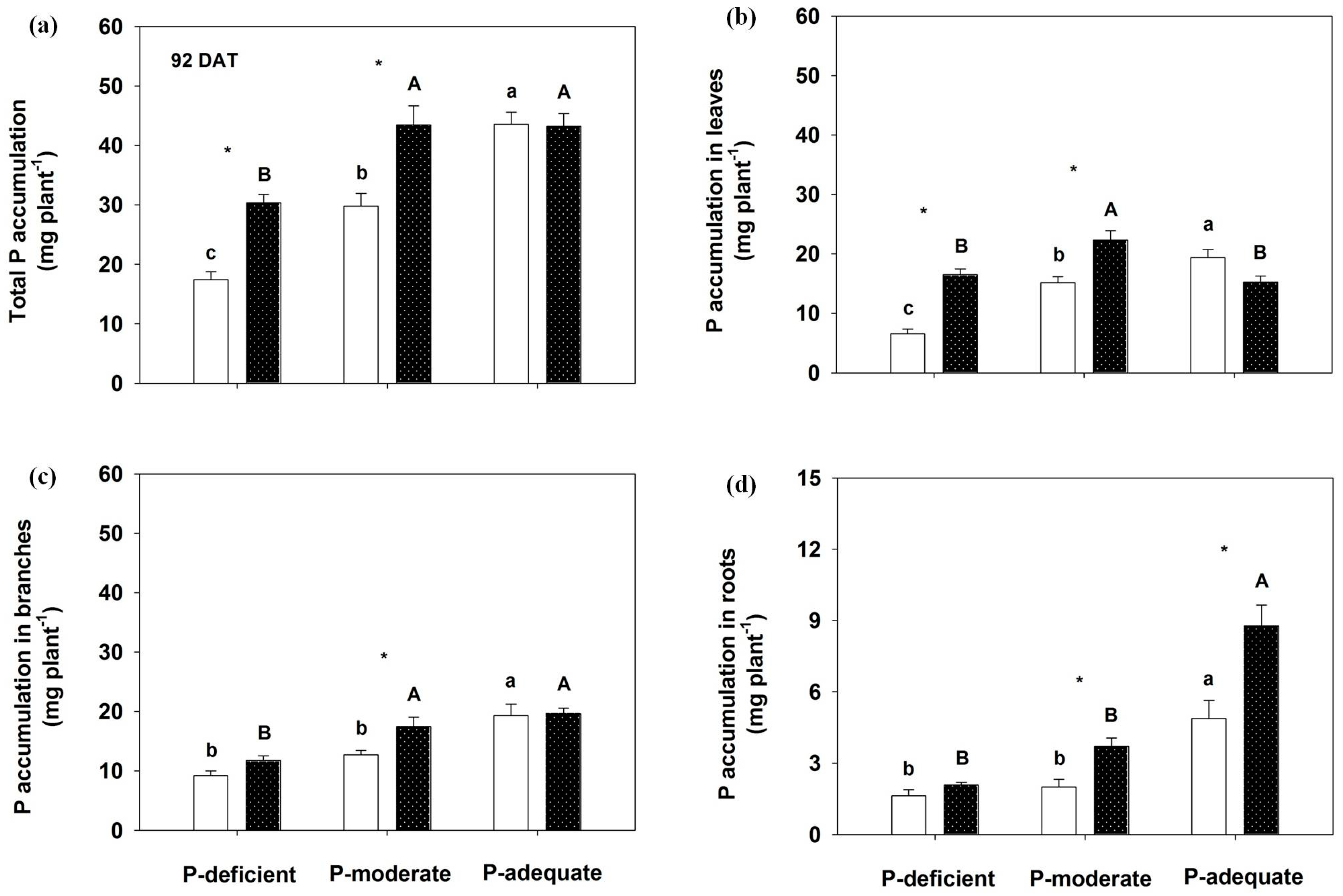
Figure 4.
Effects on total P accumulation (a), annual leaves (b), branches (c) and roots (d) by Rosa multiflora at 92 DAT. Mean values + SE are presented. For an explanation of the symbols, see Figure 1.
At 92 DAT, total P accumulation was 74.0% greater in localized P-deficient and 45.8% higher in localized P-moderate than in the respective mixed application. P accumulation levels in leaves, branches and roots were significantly higher in the localized P-moderate condition than in its mixed treatment. With mixed application, P accumulation levels in the organs were highest in the P-adequate condition, followed by P-moderate and P-deficient. No significant differences were observed in leaves, branches and total P accumulation in the P-adequate condition between the two supply methods, except for higher P accumulation in roots with localized application. With localized application, leaves, branches and total P accumulation did not differ between the localized P-moderate and P-adequate treatments, except an increased P accumulation in roots with the localized P-adequate treatment.
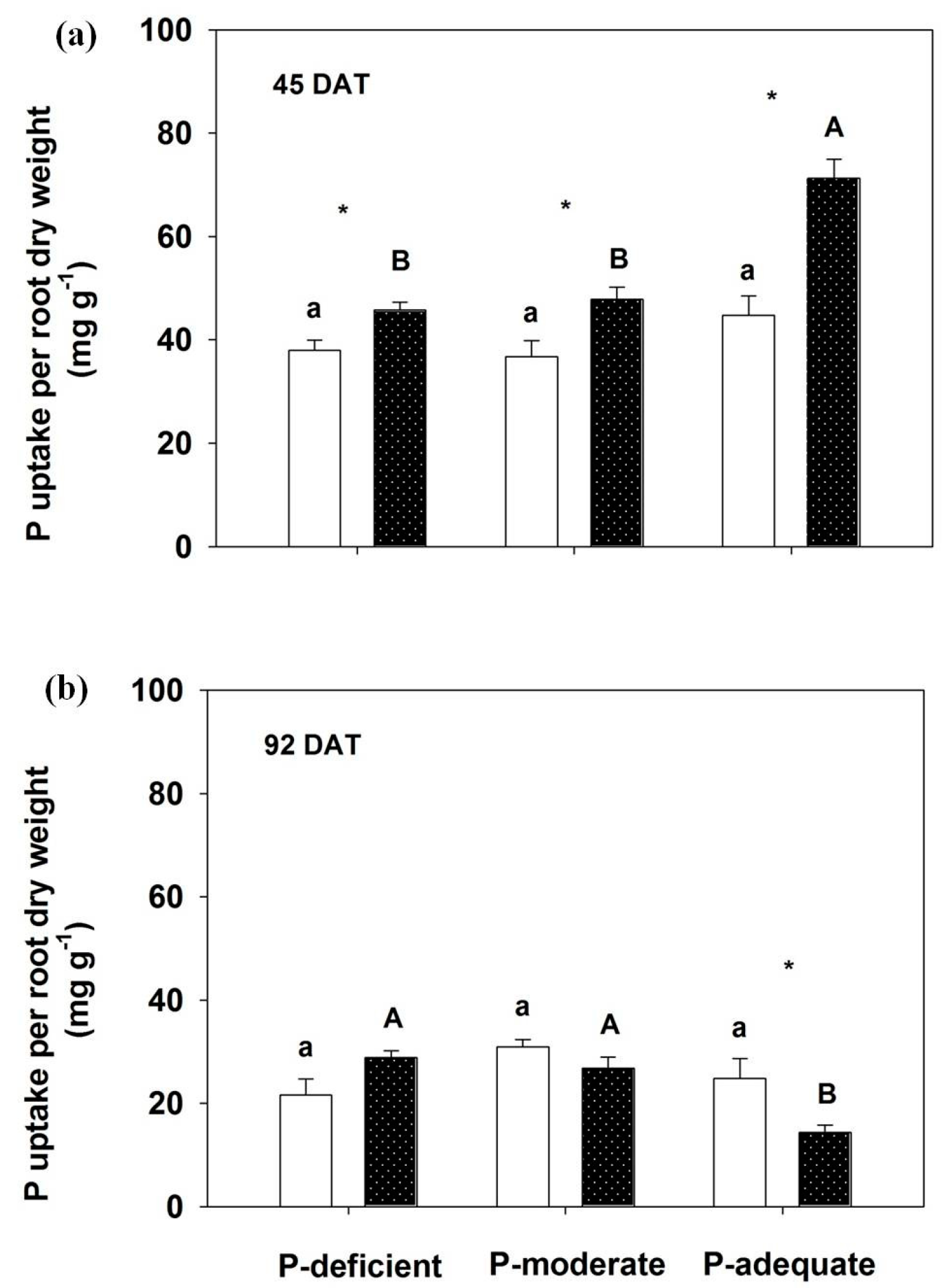
At 45 DAT, P uptake per root dry weight significantly increased in the localized P-deficient, -moderate and -adequate conditions compared with respective mixed treatments (Figure 5). No significant difference was observed among the three P levels within either supply method at 45 DAT, except P-adequate condition with localized application. In contrast, P uptake per root dry weight significantly decreased with localized P-adequate treatment compared with its mixed application, which was also lower than the other localized treatments at 92 DAT.
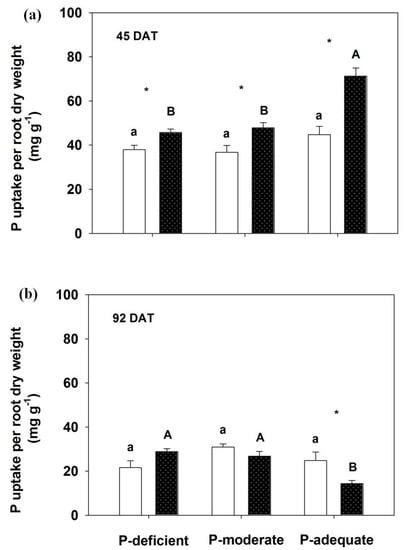
Figure 5.
Effects of different P supply (a) on P uptake (b) per root dry weight. Mean values + SE are presented. For an explanation of the symbols, see Figure 1.
3.5. Relationship Between Soil Nutrients (Nmin and Olsen-P), Root Length and P Accumulation
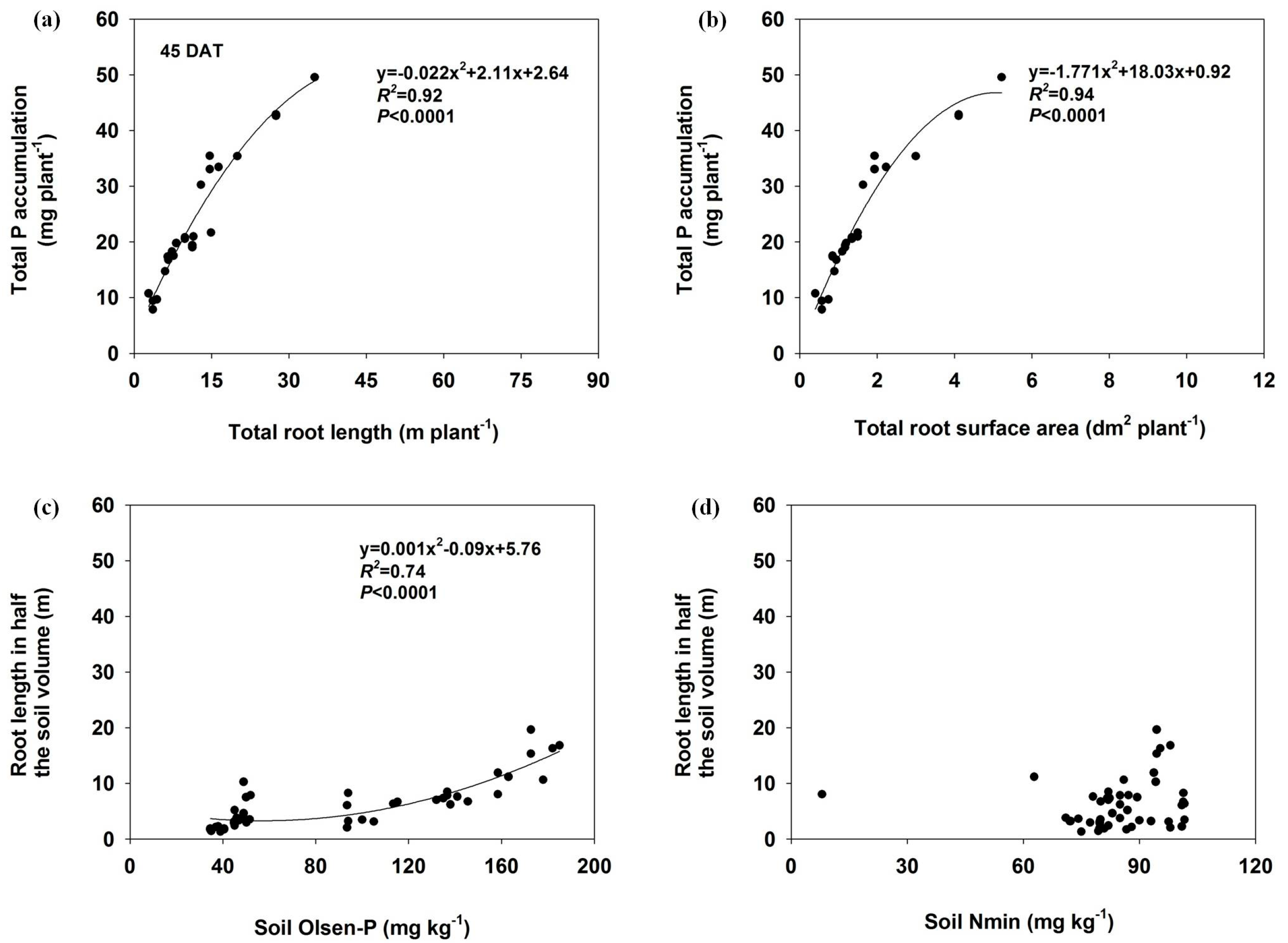
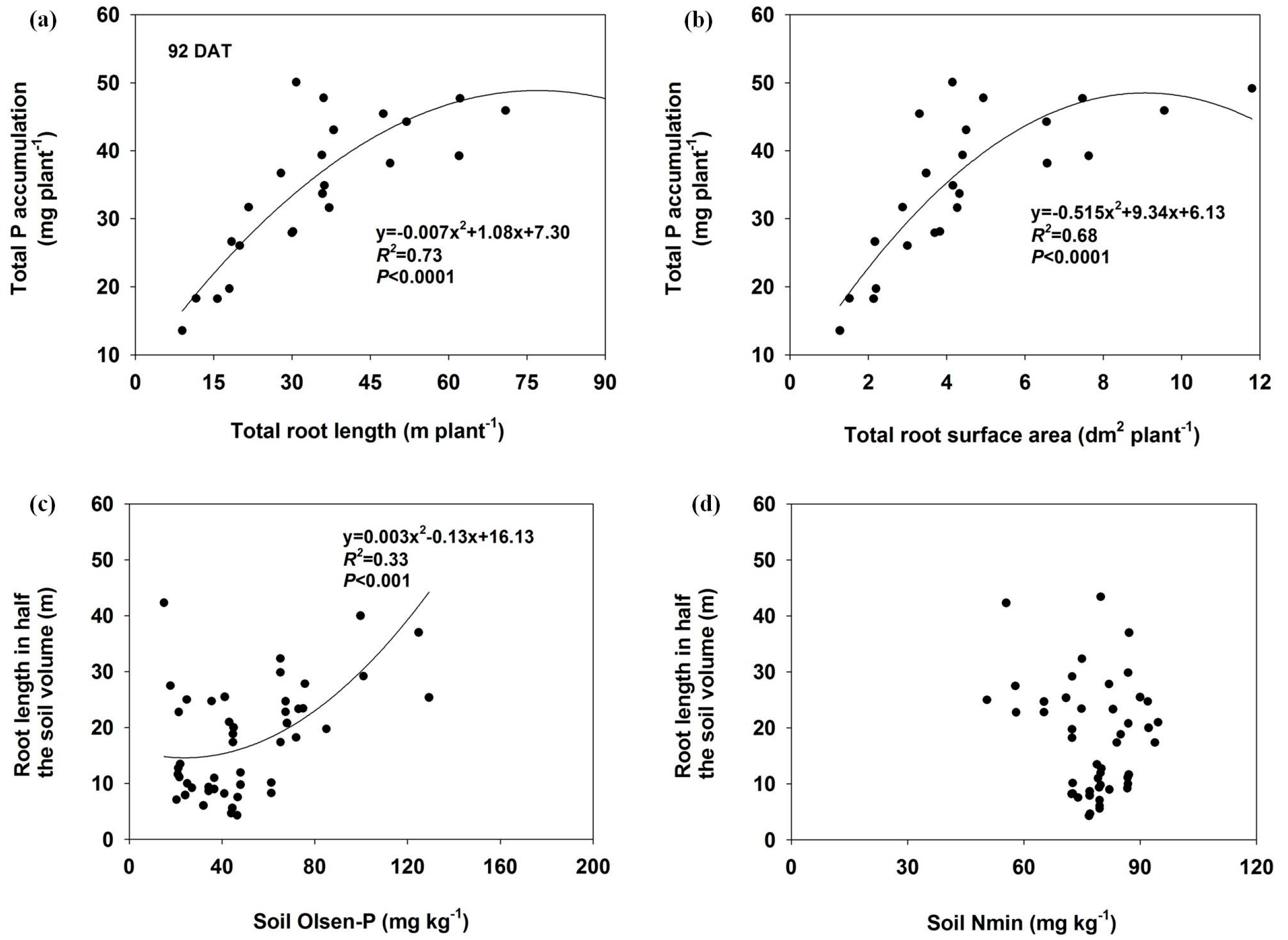
The total P accumulation significantly correlated with total root length and total root surface area (p ≤ 0.01) in both harvests (Figure 6 and Figure 7). There was also a significant relationship between soil Olsen-P concentration and root length in half of the soil volume at 45 DAT (R2 = 0.74), but the correlation coefficient decreased at 92 DAT (R2 = 0.33). No significant relationships were found between the soil Nmin and the root length in either harvest.
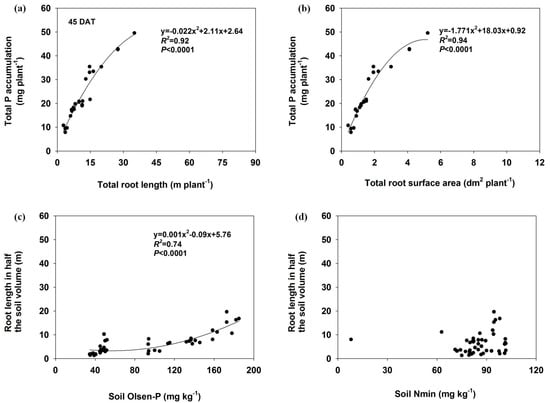
Figure 6.
The relationship between total P accumulation and total root length (a) or total root surface area (b) and as well as the root length as influenced by Olsen-P (c) or Nmin (d) in half of the soil at 45 DAT.
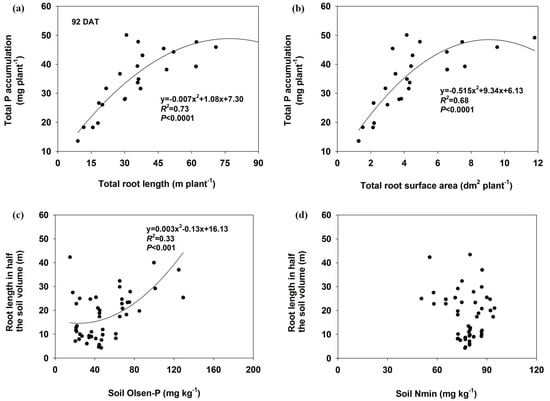
Figure 7.
The relationship between total P accumulation and total root length (a) or total root surface area (b) and as well as the root length as influenced by Olsen-P (c) or Nmin (d) in half of the soil at 92 DAT.
4. Discussion
Compared to other macronutrients, plant-available P is the most limiting factor for plant growth in most soils [1,2]. The initial soil used in the present study was collected from a typical mixed forest in North China, and the Olsen-P was only 3.25 mg kg−1, indicating that it was an extremely P-deficient soil. Recently, it has been suggested that not only cropland but also grassland and forestland require improved nutrient management, with inputs of P fertilizer to bring P into balance with N and other nutrients [3,36].
Management of P fertilizer has the potential to significantly affect root morphology and plant growth [37,38]. Significantly increased total root length and surface area were observed in localized P-deficient and P-moderate conditions compared with the respective mixed treatments, which were mainly associated with root proliferation in P-rich patches (Figure 1 and Figure 2). In P-rich soil patches, lateral root proliferation was one of the most important morphological responses of the root system [20,39]. It has been suggested that plants can sense and coordinate root growth based on available soil P and a nutrient supply method [7]. Root length and biomass were higher in heterogeneous P (i.e., patch, banding or localized P supply) treatment than in homogeneous treatment [17,29]. Soil available P in the P-rich zone may have contributed to increased root growth in localized P-deficient and P-moderate treatments, and a positive correlation was observed between root length and soil Olsen-P (Figure 6 and Figure 7).
In addition to supply methods, P supply levels also had an important effect on root growth. Significant differences in root growth were found with a localized P-deficient or P-moderate supply compared with their mixed application counterparts. No significant difference in root morphological characteristics was observed in the P-adequate condition between the two supply methods at 92 DAT, although there was a significant difference at 45 DAT. Previous research has indicated a positive effect of localized ammonium plus P fertilizer application on root growth was evident at the 8- to 10-leaf stage, but there was no significant effect afterwards [29]. Gao et al. reported that the total root length and surface of maize/faba bean were not found to be different between two P supply treatments (homogenous and heterogeneous) [27]. In the present study, the similar root characteristics between two supply methods with P-adequate condition at 92 DAT could be explained in two ways. First, root emission and organogenesis are highly energy- and carbon-demanding developmental processes in plant biology [40], thus significantly increasing root DW in localized P-adequate treatment, and then affecting carbon allocation and shoot biomass (Table 2). Second, systemic sensing may be induced in the mixed treatment with an adequate P supply, and phosphate2 may function as a systemic root P sensor [3,41].
Compared with the respective mixed treatments, total P accumulation was markedly higher with the localized P-deficient and P-moderate conditions at both harvests. This result agrees with previous research in which banded P supply increased the early growth and P uptake compared with broadcast applications [22,23]. Possible explanations for the greater P accumulation in localized P-deficient or P-moderate conditions may include the following: (1) fine roots are important organs that absorb mineral nutrients and water [7,39], and the increased total length of fine roots (diameter < 0.2 mm) with localized P-deficient and P-moderate supplies may contribute to increased nutrient uptake in the present study; (2) localized P reduces contact with the soil and results in increased soil available P [5,14], which may be effective in inducing root proliferation, and there was a positive correlation between total root length and soil Olsen-P (Figs. 6 and 7); and (3) deep placement of P significantly decreased P loss, with increased P acquisition especially in the low-P treatments [42]. Moreover, increases in P uptake and accumulation will improve a plants’ requirement for other mineral elements, such as N [43]; higher N accumulation was observed in the localized P-deficient and P-moderate conditions than in the respective mixed application in the present study (data not shown).
It is generally accepted that plant P uptake increases with increasing P input [44]. Similar results were found in the present study with mixed P application at three supply levels. However, with localized application, no significant difference in total P accumulation was observed between P-moderate and P-adequate levels at 92 DAT. Jing et al. observed that, with adequate P supply, no significant differences were found in total root length or P uptake in broadcast or localized application [29]. Hansel et al. also reported that, with band application 5 cm deep and 5 cm to the side of soybean seeds, shoot DW and shoot P uptake were similar between two usage rates of 60 and 120 kg P2O5 ha−1 [31]. The depressive effects in shoot growth and P accumulation in P-adequate treatments were possibly due to high P-induced N deficiency [45,46]. Research has reported that N and P show strong relationships to plant growth and nutrient accumulation [47]. Furthermore, localized application of P fertilizer could increase nutrient acquisition and nutrient-use efficiency, as well as dry matter yields and grain yield, which was associated with improving root traits and spatial distribution [48,49,50]. In the present study, there was a significant interactive effect of P fertilizer supply methods, levels and days on plant growth and nutrient uptake (Table 1). It suggested that quantification of multiple factors and interaction effects on plant growth could be important to optimize plant growth and nutrient-use efficiency.
Localized application at the moderate P supply significantly stimulated root proliferation and improved root spatial distribution, which can be an effective management strategy for enhancing Rosa multiflora growth and nutrient uptake. A greater understanding of P accumulation by field-grown Rosa multiflora in relation to soil P properties is needed for better defining optimum P fertilizer rate recommendations.
5. Conclusions
A significant interaction was observed among treatment days, P supply methods and levels (P ≤ 0.05) on plant growth and total P accumulation in Rosa multiflora. Plant P uptake increases with increasing P input from P-deficient to P-adequate level at 45 DAT. Total P accumulation significantly increased when P levels increased from the P-deficient to P-moderate condition, but not from P-moderate to P-adequate with localized supply at 92 DAT. Furthermore, significantly increased P uptake rate per root dry weight and greater shoot dry weight and leaf P accumulation were observed in the localized P-moderate treatment compared to its mixed treatment and localized P-adequate treatment at 92 DAT, which was associated with lateral root proliferation and increased total fine root length in fertilized zone. Results suggest that an efficient root system development response to localized application at the moderate P supply may be important for enhancing P accumulation in Rosa multiflora.
Author Contributions
Q.M. and Y.Z. (Yongan Zhang) designed the study; Q.M., L.C. and M.D. were in charge of the data collection; Q.M. analyzed the data and drafted the paper; Y.Z. (Yongan Zhang) and Y.Z. (Yaoxiang Zhang) reviewed and edited the final version of paper; Q.M. acquired the funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chinese National Natural Science Foundation (31700559) and the Science Foundation of the Chinese Academy of Forestry (CAFYBB2017MA017, CAFYBB2017ZC006).
Acknowledgments
We thank Hongliang Tang, Mingbao Luan, the professor of the Hebei University and the researcher of the Chinese Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the roots. Annu. Plant Rev. Online 2018, 48, 3–22. [Google Scholar]
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a signaling molecule and its sensing mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its efficient use in agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar]
- Herrera-Estrella, L.; López-Arredondo, D. Phosphorus: The underrated element for feeding the world. Trends Plant Sci. 2016, 21, 461–463. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, J.; Zhang, T.; Hao, F.; Jose, S.; Zhang, S. Soil degradation and the decline of available nitrogen and phosphorus in soils of the main forest types in the Qinling Mountains of China. Forests 2017, 8, 460. [Google Scholar] [CrossRef]
- Heinonen, T.; Pukkala, T.; Asikainen, A.; Peltola, H. Scenario analyses on the effects of fertilization, improved regeneration material, and ditch network maintenance on timber production of Finnish forests. Eur. J. Forest Res. 2018, 137, 93–107. [Google Scholar] [CrossRef]
- de Melo, E.A.S.C.; de Gonçalves, J.L.M.; Rocha, J.H.T.; Hakamada, R.E.; Bazani, J.H.; Wenzel, A.V.A.; Arthur, J.C., Jr.; Borges, J.S.; Malheiros, R.; de Lemos, C.C.Z.; et al. Responses of clonal eucalypt plantations to N, P and K fertilizer application in different edaphoclimatic conditions. Forests 2015, 7, 2. [Google Scholar] [CrossRef]
- Wu, P.F.; Ma, X.Q.; Tigabu, M.; Huang, Y.; Zhou, L.L.; Cai, L.; Oden, P.C. Comparative growth, dry matter accumulation and photosynthetic rate of seven species of Eucalypt in response to phosphorus supply. J. Forestry Res. 2014, 25, 377–383. [Google Scholar] [CrossRef]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Plassard, C.; Dell, B. Phosphorus nutrition of mycorrhizal trees. Tree Physiol. 2010, 30, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G.; Gama-Rodrigues, A.C.; Gonçalves, J.L.D.M.; Gama-Rodrigues, E.F.; Sales, M.V.D.S.; Aleixo, S. Labile and non-labile fractions of phosphorus and its transformations in soil under eucalyptus plantations, Brazil. Forests 2016, 7, 15. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of NH4+-N plus P at the seedling and later growth stages enhances nutrient uptake and maize yield by inducing lateral root proliferation. Plant Soil 2013, 372, 65–80. [Google Scholar] [CrossRef]
- Kaiser, D.; Mallarino, A.P.; Bermudez, M. Corn grain yield, early growth, and early nutrient uptake as affected by broadcast and in-furrow starter fertilization. Agron. J. 2005, 97, 620–626. [Google Scholar] [CrossRef]
- Roth, G.W.; Beegle, D.B.; Heinbaugh, S.M.; Antle, M.E. Starter fertilizers for corn on soils testing high in phosphorus in the northeastern USA. Agron. J. 2006, 98, 1121–1127. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, W.; Li, Y.; Hou, X.; Liu, A.; Cai, L. Morphological plasticity and phosphorus uptake mechanisms of hybrid Eucalyptus roots under spatially heterogeneous phosphorus stress. J. Forestry Res. 2017, 28, 713–724. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Tang, X.; Li, H.; Zhang, F.; Rengel, Z.; Shen, J. Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol. 2016, 209, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.D.; Dunsford, K.; McLaughlin, M.J.; McBeath, T.; Mason, S.; Dunbabin, V.M. Phosphorus and nitrogen fertiliser use efficiency of wheat seedlings grown in soils from contrasting tillage systems. Plant Soil 2015, 396, 297–309. [Google Scholar] [CrossRef]
- Li, H.; Mollier, A.; Ziadi, N.; Shi, Y.; Parent, L.; Morel, C. The long-term effects of tillage practice and phosphorus fertilization on the distribution and morphology of corn root. Plant Soil 2017, 412, 97–114. [Google Scholar] [CrossRef]
- Weligama, C.; Tang, C.; Sale, P.W.G.; Conyers, M.; Liu, D. Localised nitrate and phosphate application enhances root proliferation by wheat and maximises rhizosphere alkalisation in acid subsoil. Plant Soil 2008, 312, 101–115. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crop. Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Gao, W.; Blaser, S.R.; Schlüter, S.; Shen, J.; Vetterlein, D. Effect of localised phosphorus application on root growth and soil nutrient dynamics in situ–comparison of maize (Zea mays) and faba bean (Vicia faba) at the seedling stage. Plant Soil 2019, 441, 469–483. [Google Scholar] [CrossRef]
- Mallarino, A.P.; Bergmann, N.; Kaiser, D.E. Corn responses to in-furrow phosphorus and potassium starter fertilizer applications. Agron. J. 2011, 103, 685–694. [Google Scholar] [CrossRef]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crop. Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Su, W.; Liu, B.; Liu, X.; Li, X.; Ren, T.; Cong, R.; Lu, J. Effect of depth of fertilizer banded-placement on growth, nutrient uptake and yield of oilseed rape (Brassica napus L.). Eur. J. Agron. 2015, 62, 38–45. [Google Scholar] [CrossRef]
- Hansel, F.; Diaz, D.; Rosa, A.; Moorberg, C. Phosphorus fertilizer placement and rate affect soybean root growth and nutrient uptake in soil with high fertility. Agron. Sci. Biotechnol. 2019, 5, 62–73. [Google Scholar] [CrossRef]
- Feng, H.; Wang, M.L.; Cong, R.C.; Dai, S.L. Colchicine-and trifluralin-mediated polyploidization of Rosa multiflora Thunb. var. inermis and Rosa roxburghii f. normalis. J. Hortic. Sci. Biotech. 2017, 92, 279–287. [Google Scholar] [CrossRef]
- Otiende, M.A.; Nyabundi, J.O.; Ngamau, K.; Opala, P. Effects of cutting position of rose rootstock cultivars on rooting and its relationship with mineral nutrient content and endogenous carbohydlevels. Sci. Hortic. 2017, 225, 204–212. [Google Scholar] [CrossRef]
- Westerman, R.L. Soil Testing and Plant Analysis, 3rd ed.; American Society of Agronomy and Soil Science of America: Madison, WI, USA, 1991. [Google Scholar]
- Johnson, C.M.; Ulrich, A. Analytical Methods for Use in Plant Analysis. Bull. Calif. Agric. Exp. Stn. 1959, 766, 78. [Google Scholar]
- Sattari, S.Z.; Bouwman, A.F.; Rodriguez, R.M.; Beusen, A.H.W.; Van Ittersum, M.K. Negative global phosphorus budgets challenge sustainable intensification of grasslands. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, C.; Ma, X.; Wu, P. Root morphology and seedling growth of three tree species in southern China in response to homogeneous and heterogeneous phosphorus supplies. Trees 2019, 33, 1283–1297. [Google Scholar] [CrossRef]
- Pan, F.; Liang, Y.; Wang, K.; Zhang, W. Responses of fine root functional traits to soil nutrient limitations in a karst ecosystem of Southwest China. Forests 2018, 9, 743. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Rose, T.J.; Rengel, Z.; Ma, Q.; Bowden, J.W. Phosphorus accumulation by field-grown canola crops and the potential for deep phosphorus placement in a Mediterranean-type climate. Crop. Pasture Sci. 2009, 60, 987–994. [Google Scholar] [CrossRef]
- Ågren, G.I.; Wetterstedt, J.M.; Billberger, M.F. Nutrient limitation on terrestrial plant growth–modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, H.; Shen, J.; Rengel, Z. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Scott, D.A.; Bliss, C.M. Phosphorus fertilizer rate, soil P availability, and long-term growth response in a loblolly pine plantation on a weathered ultisol. Forests 2012, 3, 1071–1085. [Google Scholar] [CrossRef]
- Song, X.; Wan, F.; Chang, X.; Zhang, J.; Sun, M.; Liu, Y. Effects of nutrient deficiency on root morphology and nutrient allocation in Pistacia chinensis bunge Seedlings. Forests 2019, 10, 1035. [Google Scholar] [CrossRef]
- Hu, B.; Chu, C. Nitrogen–phosphorus interplay: Old story with molecular tale. New Phytol. 2020, 225, 1455–1460. [Google Scholar] [CrossRef]
- Robinson, D. The responses of plants to non-uniform supplies of nutrients. New Phytol. 1994, 127, 635–674. [Google Scholar] [CrossRef]
- Graciano, C.; Tambussi, E.A.; Castán, E.; Guiamet, J.J. Dry mass partitioning and nitrogen uptake by Eucalyptus grandis plants in response to localized or mixed application of phosphorus. Plant Soil 2009, 319, 175–184. [Google Scholar] [CrossRef]
- Mazlouzi, M.E.; Morel, C.; Robert, T.; Yan, B.; Mollier, A. Phosphorus uptake and partitioning in two durum wheat cultivars with contrasting biomass allocation as affected by different P supply during grain filling. Plant Soil 2020, 449, 179–192. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).