Comparison of Mangrove Stand Development on Accretion and Erosion Sites in Ca Mau, Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Plot Establishment and Measurement of Trees, Saplings, and Seedlings
2.3. Data Analysis
3. Results
3.1. Grouping of Stands
3.2. Structural Characteristics of Canopy Layer
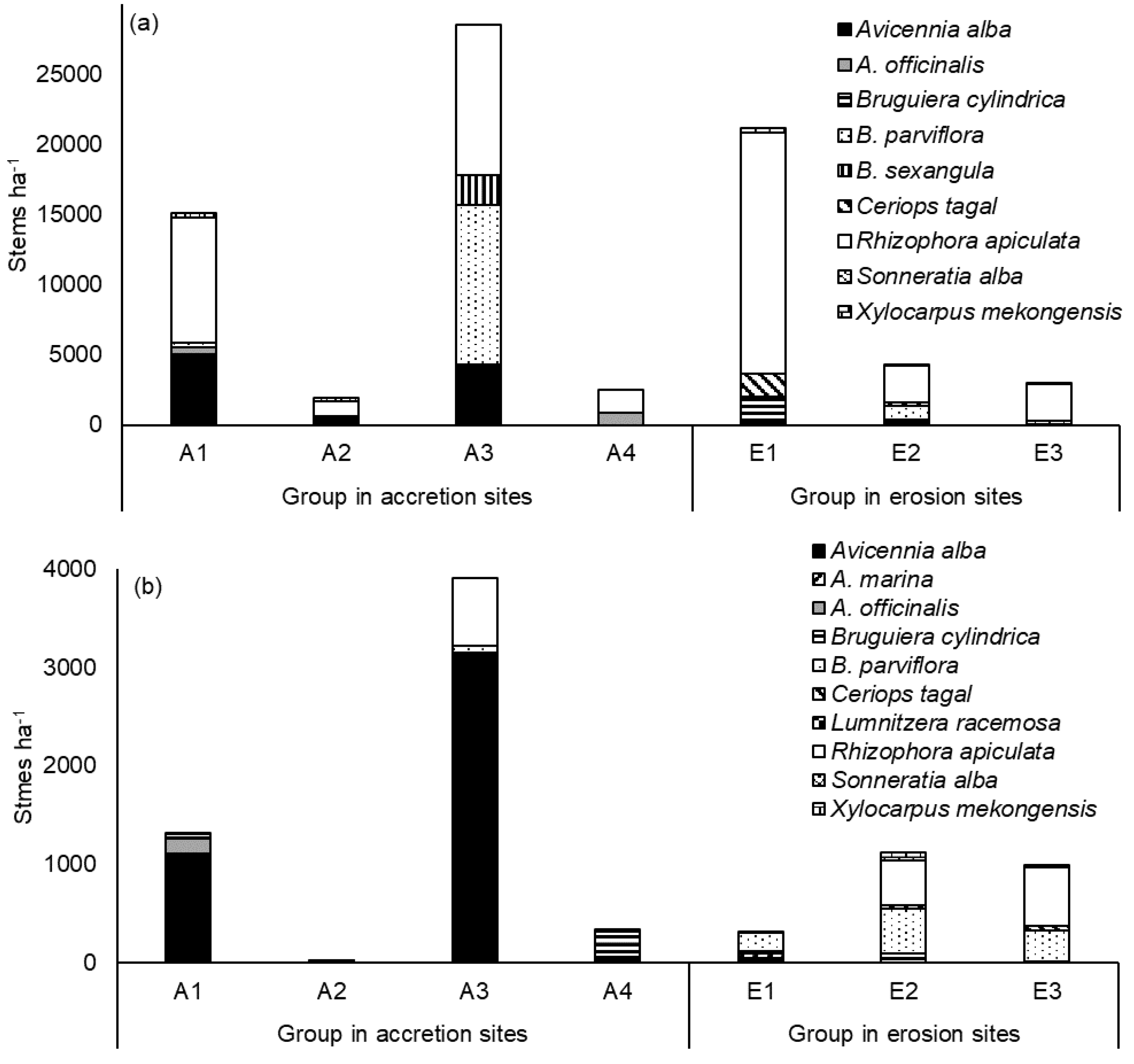
3.2.1. Species Composition in Each Group
3.2.2. DBH Distribution by Species in Accretion Sites
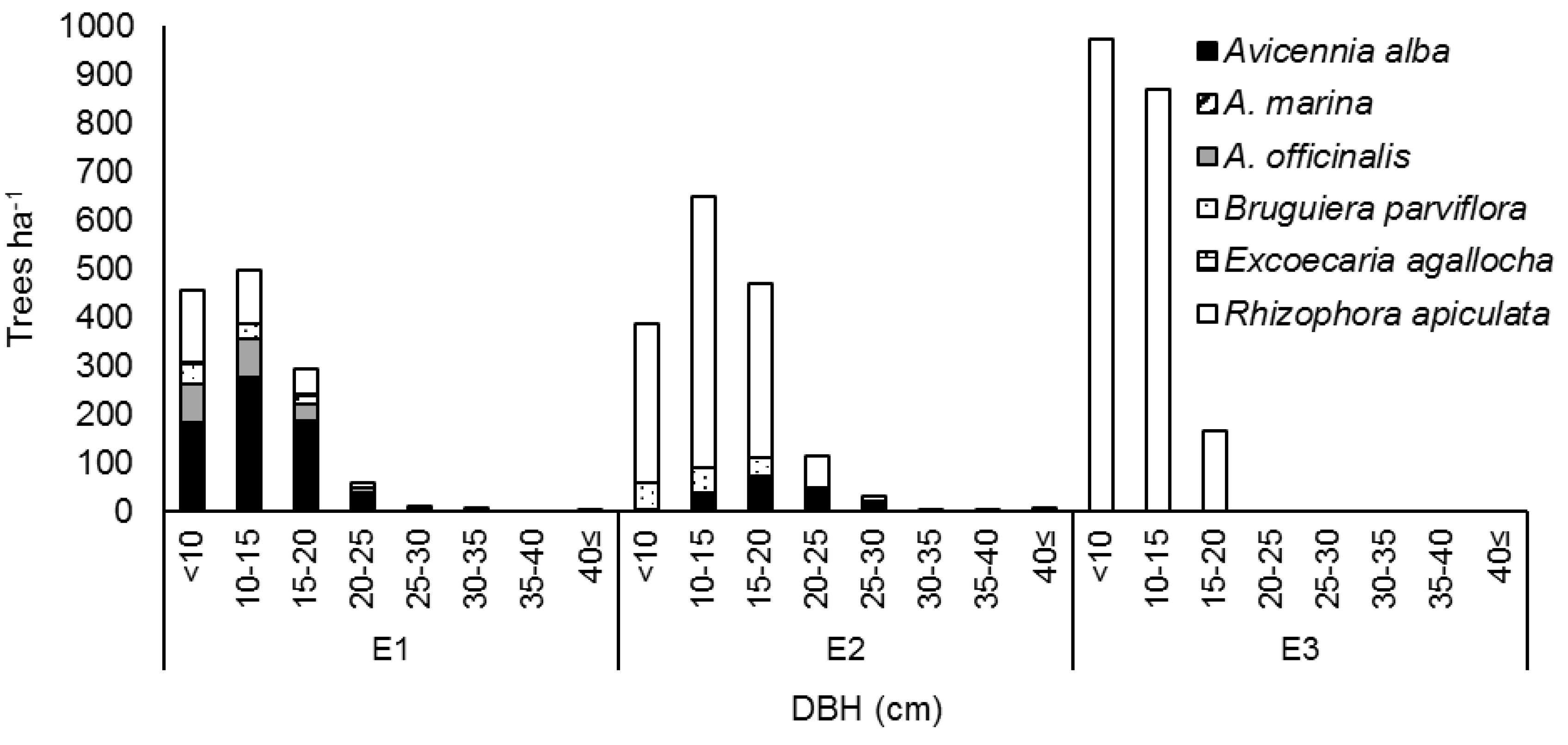
3.2.3. DBH Distribution by Species in Erosion Sites
3.3. Regeneration on Accretion and Erosion Sites
3.4. Correlation of Structural Variables
4. Discussion
4.1. Effects of Accretion and Erosion on Mangrove Structure
4.2. Development of Estuarian Mangrove Forests on Accretion Sites
4.3. Mosaic Pattern of Mangrove Forests on Erosion Sites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Andreetta, A.; Fusi, M.; Cameldi, I.; Cimò, F.; Carnicelli, S.; Cannicci, S. Mangrove carbon sink. Do burrowing crabs contribute to sediment carbon storage? Evidence from a Kenyan mangrove system. J. Sea Res. 2014, 85, 524–533. [Google Scholar] [CrossRef]
- Li, Y.-F.; Du, F.-Y.; Gu, Y.-G.; Ning, J.-J.; Wang, L.-G. Changes of the macrobenthic faunal community with stand age of a non-native mangrove species in Futian Mangrove National Nature Reserve, Guangdong, China. Zool. Stud. 2017, 56, e19. [Google Scholar] [PubMed]
- Lugo, A.E.; Snedaker, S.C. The ecology of mangroves. Ann. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Emma, A.; Richard, L.; Catherine, T.; Peter, B. Mangrove response to environmental change in Australia’s Gulf of Carpentaria. Ecol. Evol. 2016, 6, 3523–3539. [Google Scholar]
- Nitto, D.D.; Neukermans, G.; Koedam, N.; Defever, H.; Pattyn, F.; Kairo, J.G.; Dahdouh-Guebas, F. Mangroves facing climate change: Landward migration potential in response to projected scenarios of sea level rise. Biogeosciences 2014, 11, 857–871. [Google Scholar] [CrossRef]
- Dyer, K.R. Fine sediment particle transport in estuaries. In Physical Processes in Estuaries; van Leussen, W., Dronkers, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 295–310. [Google Scholar]
- Baas, A.C.W. Chaos, fractals and self-organization in coastal geomorphology: Simulating dune landscapes in vegetated environments. Geomorphology 2002, 48, 309–328. [Google Scholar] [CrossRef]
- Plaziat, J.; Augustinus, P. Evolution of progradation/erosion along the French Guiana mangrove coast: A comparison of mapped shorelines since the 18th century with Holocene data. Mar. Geol. 2004, 208, 127–143. [Google Scholar] [CrossRef]
- Nascimento, W.R., Jr.; Souza-Filho, P.W.M.; Proisy, C.; Lucas, R.M.; Rosenqvist, A. Mapping changes in the largest continuous Amazonian mangrove belt using object-based classification of multisensor satellite imagery. Estuar. Coast. Shelf Sci. 2013, 117, 83–93. [Google Scholar] [CrossRef]
- Asp, N.E.; Gomes, V.J.C.; Schettini, C.A.F.; Souza-Filho, P.W.M.; Siegle, E.; Ogston, A.S.; Nittrouer, C.A.; Silva, J.N.S.; Nascimento, W.R., Jr.; Souza, S.R.; et al. Sediment dynamics of a tropical tide-dominated estuary: Turbidity maximum, mangroves and the role of the Amazon River sediment load. Estuar. Coast. Shelf Sci. 2018, 214, 10–24. [Google Scholar] [CrossRef]
- Carlton, J. Land-building and stabilization by mangroves. Environ. Conserv. 1974, 1, 285–294. [Google Scholar] [CrossRef]
- Rogers, K.; Saintilan, N.; Heijnis, H. Mangrove encroachment of salt marsh in Western Port Bay, Victoria: The role of sedimentation, subsidence, and sea level rise. Estuaries 2005, 28, 551–559. [Google Scholar] [CrossRef]
- Krishna Prasad, M.B.; Ramanathan, A.L. Sedimentary nutrient dynamics in a tropical estuarine mangrove ecosystem. Estuar. Coast. Shelf Sci. 2008, 80, 60–66. [Google Scholar] [CrossRef]
- Reed, D.J. The impact of sea-level rise on coastal salt marshes. Prog. Phys. Geog. 1990, 14, 465–481. [Google Scholar] [CrossRef]
- Trincardi, F.; Correggiari, A.; Roveri, M. Late Quaternary transgressive erosion and deposition in a modern epicontinental shel: The Adriatic semienclosed basin. Geo-Mar. Lett. 1994, 14, 41–51. [Google Scholar] [CrossRef]
- Choowong, M.; Murakoshi, N.; Hisada, K.; Charusiri, P.; Daorerk, V.; Charoentitirat, T.; Chutakositkanon, V.; Jankaew, K.; Kanjanapayont, P. Erosion and deposition by the 2004 Indian Ocean tsunami in Phuket and Phang-nga Provinces, Thailand. J. Coast. Res. 2007, 23, 1270–1276. [Google Scholar] [CrossRef]
- Breanyn, M.; Joanne, B.; Pinegina, T.K.; Ekaterina, A.K. Tsunami geomorphology: Erosion and deposition from the 15 November 2006 Kuril Island tsunami. Geology 2009, 37, 995–998. [Google Scholar]
- Malini, B.H.; Rao, K.N. Coastal erosion and habitat loss along the Godavari delta front—A fallout of dam construction. Curr. Sci. 2004, 87, 1232–1236. [Google Scholar]
- Saengsupavanich, C.; Chonwattana, S.; Naimsampao, T. Coastal erosion through integrated management: A case of Southern Thailand. Ocean Coast. Manag. 2009, 52, 307–316. [Google Scholar] [CrossRef]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Thomas, N.; Lucas, R.; Bunting, P.; Hardy, A.; Rosenqvist, A.; Simard, M. Distribution and drivers of global mangrove forest change, 1996–2010. PLoS ONE 2017, 12, e0179302. [Google Scholar] [CrossRef] [PubMed]
- Mazda, Y.; Magi, M.; Nanao, H.; Kogo, M.; Miyagi, T.; Kanazawa, N.; Kobashi, D. Coastal erosion due to long-term human impact on mangrove forests. Wetl. Ecol. Manag. 2002, 10, 1–9. [Google Scholar] [CrossRef]
- Semeniuk, V. Mangrove zonation along an eroding coastline in King Sound, North-Western Australia. J. Ecol. 1980, 68, 789–812. [Google Scholar] [CrossRef]
- Nardin, W.; Locatelli, S.; Pasquarella, V.; Rulli, M.C.; Woodcock, C.E.; Fagherazzi, S. Dynamics of a fringe mangrove forest detected by Landsat images in the Mekong River Delta, Vietnam. Earth Surf. Proc. Landf. 2016, 41, 2024–2037. [Google Scholar] [CrossRef]
- Thampanya, U.; Vermaat, J.E.; sinsakul, S.; Panapitukkul, N. Coastal erosion and mangrove progradation of Southern Thailand. For. Ecol. Manag. 2006, 68, 75–85. [Google Scholar] [CrossRef]
- Nguyen, H.-H.; Nghia, N.H.; Nguyen, H.T.T.; Le, A.T.; Tran, L.T.N.; Duong, L.V.K.; Bohm, S.; Furniss, M.J. Classification methods for mapping mangrove extents and drivers of change in Thanh Hoa Province, Vietnam during 2005–2018. For. Soc. 2020, 4, 225–242. [Google Scholar]
- Baldwin, H.A.; Egnotovich, M.; Ford, M.; Platt, W.J. Regeneration in fringe mangrove forests damaged by Hurricane Andrew. Plant Ecol. 2001, 157, 151–164. [Google Scholar] [CrossRef]
- Clarke, P.J.; Allaway, W.G. The regeneration niche of the grey mangrove (Avicennia marina): Effects of salinity, light and sediment factors on establishment, growth and survival in the field. Oecologia 1993, 93, 548–556. [Google Scholar] [CrossRef]
- Sherman, R.E.; Fahey, T.J.; Battles, J.J. Small-scale disturbance and regeneration dynamics in a neotropical mangrove forest. J. Ecol. 2000, 88, 165–178. [Google Scholar] [CrossRef]
- Joshi, H.; Ghose, M. Forest structure and species distribution along soil salinity and pH gradient in mangrove swamps of the Sundarbans. J. Trop. Ecol. 2003, 2, 197–206. [Google Scholar]
- Wakushima, S.; Kuraishi, S.; Sakurai, N. Soil salinity and pH in Japanese mangrove forests and growth of cultivated mangrove plants in different soil conditions. J. Plant Res. 1994, 107, 39–46. [Google Scholar] [CrossRef]
- Kitaya, Y.; Jintana, V.; Piriyayotha, S.; Jaijing, D.; Yabuki, K.; Izutani, S.; Nishimiya, A.; Iwasaki, M. Early growth of seven mangrove species planted at different elevations in a Thai estuary. Trees 2002, 16, 150–154. [Google Scholar] [CrossRef]
- Pi, N.; Tam, N.F.Y.; Wu, Y.; Wrong, M.H. Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquat. Bot. 2009, 90, 222–230. [Google Scholar] [CrossRef]
- Estrada, G.; Mário, L.; Filipe, O.; Viviane, F. Analysis of the structural variability of mangrove forests through the physiographic types approach. Aquat. Bot. 2013, 111, 135–143. [Google Scholar] [CrossRef]
- Fromard, F.; Puig, H.; Mougin, E.; Marty, G.; Betoulle, J.L.; Cadamuro, L. Structure, above-ground biomass and dynamics of mangrove ecosystems: New data from French Guiana. Oecologia 1998, 115, 39–53. [Google Scholar] [CrossRef]
- Southern Institute of Water Resources Planning. Final Report to Japan International Cooperation Agency: The Project for Climate Change Adaptation for Agriculture and Rural Sustainable Development of Coastal Area of Cuu Long Delta, Vietnam; Southern Institute of Water Resources Planning: Ho Chi Minh, Vietnam, 2013; p. 252. [Google Scholar]
- Thao, V.N. Research on the Structure and Nutrient at Ong Trang Mangrove Islet, Ca Mau Province, in Environmental Soil and Water. Ph.D. Thesis, Can Tho University, Can Tho, Vietnam, 2017. [Google Scholar]
- Vu, P.T.; Minh, V.Q.; Tri, L.Q.; Thang, Q.T. Soils of the Mekong delta classified by WRB-FAO (2006) classification system. Can Tho Univ. J. Sci. 2011, 18b, 10–17. [Google Scholar]
- Hien, N.M. Research the Changes in Coastal Shoreline of Ca Mau, Vietnam from Landsat Database; Hanoi University of Natural Resources and Environment: Hanoi, Vietnam, 2017. [Google Scholar]
- Ministry of Agriculture and Rural Department. Mangrove Forest Inventory Report; Ministry of Agriculture and Rural Department: Hanoi, Vietnam, 2016.
- Tri, N.H. Mangrove Species of Vietnam; Education Publishing House of Hanoi: Hanoi, Vietnam, 1996. [Google Scholar]
- Southern Institute of Water Resources Research. Verifying the Erosion Status in Provinces of Bac Lieu and Ca Mau; Southern Institute of Water Resources Planning: Ho Chi Minh, Vietnam, 2018. [Google Scholar]
- Tran Thi, V.; Tien Thi Xuan, A.; Phan Nguyen, H.; Dahdouh-Guebas, F.; Koedam, N. Application of remote sensing and GIS for detection of long-term mangrove shoreline changes in Mui Ca Mau, Vietnam. Biogeosciences 2014, 11, 3781–3795. [Google Scholar] [CrossRef]
- Lap, N.V.; Oanh, N.T.K. Sedimentary characteristics of tidal flats and coastline changes in Ca Mau coastal area, Mekong River Delta. Vietnam J. Earth Sci. 2012, 34, 1–9. [Google Scholar]
- Ohira, W.; Honda, K.; Nagai, M.; Ratanasuwan, A. Mangrove stilt root morphology modeling for estimating hydraulic drag in tsunami inundation simulation. Trees 2013, 27, 141–148. [Google Scholar] [CrossRef]
- Abonyi, J.; Feil, B. Cluster Analysis for Data Mining and System Identification; Birkhäuser Verlag AG: Berlin, Germany, 2007. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Twilley, R.R. Mangrove wetlands. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2198–2208. [Google Scholar]
- Duke, N. Gap creation and regenerative processes driving diversity and structure of mangrove ecosystems. Wetl. Ecol. Manag. 2001, 9, 267–279. [Google Scholar] [CrossRef]
- Terrados, J.; Thampanya, U.; Srichai, N.; Kheowvongsri, P.; Geertz-Hansen, O.; Boromthanarath, S.; Panapitukkul, N.; Duarte, C.M. The effect of increased sediment accretion on the survival and growth of Rhizophora apiculata seedlings. Estuar. Coast. Shelf Sci. 1997, 45, 697–701. [Google Scholar] [CrossRef]
- Affandi, N.A.M.; Kamali, B.; Mz, R.; Tamin, N.M.; Hashim, R. Early growth and survival of Avicennia alba seedlings under excessive sedimentation. Sci. Res. Essays 2010, 5, 2801–2805. [Google Scholar]
- Balke, T.; Webb, E.; Elzen, E.; Galli, D.; Herman, P.; Bouma, T. Seedling establishment in a dynamic sedimentary environment: A conceptual framework using mangroves. J. Appl. Ecol. 2013, 50, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, S.; Nordhaus, I.; Geist, S.J. Status, diversity and distribution patterns of mangrove vegetation in the Segara Anakan lagoon, Java, Indonesia. Reg. Environ. Chang. 2009, 9, 275–289. [Google Scholar] [CrossRef]
- Panapitukkul, N.; Duarte, C.M.; Thampanya, U.; Kheowvongsri, P.; Srichai, N.; Geertz-Hansen, O.; Terrados, J.; Boromthanarath, S. Mangrove colonization: Mangrove progression over the growing Pak Phanang (SE Thailand) mud flat. Estuar. Coast. Shelf Sci. 1998, 47, 51–61. [Google Scholar] [CrossRef]
- Lovelock, C.; Sorrell, B.; Hancock, N.; Hua, Q.; Swales, A. Mangrove forest and soil development on a rapidly accreting shore in New Zealand. Ecosystems 2010, 13, 437–451. [Google Scholar] [CrossRef]
- Faas, R.W. Mudbanks of the southwest coast of India III: Role of non-Newtonian flow properties in the generation and maintenance of mudbanks. J. Coast. Res. 1995, 11, 911–917. [Google Scholar]
- Hatton, J.C.; Couto, A.L. The effect of coastline changes on mangrove community structure, Portuguese Island, Mozambique. Hydrobiologia 1992, 247, 49–57. [Google Scholar] [CrossRef]
- Rodtassana, C.; Poungparn, S. Quantitative analysis of the root system of Avicennia alba based on the pipe model theory. Sci. Asia 2012, 38, 414–418. [Google Scholar] [CrossRef]
- Dalling, J.W. Pioneer species. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2779–2782. [Google Scholar]
- Tan, T.D. Research on Influence of Ecological Environmental Drivers on Adaptability of Mangrove Species at Con Ngoai Ong Trang, Ca Mau; Forest Science Institute of South Vietnam: Ho Chi Minh, Vietnam, 2008.
- Lee, K.; Tan, W.H.; Havanond, S. Regeneration and colonisation of mangrove on clay-filled reclaimed land in Singapore. Hydrobiologia 1996, 319, 23–35. [Google Scholar] [CrossRef]
- Koh, H.; Teh, S.; Kh’ng, X.; Raja, B.R. Protection against and resilience to coastal disturbances mangrove forests. J. Trop. For. Sci. 2018, 30, 446–460. [Google Scholar] [CrossRef]
- Matsui, N.; Songsangjinda, P.; Keiyo, M. Mangrove rehabilitation on highly eroded coastal shorelines at Samut Sakhon, Thailand. Int. J. Ecol. 2012, 2012, 1–11. [Google Scholar]
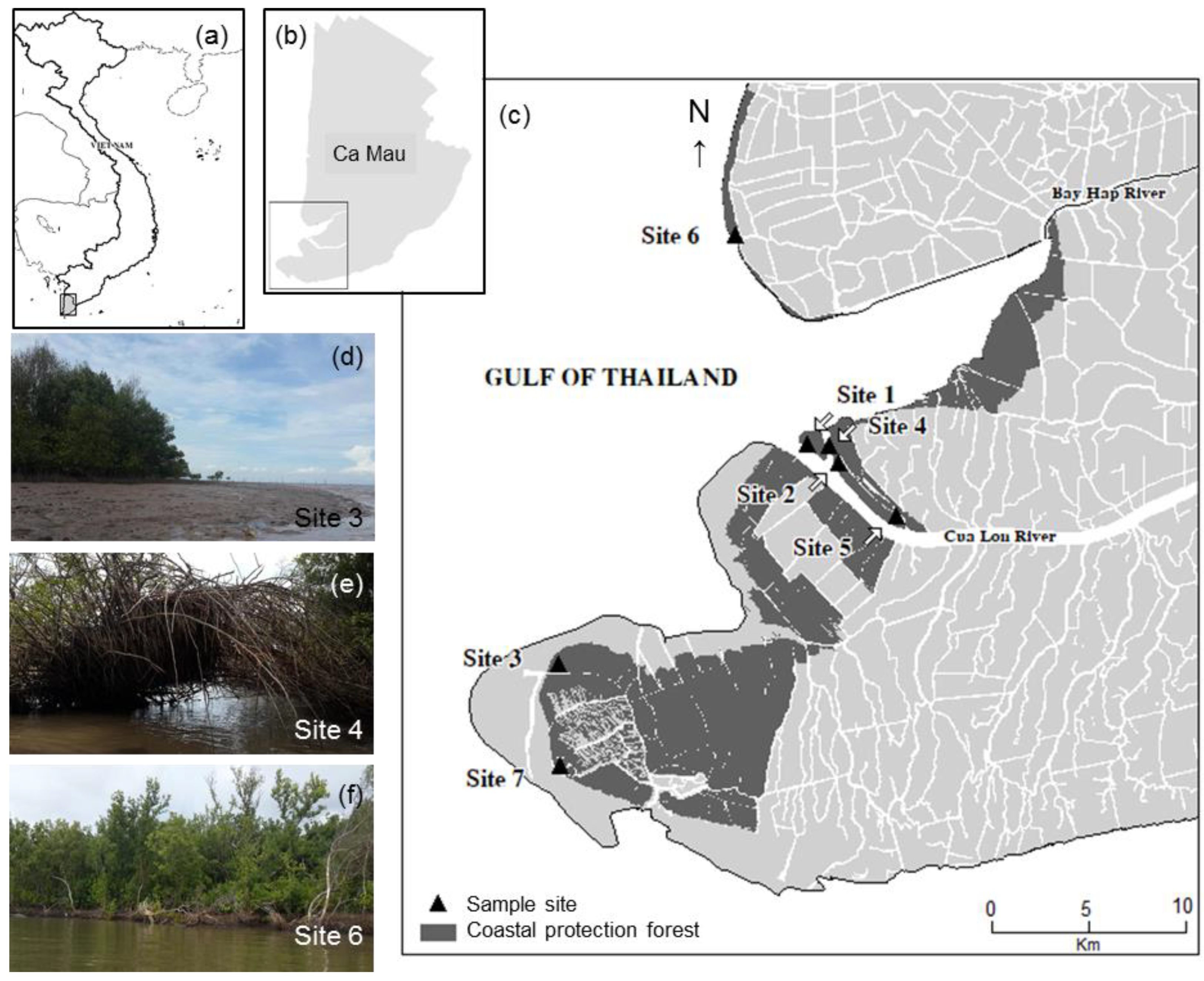
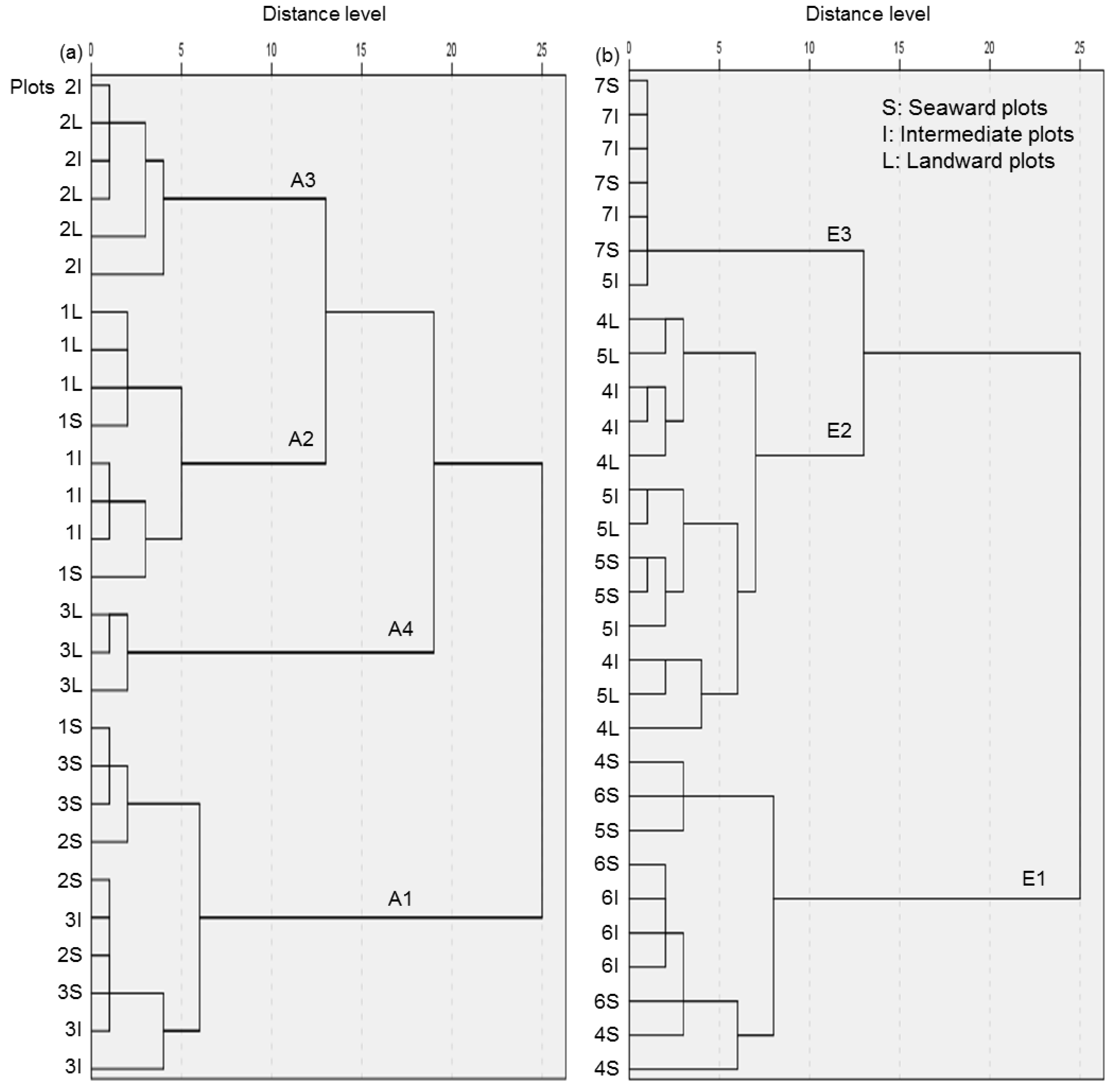
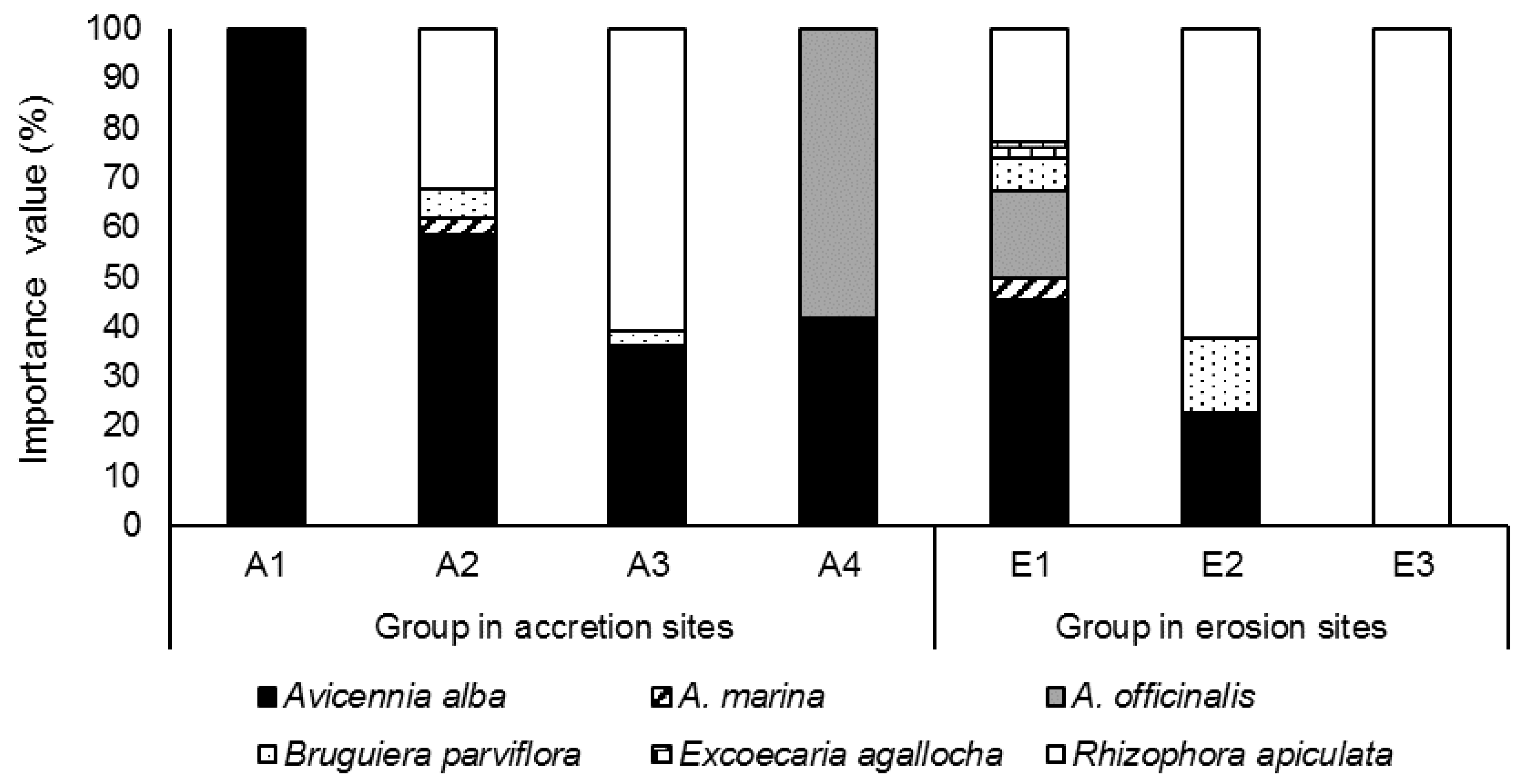
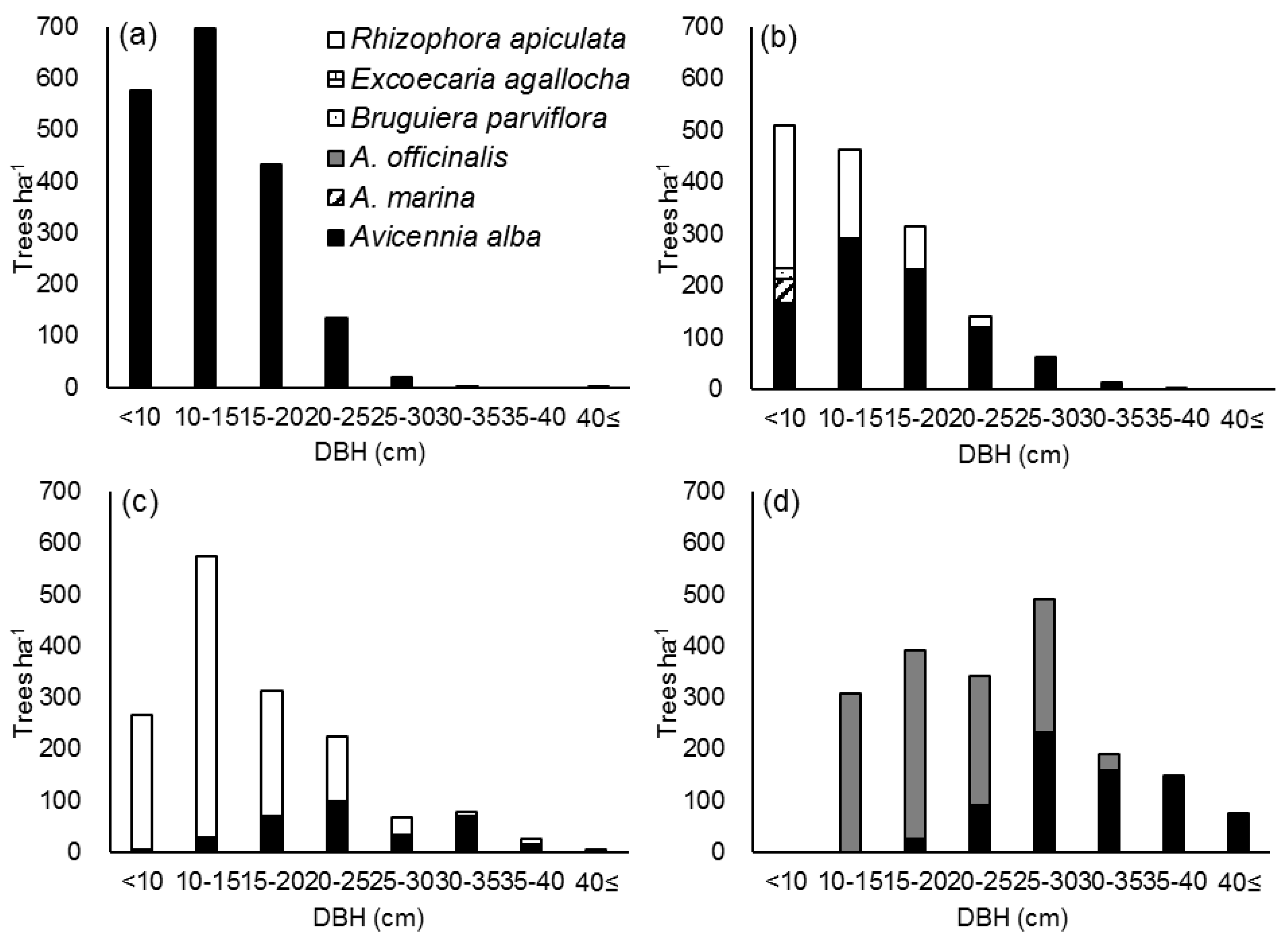
| Site | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Location | Seaward side of Con Ngoai Ong Trang Island: NW bank, Cua Lon Estuary | Seaward side of Con Trong Ong Trang Island: NW bank, Cua Lon Estuary | Dat Mui National Park, at the border between Gulf of Thailand and East Sea | SE bank of Con Ngoai Ong Trang Island, Cua Lon Estuary | SE bank of Con Trong Ong Trang Island, Cua Lon Estuary | Coastline of Gulf of Thailand, in front of sea dam | Dat Mui National Park, behind the sea dam |
| Accretion/erosion | Accretion | Accretion | Accretion | Erosion | Erosion | Erosion | Erosion |
| Formed | 1980s | 1960s | Before 1953 | Since 2014 | Before 2004 | After 1998 | 1940–1985 |
| Rate (m yr−1) | 7.38 ± 7.61 (1992–2011) [45] | 48.69 ± 3.01 (1979–2011) [45] | 44.74 ± 26.76 (1953–2011) [45] | NA | NA | −40.8 (1998–2002) [46] | −10.28 ± 2.64 (1953–2011) [45] |
| Mean altitude (asl, m) | −1.9 | −2.0 | −3.0 | −1.8 | 2.2 | 1.3 | 1.3 |
| Soil texture | Silt loam or silt clay loam [39] | Silt loam or silt clay loam [39] | Silt loam or silt clay loam [39] | Clay | Clay | Clay | Clay |
| Soil salinity (‰) | 32.87 | 30.32 | 35.15 | 35.85 | 33.54 | 40.94 | 35.39 |
| Variable | Description |
|---|---|
| Number of species | Number of species in a plot |
| Relative density of Avicennia alba | |
| Relative density of Rhizophora apiculata | |
| Ratio of A. alba basal area to total stand basal area | |
| Ratio of R. apiculata basal area to total stand basal area | |
| Number of A. alba ≥ 20 cm DBH | Number of A. alba with DBH ≥ 20 cm |
| Number of R. apiculata ≥ 20 cm DBH | Number of R. apiculata with DBH ≥ 20 cm |
| Number of other species ≥ 20 cm DBH | Number of stems of tree species with DBH ≥ 20 cm except A. alba and R. apiculata |
| Number of A. alba < 10 cm DBH | Number of A. alba with DBH < 10 cm |
| # Species | RD Aa | RD Ra | RBAAa | RBARa | RBAOthers | Aa ≥ 20 | Ra ≥ 20 | Others ≥ 20 | Aa < 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| RD Aa | −0.145 | |||||||||
| RD Ra | 0.069 | −0.916 ** | ||||||||
| RBAAa | −0.153 | 0.980 ** | −0.854 ** | |||||||
| RBARa | 0.099 | −0.936 ** | 0.987 ** | −0.888 ** | ||||||
| RBAOthers | 0.754 ** | −0.103 | −0.176 | −0.189 | −0.116 | |||||
| Aa ≥ 20 | 0.123 | 0.309 * | −0.286 * | 0.328 * | −0.349 ** | −0.022 | ||||
| Ra ≥ 20 | 0.525 ** | −0.251 | 0.318 * | −0.211 | 0.278 * | 0.170 | 0.241 | |||
| Others ≥ 20 | 0.251 | 0.026 | −0.266 * | −0.076 | −0.246 | 0.509 ** | 0.178 | 0.022 | ||
| Aa < 10 | −0.132 | 0.852 ** | −0.776 ** | 0.845 ** | −0.770 ** | −0.065 | −0.065 | −0.325 * | −0.071 | |
| Density | −0.019 | 0.205 | −0.213 | 0.212 | −0.223 | 0.080 | 0.012 | −0.264 | −0.101 | 0.211 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.M.; Hoang, H.T.; Ta, H.V.; Park, P.S. Comparison of Mangrove Stand Development on Accretion and Erosion Sites in Ca Mau, Vietnam. Forests 2020, 11, 615. https://doi.org/10.3390/f11060615
Nguyen LTM, Hoang HT, Ta HV, Park PS. Comparison of Mangrove Stand Development on Accretion and Erosion Sites in Ca Mau, Vietnam. Forests. 2020; 11(6):615. https://doi.org/10.3390/f11060615
Chicago/Turabian StyleNguyen, Linh Thuy My, Hanh Thi Hoang, Han Van Ta, and Pil Sun Park. 2020. "Comparison of Mangrove Stand Development on Accretion and Erosion Sites in Ca Mau, Vietnam" Forests 11, no. 6: 615. https://doi.org/10.3390/f11060615
APA StyleNguyen, L. T. M., Hoang, H. T., Ta, H. V., & Park, P. S. (2020). Comparison of Mangrove Stand Development on Accretion and Erosion Sites in Ca Mau, Vietnam. Forests, 11(6), 615. https://doi.org/10.3390/f11060615






