An Efficient Tool for the Maintenance of Thermophilous Oak Forest Understory—Sheep or Brush Cutter?
Abstract
:1. Introduction
2. Materials and Methods
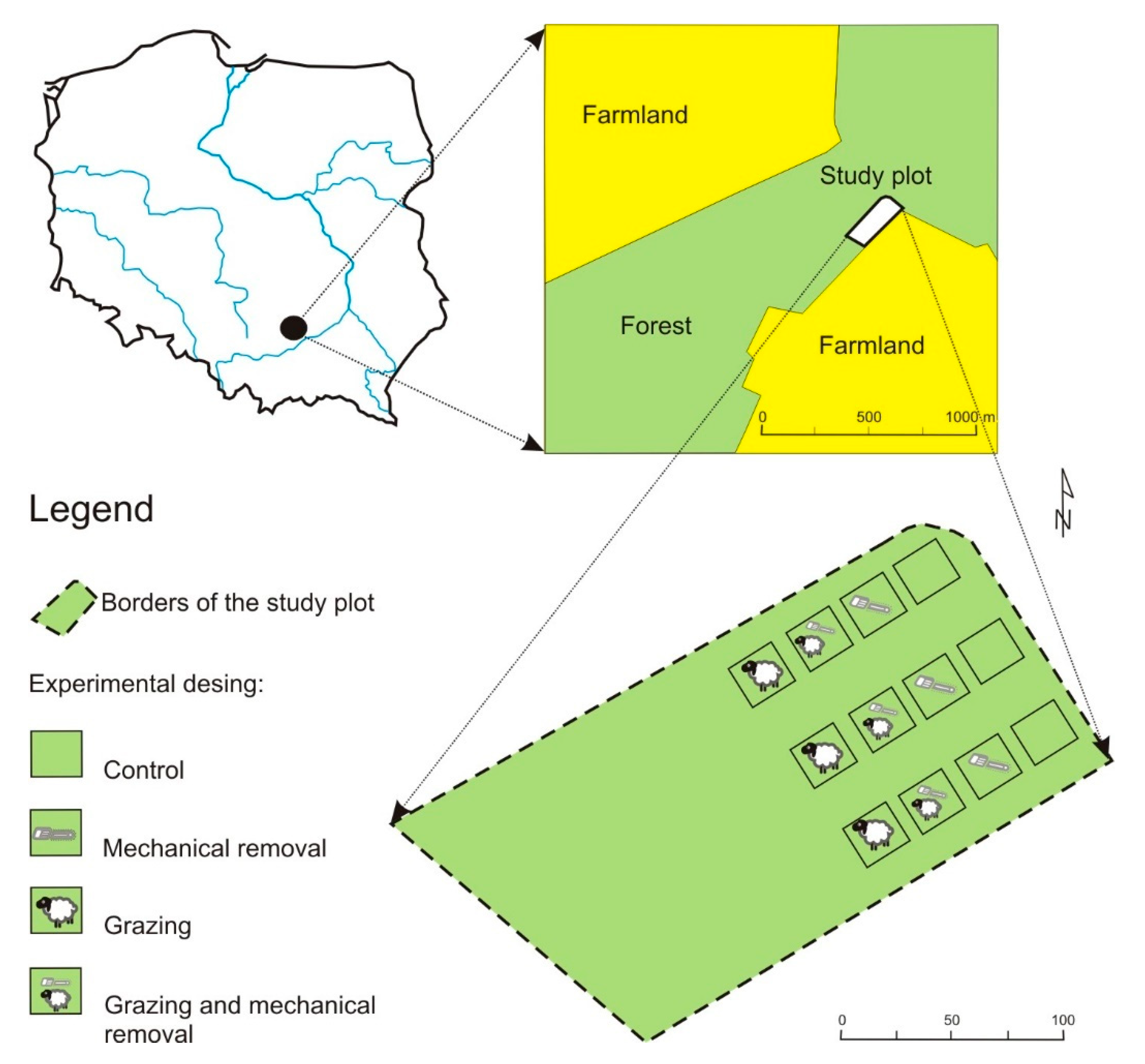
2.1. Study Area: History and Location
2.2. Methods
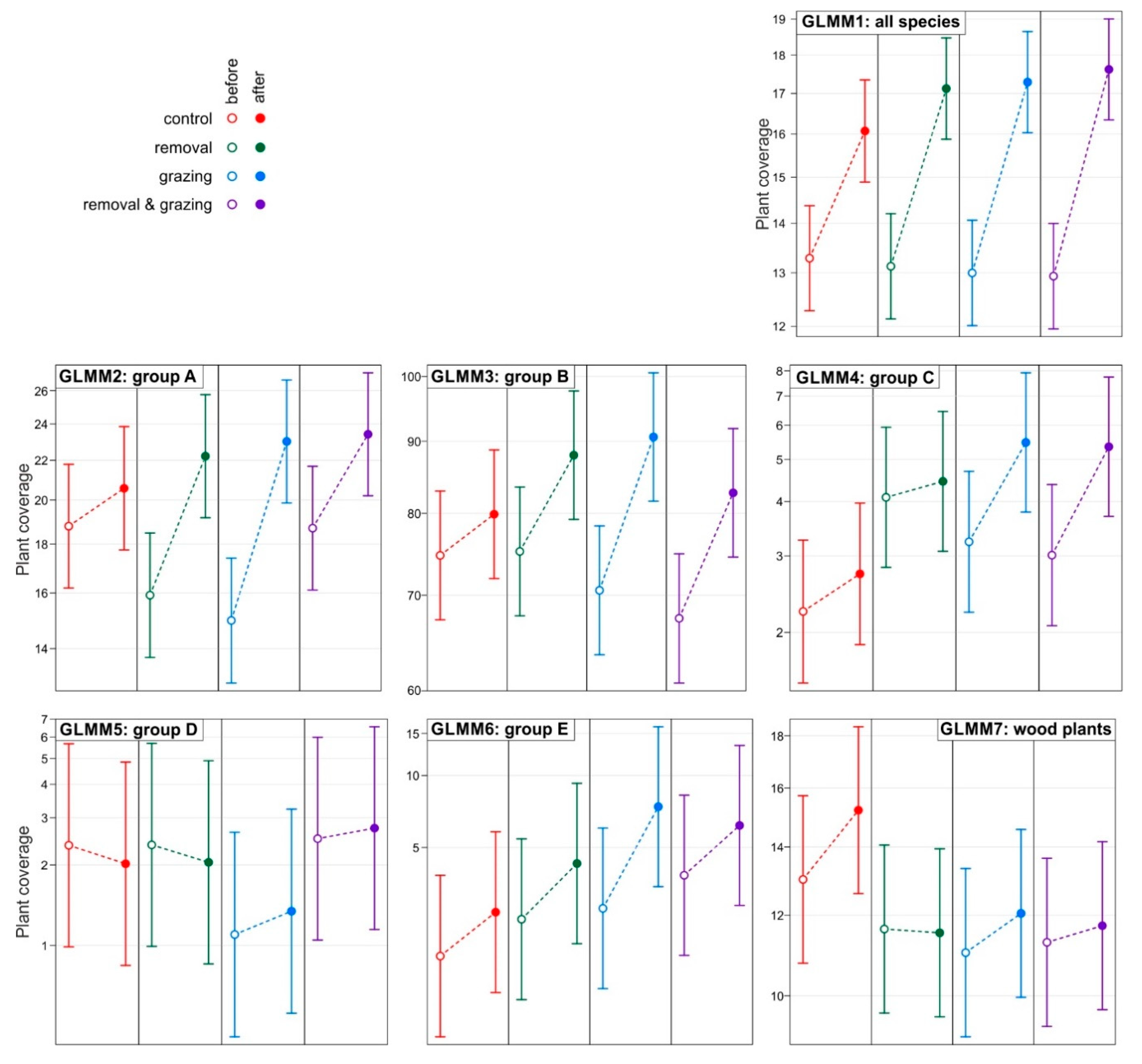
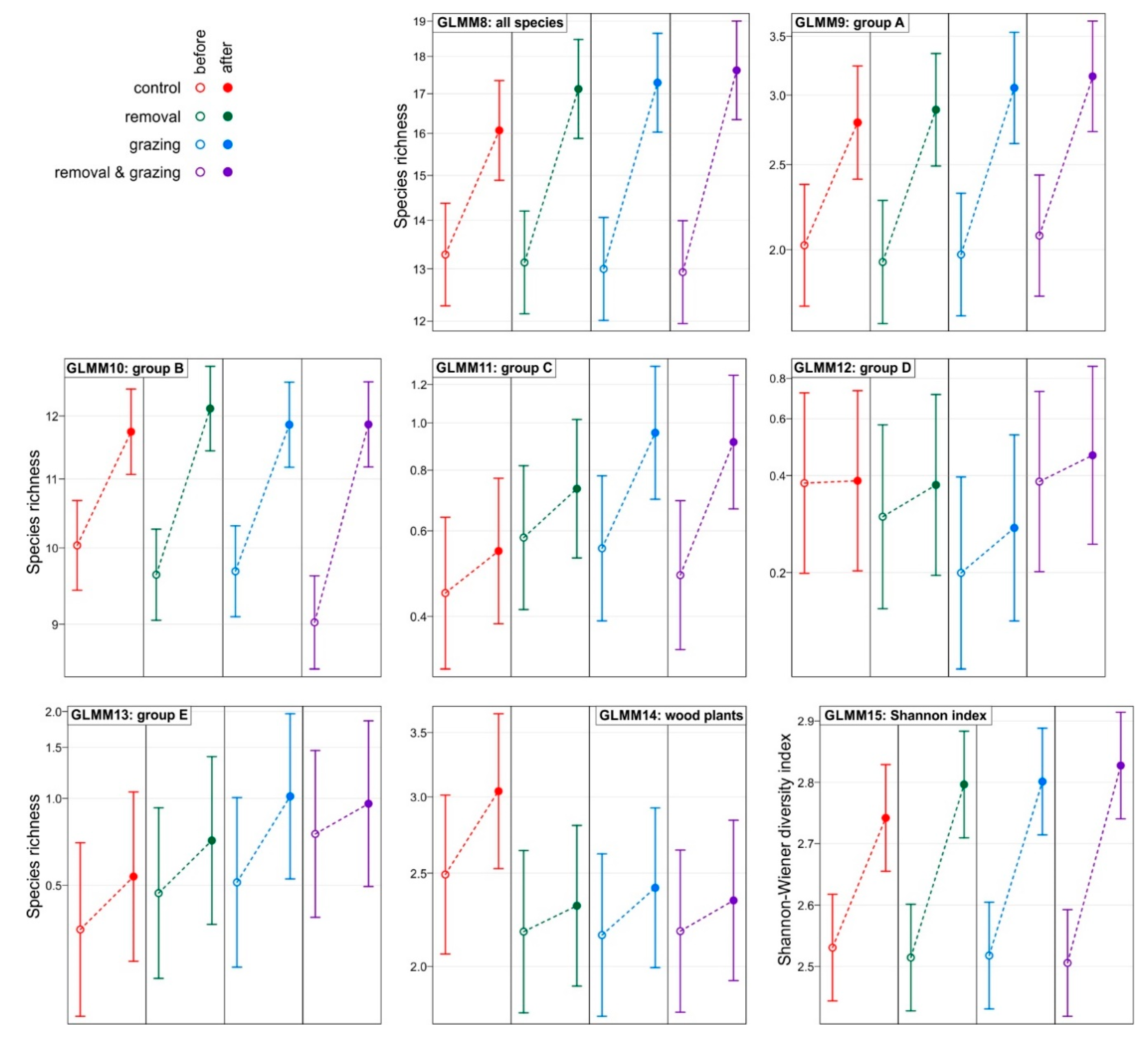
- A—species of thermophilous oak forests—stenotopic species whose occurrence defines the priority habitat “thermophilous oak forest (91I0)” from the list of natural habitats of Community importance.
- B—species of temperate deciduous forests—species with a wide ecological range growing in eutrophic and mesotrophic deciduous forests, mainly in oak-lime-hornbeam mixed deciduous forests and beech forests.
- C—species of meadows and xerothermic grasslands, typical of open habitats—xerothermic grasslands, ecotone communities between xerothermic grassland and forest, thermophilus bush communities and forest clearings.
- D—species with a wide ecological range, associated mainly with wet places, growing in alluvial forests and nitrophilic riverside thickets in shaded places.
- E—weeds (plants associated with agriculture) and alien species.
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matuszkiewicz, J.M.; Kozłowska, A.B. Przegląd fitosocjologiczny zbiorowisk leśnych Polski–ciepłolubne dąbrowy. Fragm. Flor. Geobot. 1991, 36, 203–256. [Google Scholar]
- Jakubowska-Gabara, J. Recesja Zespołu Świetlistej Dąbrowy Potentilloalbae-Queretum (Libb. 1933) w Polsce; Uniwersytet Łódzki: Łódź, Poland, 1993; pp. 1–190. [Google Scholar]
- Kurowski, J.K. Roślinność leśna. In Szata Roślinna Polski Środkowej; Kurowski, J.K., Ed.; Wydawnictwo EKO-GRAF: Łódź, Poland, 2009; pp. 103–123. [Google Scholar]
- Jakubowska-Gabara, J.; Kiedrzyński, M. Dąbrowa świetlista (ciepłolubna) Potentilloalbae—Quercetum (Libb.1933). In Czerwona Księga Roślin Województwa Łódzkiego. Zagrożone Rośliny Naczyniowe. Zagrożone Zbiorowiska Roślinne; Olaczek, R., Ed.; Ogród Botaniczny w Łodzi, Uniwersytet Łódzki: Łódź, Poland, 2012; pp. 228–229. [Google Scholar]
- Kwiatkowska, A.J.; Solińska−Górnicka, B. Changes in typological and spatial boundaries between neighbouring communities of Potentilloalbae−Quercetum and Tilio−Carpinetum. Acta Soc. Bot. Pol. 1993, 62, 59–66. [Google Scholar] [CrossRef]
- Kaźmierczakowa, R. Przemiany zespołu świetlistej dąbrowy w rezerwacie Kwiatówka na Wyżynie Małopolskiej w ciągu 25 lat ochrony. Prądnik. Prace i Materiały Muzeum im. Prof. Wł. Szafera 1991, 4, 39–48. [Google Scholar]
- Jakubowska-Gabara, J. Decline of Potentilloalbae−Quercetum (Libb. 1933) phytocoenoses in Poland. Vegetatio 1996, 124, 45–59. [Google Scholar] [CrossRef]
- Matuszkiewicz, J.M. (Ed.) Geobotaniczne Rozpoznanie Tendencji Rozwojowych Zbiorowisk Leśnych w Wybranych Regionach Polski; Monografie; PAN IGiPZ: Warszawa, Poland, 2007; Volume 8, pp. 1–978. [Google Scholar]
- Bobiec, A.; Podlaski, R.; Ortyl, B.; Korol, M.; Havryliuk, S.A.; Öllerer, K.; Ziobro, J.M.; Pilch, K.; Dychkevych, V.; Dudek, T.; et al. Top-down segregated policies undermine the maintenance of traditional wooded landscapes: Evidence from oaks at the European Union’s eastern border. Landsc. Urban Plan. 2019, 189, 247–259. [Google Scholar] [CrossRef]
- Bobiec, A.; Reif, A.; Öllerer, K. Seeing the oakscape beyond the forest: A landscape approach to the oak regeneration in Europe. Landsc. Ecol. 2018, 33, 513–528. [Google Scholar] [CrossRef] [Green Version]
- Lasota, J.; Błońska, E.; Pacanowski, P. Forest sites and forest types on rendzinas in Poland. Soil Sci. Annu. 2018, 69, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Plue, J.; Van Gils, B.; De Schrijver, A.; Peppler-Lisbach, C.; Verheyen, K.; Hermy, M. Forest herb layer response to long-term light deficit along a forest developmental series. Acta Oecologica 2013, 53, 63–72. [Google Scholar] [CrossRef]
- Brunet, J.; Falkengren-Grerup, U.; Tyler, G. Herb layer vegetation of south Swedish beech and oak forests—effects of management and soil acidity during one decade. For. Ecol. Manag. 1996, 88, 259–272. [Google Scholar] [CrossRef]
- Kwiatkowska, A.J. Zmiana presji roślinożerców jako przyczyna regresji dąbrów świetlistych w Puszczy Białowieskiej. Wiad. Ekol. 1996, 42, 137–162. [Google Scholar]
- Kwiatkowska−Falińska, A.J. The pressure of dominant herbivores and the process of secondary succession of Potentillo albae−Quercetum in Białowieża Primeval Forest. Coll. Phytosoc. 2006, 2, 185–212. [Google Scholar]
- Panter, C.J.; Dolman, P.M. Mammalian herbivores as potential seed dispersal vectors in ancient woodland fragments. Wildl. Biol. 2012, 18, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Albert, A.; Auffret, A.G.; Cosyns, E.; Cousins, S.A.O.; D’Hondt, B.; Eichberg, C.; Eycott, A.E.; Heinken, T.; Hoffmann, M.; Jaroszewicz, B.; et al. Seed dispersal by ungulates as an ecological filter: A trait-based meta-analysis. Oikos 2015, 124, 1109–1120. [Google Scholar] [CrossRef]
- Kiedrzyński, M. The impact of forest management on the flora and vegetation of old oak-stands (an example from The Spała Forests, central Poland). Nat. Conserv. 2008, 65, 51–62. [Google Scholar]
- Ávila-Ramírez, D.N.; Lara-Bueno, A.; Krishnamurthy, L.; Espinosa-Aviña, F.; Escutia-Sánchez, J.A.; Uribe-Gómez, M. Seasonal silvopastoral system with sheep in pine-oak forest: Effects on soil and vegetation. Agrofor. Syst. 2019, 93, 1637–1645. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Brunet, J.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; De Groote, R.; Verheyen, K. Interactive effects of past land use and recent forest management on the understorey community in temperate oak forests in South Sweden. J. Veg. Sci. 2019, 30, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Debussche, M.; Debussche, G.; Lepart, J. Changes in the Vegetation of Quercus pubescens Woodland after Cessation of Coppicing and Grazing. J. Veg. Sci. 2001, 12, 81. [Google Scholar] [CrossRef]
- Douglas, J.S.; de Hart, J.R.A. Forest Farming: Towards a Solution to Problems of World Hunger and 403 Conservation; Intermediate Technology Publications: London, UK, 1984. [Google Scholar]
- Nieppola, J. Long-term vegetation changes in stands of Pinus sylvestrisin southern Finland. J. Veg. Sci. 1992, 3, 475–484. [Google Scholar] [CrossRef]
- Graae, B.J.; Heskær, V.S. A comparison of understory vegetation between untouched and managed 479 deciduous forest in Denmark. For. Ecol. Manag. 1997, 96, 111–123. [Google Scholar] [CrossRef]
- Vera, F.W.M. Grazing Ecology and Forest History; C.A.B. International: Wallingford, CT, UK, 2000; pp. 1–385. [Google Scholar]
- Van Oene, H.; Van Deursen, E.J.M.; Berendse, F. Plant-Herbivore Interaction and Its Consequences for Succession in Wetland Ecosystems: A Modeling Approach. Ecosystems 1999, 2, 122–138. [Google Scholar] [CrossRef]
- Courchamp, F.; Chapuis, J.-L.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugalho, M.; LeComte, X.; Gonçalves, M.; Caldeira, M.; Branco, M. Establishing grazing and grazing-excluded patches increases plant and invertebrate diversity in a Mediterranean oak woodland. For. Ecol. Manag. 2011, 261, 2133–2139. [Google Scholar] [CrossRef]
- Chaideftou, E.; Thanos, C.A.; Bergmeier, E.; Kallimanis, A.S.; Dimopoulos, P. Seed bank composition and above-ground vegetation in response to grazing in sub-Mediterranean oak forests (NW Greece). Plant Ecol. 2009, 201, 255–265. [Google Scholar] [CrossRef]
- Ford, H.; Healey, J.; Markesteijn, L.; Smith, A.R. How does grazing management influence the functional diversity of oak woodland ecosystems? A plant trait approach. Agric. Ecosyst. Environ. 2018, 258, 154–161. [Google Scholar] [CrossRef]
- Kwiatkowska, A.J.; Wyszomirski, T. Species deletion in Potentillo albae-Quercetum phytocoenoses reversed by the removal of Carpinus betulus. Vegetatio 1990, 87, 115–126. [Google Scholar] [CrossRef]
- Kwiatkowska, A.J. Changes in the species richness, spatial pattern and species frequency associated with the decline of oak forest. Vegetatio 1994, 112, 171–180. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Alard, D.; Saguez, R.; Wattez-Franger, A.; De Foucault, B.; Delelis-Dusollier, A.; Bardat, J. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. J. Appl. Ecol. 2004, 41, 1065–1079. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Abrams, M.D. Recognizing loss of open forest ecosystems by tree densification and land use intensification in the Midwestern USA. Reg. Environ. Chang. 2018, 18, 1731–1740. [Google Scholar] [CrossRef]
- Andrzejewski, H.; Kiedrzyński, M.; Jakubowska-Gabara, J. Czynna ochrona dąbrowy świetlistej Potentillo albae Quercetum w rezerwacie Napoleonów (Polska Środkowa)—Rezultaty jednorazowego zabiegu. Studia i Materiały CEPL w Rogowie 2015, 17, 125–131. [Google Scholar]
- Daryanto, S.; Eldridge, D.J.; Throop, H.L. Managing semi-arid woodlands for carbon storage: Grazing and shrub effects on above and belowground carbon. Agric. Ecosyst. Environ. 2013, 169, 1–11. [Google Scholar] [CrossRef]
- Paszyński, J.; Kluge, M. Klimat Niecki Nidziańskiej. Studia Ośrodka Dokumentacji Fizjograficznej PAN 1986, 14, 23–43. [Google Scholar]
- Świercz, A.; Urban, J. Budowa geologiczna i pokrywa glebowa województwa świętokrzyskiego. In Ochrona Bioróżnorodności Flory Województwa Świętokrzyskiego; Jankowska-Błaszczuk, M., Czerwik-Marcinkowska, J., Eds.; Wojewódzki Fundusz Ochrony Środowiska i Gospodarki Wodnej w Kielcach: Kielce, Poland, 2014; pp. 33–74. [Google Scholar]
- Kostrowicki, A.; Solon, J. Studium Geobotaniczno-Krajobrazowe Okolic Pińczowa. Dokumentacja Geograficzna ½; PAN IgiPZ: Warszawa, Poland, 1994; pp. 151–169. [Google Scholar]
- Londo, G. The decimal scale for releves of permanent quadrats. Vegetatio 1976, 33, 61–64. [Google Scholar] [CrossRef]
- Matuszkiewicz, J.M. Zespoły Leśne Polski; Wyd. Nauk. PWN: Warszawa, Poland, 2001; pp. 1–360. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland—A Checklist; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2003; pp. 1–442. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 6 April 2020).
- RStudio Team. RStudio: Integrated Development for R; RStudio Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 6 April 2020).
- Lorite, J.; Serrano, F.; Lorenzo, A.; Cañadas, E.M.; Ballesteros, M.; Peñas, J. Rock climbing alters plant species composition, cover, and richness in Mediterranean limestone cliffs. PLoS ONE 2017, 12, e0182414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, F.J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package Version 2.5-6. 2019. Available online: https://github.com/vegandevs/vegan (accessed on 6 April 2020).
- Andrzejewski, H. Changes in the species composition and structure of the herb layer of a thermophilous oak forest subject to clear cutting. Acta Soc. Bot. Pol. 2014, 56, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowska−Falińska, A.J.; Panufnik−Mędrzycka, D.; Wódkiewicz, M.; Sondej, I.; Jaroszewicz, B. Ancient forest species and the diversity of vegetation and seed bank indicate the aptitude of transformed thermophilous oak wood patches for restoration. Pol. J. Ecol. 2013, 61, 65–78. [Google Scholar]
- Kwiatkowska, A.J.; Spalik, K.; Michalak, E.; Palińska, A.; Panufnik-Mędrzycka, D. Influence of the size and density of Carpinus betulus on the spatial distribution and rate of deletion of forest-floor species in thermophilous oak forest. Plant Ecol. 1997, 129, 1–10. [Google Scholar] [CrossRef]
- Kwiatkowska-Falińska, A.J.; Panufnik-Mędrzycka, D.; Wodkiewicz, M.; Sondej, I.; Jaroszewicz, B. The effects of different types of woodstand disturbance on the persistence of soil seed banks. Acta Soc. Bot. Pol. 2011, 80, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, J.; Hošek, J.; Modrý, M.; Roleček, J. The influence of light and nutrient availability on herb layer species richness in oak-dominated forests in central Bohemia. Plant Ecol. 2009, 205, 57–75. [Google Scholar] [CrossRef]
- Axmanová, I.; Zelený, D.; Li, C.-F.; Chytrý, M. Environmental factors influencing herb layer productivity in Central European oak forests: Insights from soil and biomass analyses and a phytometer experiment. Plant Soil 2011, 342, 183–194. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Czerepko, J.; et al. Light availability and land-use history drive biodiversity and functional changes in forest herb layer communities. J. Ecol. 2020. [Google Scholar] [CrossRef]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2011, 100, 352–365. [Google Scholar] [CrossRef]
- McEvoy, P.; Flexen, M.; McAdam, J. The effects of livestock grazing on ground flora in broadleaf woodlands in Northern Ireland. For. Ecol. Manag. 2006, 225, 39–50. [Google Scholar] [CrossRef]
- Bobiec, A.; van der Burgt, H.; Meijer, K.; Zuyderduyn, C.; Haga, J.; Vlaanderen, B. Rich deciduous forests in Bialowieza as a dynamic mosaic of developmental phases: Premises for nature conservation and restoration management. For. Ecol. Manag. 2000, 130, 159–175. [Google Scholar] [CrossRef]
- Král, K.; Daněk, P.; Janík, D.; Krůček, M.; Vrška, T. How cyclical and predictable are Central European temperate forest dynamics in terms of development phases? J. Veg. Sci. 2017, 29, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Hermy, M.; Honnay, O.; Firbank, L.; Grashof-Bokdam, C.; Lawesson, J.E.; Firbank, L. An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol. Conserv. 1999, 91, 9–22. [Google Scholar] [CrossRef]
- De Frenne, P.; Baeten, L.; Graae, B.J.; Brunet, J.; Wulf, M.; Orczewska, A.; Kolb, A.; Jansen, I.; Jamoneau, A.; Jacquemyn, H.; et al. Interregional variation in the floristic recovery of post-agricultural forests. J. Ecol. 2010, 99. [Google Scholar] [CrossRef]
- Vandvik, V.; Klanderud, K.; Meineri, E.; Måren, I.; Töpper, J. Seed banks are biodiversity reservoirs: Species-area relationships above versus below ground. Oikos 2015, 125, 218–228. [Google Scholar] [CrossRef] [Green Version]

| Model: | GLMM1 | GLMM2 | GLMM3 | GLMM4 | GLMM5 | GLMM6 | GLMM7 |
|---|---|---|---|---|---|---|---|
| Response: | All Species | Group A | Group B | Group C | Group D | Group E | Wood |
| Main effects | |||||||
| Intercept | 1.95 (0.03) *** | 2.93 (0.08) *** | 4.31 (0.06) *** | 0.80 (0.21) *** | 0.81 (0.47) ^ | 0.56 (0.41) | 2.56 (0.10) *** |
| Year: after | 0.01 (0.03) | 0.10 (0.08) | 0.07 (0.04) ^ | 0.21 (0.20) | −0.05 (0.26) | 0.42 (0.18) * | 0.17 (0.06) ** |
| Removal: yes | −0.02 (0.04) | −0.16 (0.12) | 0.01 (0.08) | 0.63 (0.30) * | −0.14 (0.67) | 0.38 (0.58) | −0.11 (0.14) |
| Grazing: yes | −0.07 (0.04) ^ | −0.22 (0.12) ^ | −0.06 (0.08) | 0.38 (0.30) | −0.85 (0.67) | 0.37 (0.58) | −0.16 (0.14) |
| 2-way interactions | |||||||
| Year * Removal | 0.11 (0.05) * | 0.23 (0.11) * | 0.09 (0.05) ^ | −0.14 (0.28) | 0.28 (0.37) | 0.07 (0.25) | −0.18 (0.09) * |
| Grazing * Removal | 0.07 (0.06) | 0.38 (0.16) * | −0.05 (0.11) | −0.69 (0.42) ^ | 1.07 (0.95) | −0.00 (0.82) | 0.13 (0.20) |
| Year * Grazing | 0.18 (0.05) *** | 0.33 (0.11) ** | 0.19 (0.05) *** | 0.31 (0.28) | 0.48 (0.38) | 0.69 (0.25) ** | −0.08 (0.09) |
| 3-way interaction | |||||||
| Year * Grazing * Removal | −0.19 (0.06) ** | −0.43 (0.16) ** | −0.14 (0.07) ^ | 0.18 (0.39) | −0.57 (0.53) | −0.65 (0.35) ^ | 0.13 (0.13) |
| Model: | GLMM8 | GLMM9 | GLMM10 | GLMM11 | GLMM12 | GLMM13 | GLMM14 |
|---|---|---|---|---|---|---|---|
| Response: | All Species | Group A | Group B | Group C | Group D | Group E | Wood |
| Main effects | |||||||
| Intercept | 2.59 (0.04) *** | 0.70 (0.08) *** | 2.31 (0.03) *** | −0.81 (0.18) *** | −0.97 (0.33) ** | −1.05 (0.35) ** | 0.91 (0.10) *** |
| Year: after | 0.19 (0.03) *** | 0.32 (0.08) *** | 0.16 (0.04) *** | 0.20 (0.16) | 0.02 (0.18) | 0.42 (0.17) * | 0.20 (0.07) ** |
| Removal: yes | −0.01 (0.06) | −0.04 (0.12) | −0.04 (0.05) | 0.26 (0.25) | −0.24 (0.47) | 0.29 (0.50) | −0.14 (0.14) |
| Grazing: yes | −0.02 (0.06) | −0.02 (0.12) | −0.04 (0.05) | 0.21 (0.25) | −0.64 (0.48) | 0.38 (0.50) | −0.14 (0.14) |
| 2-way interactions | |||||||
| Year * Removal | 0.08 (0.04) ^ | 0.08 (0.11) | 0.07 (0.05) | 0.03 (0.21) | 0.21 (0.26) | −0.00 (0.22) | −0.14 (0.10) |
| Grazing * Removal | 0.01 (0.08) | 0.09 (0.16) | −0.03 (0.06) | −0.39 (0.36) | 0.89 (0.67) | 0.10 (0.69) | 0.15 (0.20) |
| Year * Grazing | 0.10 (0.04) * | 0.12 (0.11) | 0.05 (0.05) | 0.35 (0.21) | 0.30 (0.28) | 0.26 (0.22) | −0.09 (0.10) |
| 3-way interaction | |||||||
| Year * Grazing * Removal | −0.05 (0.06) | −0.10 (0.15) | −0.00 (0.07) | 0.05 (0.29) | −0.34 (0.37) | −0.44 (0.28) | −0.10 (0.15) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroszewicz, B.; Jankowska-Błaszczuk, M.; Żmihorski, M.; Hałatkiewicz, T. An Efficient Tool for the Maintenance of Thermophilous Oak Forest Understory—Sheep or Brush Cutter? Forests 2020, 11, 582. https://doi.org/10.3390/f11050582
Jaroszewicz B, Jankowska-Błaszczuk M, Żmihorski M, Hałatkiewicz T. An Efficient Tool for the Maintenance of Thermophilous Oak Forest Understory—Sheep or Brush Cutter? Forests. 2020; 11(5):582. https://doi.org/10.3390/f11050582
Chicago/Turabian StyleJaroszewicz, Bogdan, Małgorzata Jankowska-Błaszczuk, Michał Żmihorski, and Tomasz Hałatkiewicz. 2020. "An Efficient Tool for the Maintenance of Thermophilous Oak Forest Understory—Sheep or Brush Cutter?" Forests 11, no. 5: 582. https://doi.org/10.3390/f11050582
APA StyleJaroszewicz, B., Jankowska-Błaszczuk, M., Żmihorski, M., & Hałatkiewicz, T. (2020). An Efficient Tool for the Maintenance of Thermophilous Oak Forest Understory—Sheep or Brush Cutter? Forests, 11(5), 582. https://doi.org/10.3390/f11050582






