Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Application Software
2.2. Species Occurrence Data
2.3. Environmental Variables
2.4. Setting of MaxEnt Software Parameters
2.5. GIS Analysis
2.6. Model Result Evaluation
3. Results
3.1. Model Assessment
3.2. Important Environmental Variables
3.3. Predicted Distribution of Icerya Species
3.4. Future Invasion Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What\”s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [Green Version]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; Van Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Genovesi, P.; Bellingham, P.J.; Costello, M.J.; McGrannachan, C.; Sheppard, A.W. Prioritizing species, pathways, and sites to achieve conservation targets for biological invasion. Biol. Invasions 2016, 18, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.Z.; Borgemeister, C. Future risks of pest species under changing climatic conditions. PLoS ONE 2016, 11, e153237. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, C.N.; Tonkyn, D. Changes in the geographic distribution of the diana fritillary (speyeria diana: Nymphalidae) under forecasted predictions of climate change. Insects 2018, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [Green Version]
- Barbet-Massin, M.; Rome, Q.; Muller, F.; Perrard, A.; Villemant, C.; Jiguet, F. Climate change increases the risk of invasion by the yellow-legged hornet. Biol. Conserv. 2013, 157, 4–10. [Google Scholar] [CrossRef]
- García Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A Literature-Based Model of Scale Insect Biology and Systematics. Database 2016, 2016. Available online: http://scalenet.info/catalogue/ (accessed on 15 February 2020).
- Signoret, V. ESLC, Essai sur les cochenilles ou gallinsectes (Homoptères—Coccides), 16e partie. Essay on gall-forming scale insects (Homoptera—Coccidae), 16th part. Ann. de la Société Entomol. de Fr. 1876, 5, 346–373. [Google Scholar]
- Yu, Y.H. Study on Biology and Control of Icerya aegyptiaca. Dissertation; South China Agricultural University: Guangdong, China, 2005. [Google Scholar]
- Singh, S.; Kaur, G. Biodiversity of insect and mite pests infesting fig in the Indian Punjab. Acta Hortic. 2017, 1173, 257–262. [Google Scholar] [CrossRef]
- Fuller, C. XIV. Notes and descriptions of some species of western Australian Coccidae. Trans. R. Entomol. Soc. Lond. 1899, 47, 435–473. [Google Scholar] [CrossRef]
- Hall, W.J. Observations on the Coccidae of Egypt. Bulletin, ministry of agriculture, Egypt. Tech. Sci. Serv. 1922, 22, 1–54. [Google Scholar]
- Uesato, T.; Kondo, T.; Unruh, C.; Williams, D.J. Establishment and host records of Icerya aegyptiaca (Douglas) (Hemiptera: Coccoidea: Monophlebidae) in the Sakishima islands of the Ryukyu archipelago, Japan, with notes on its worldwide distribution. Entomol. Sci. 2011, 14, 49–55. [Google Scholar] [CrossRef]
- Zhang, W. Primary Investigation of Host Plants and Damage Extent for Icerya aegyptiaca (Douglas). Master’s Thesis, Anhui Agricultural Science Bulletin, Anhui, China, 2011. [Google Scholar]
- Prasad, Y.K. The role of natural enemies in controlling Icerya purchasi in South Australia. Entomophaga 1989, 34, 391–395. [Google Scholar] [CrossRef]
- Tang, F.T.; Hao, J.J. The Margarodidae and Others of China; Chinese Agricultural Science and Technology Press: Beijing, China, 1995; p. 157. [Google Scholar]
- Papadopoulou, S.; Chryssohoides, C. Icerya purchasi (Homoptera: Margarodidae) on Rosmarinus officinalis (Lamiaceae), a new host plant record for Greece. EPPO Bull. 2012, 42, 148–149. [Google Scholar] [CrossRef]
- Unruh, C.M.; Gullan, P.J. Molecular data reveal convergent reproductive strategies in Iceryine scale insects (Hemiptera: Coccoidea: Monophlebidae), allowing the re-interpretation of morphology and a revised generic classification. Syst. Entomol. 2008, 33, 8–50. [Google Scholar] [CrossRef]
- Zhao, G.S.; Zhou, W.C. Exotic pests and diseases of plants in Taiwan. Taiwan Agric. Res. 1994, 2, 25–27. [Google Scholar]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Venette, R.C.; Kriticos, D.J.; Magarey, R.D.; Koch, F.H.; Baker, R.H.A.; Worner, S.P.; Raboteaux, N.N.G.; McKenney, D.W.; Dobesberger, E.J.; Yemshanov, D.; et al. Pest risk maps for invasive alien species: A roadmap for improvement. Bioscience 2010, 60, 349–362. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. Domain: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Chejara, V.K.; Kriticos, D.J.; Kristiansen, P.; Sindel, B.M.; Whalley, R.D.B.; Nadolny, C. The current and future potential geographical distribution of Hyparrhenia. Hirta. Weed Res. 2010, 50, 174–184. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.P.; Liu, Q.; Gao, Y.B. Improving ecological niche model transferability to predict the potential distribution of invasive exotic species. Biodivers. Sci. 2014, 22, 223–230. [Google Scholar] [CrossRef]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanço, M.C. Mapping global risk levels of Bemisia tabaci in areas of suitability for open field tomato cultivation under current and future climates. PLoS ONE 2018, 13, e198925. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Q.; Zhao, W.; Zhang, H. Predicting the potential distributions of the invasive cycad scale Aulacaspis yasumatsui (Hemiptera: Diaspididae) under different climate change scenarios and the implications for management. Peerj 2018, 6, e4832. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.P. Iceryine Scale Insects Recorded from the Orient. Indian J. Entomol. 1951, 12, 39–158. Available online: https://www.cabdirect.org/cabdirect/abstract/20057007519 (accessed on 10 February 2020).
- Beardsley, J.W. Insects of Micronesia Homoptera: Coccoidea. Insects Micrones. 1966, 6, 377–562. Available online: http://hbs.bishopmuseum.org/pubs-online/pdf/iom6-9cocc.pdf (accessed on 10 February 2020).
- Ben-Dov, Y. The scale insects (Hemiptera: Coccoidea) of Israel-checklist, host plants, zoogeographical considerations and annotations on species. Isr. J. Entomol. 2012, 41, 21–48. [Google Scholar]
- Williams, D.J.; Watson, G.W. The Scale Insects of the Tropical South Pacific Region. Part 1: The Armoured Scales (Diaspididae). CAB International 1990. Available online: https://www.cabdirect.org/cabdirect/abstract/19901143648 (accessed on 10 February 2020).
- Malumphy, C.; Badmin, J.S. Scale Insects and Whiteflies (Hemiptera: Coccoidea and Aleyrodoidea) of Watsonian Kent; with a Discussion on the Impact of Naturalised Non-Native Species. Br. J. Entomol. Nat. Hist. 2012, 25, 15. Available online: https://www.researchgate.net/profile/Chris_Malumphy2/publication/311344460_Scale_insects_and_whiteflies_Hemiptera_Coccoidea_and_Aleyrodoidea_of_Watsonian_Kent_with_a_discussion_on_the_impact_of_naturalised_non-native_species/links/5864fab008ae6eb871adadf6.pdf (accessed on 20 February 2020).
- Kim, D.H.; Yang, J.Y.; Jang, Y.S.; Choi, K.S.; Hyun, H.N.; Kim, D.-S. Stage-specific population dynamics of cottony cushion scale, Icerya purchasi (Hemiptera: Monophlebidae), in citrus orchards in Jeju, Korea. J. Asia-Pac. Entomol. 2011, 14, 305–309. [Google Scholar] [CrossRef]
- Kadmon, R.; Farber, O.; Danin, A. Effect of roadside bias on the accuracy of predictive maps produced by bioclimatic models. Ecol. Appl. 2004, 14, 401–413. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Li, G.; Du, S.; Guo, K. Evaluation of limiting climatic factors and simulation of a climatically suitable habitat for Chinese sea buckthorn. PLoS ONE 2015, 10, e131659. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2014, 16, 1424–1435. [Google Scholar] [CrossRef]
- Bitz, C.M.; Shell, K.; Gent, P.R.; Bailey, D.A.; Danabasoglu, G.; Armour, K.C.; Holland, M.M.; Kiehl, J.T. Climate sensitivity of the community climate system model, version 4. J. Clim. 2012, 25, 3053–3070. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Nozawa, T.; Kawase, H.; Abe, M.; Yokohata, T.; et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 2011, 4, 845. [Google Scholar] [CrossRef] [Green Version]
- Nozawa, T.; Nagashima, T.; Shiogama, H.; Crooks, S.A. Detecting natural influence on surface air temperature change in the early twentieth century. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef] [Green Version]
- Moss, R.-H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5. [Google Scholar] [CrossRef]
- Moss, R.; Babiker, W.; Brinkman, S.; Calvo, E.; Carter, T.; Edmonds, J.; Elgizouli, I.; Emori, S.; Erda, L.; Hibbard, K.; et al. Towards new scenarios for the analysis of emissions: Climate change, impacts and response strategies. Environ. Policy Collect. 2008, 5, 399–406. [Google Scholar] [CrossRef]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. Rcp 8.5: A scenario of comparatively high greenhouse gas emissions. Clim. Chang. 2011, 109, 33. [Google Scholar] [CrossRef] [Green Version]
- Thomson, A.M.; Calvin, K.; Smith, S.J.; Kyle, P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E.; et al. Rcp4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. Maxent’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. Peerj 2017, 5, e3093. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.; Silander, J.A. A practical guide to maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Kumar, S.; Neven, L.G.; Zhu, H.; Zhang, R. Assessing the global risk of establishment of Cydia pomonella (Lepidoptera: Tortricidae) using Climex and Maxent niche models. J. Econ. Entomol. 2015, 108, 1708–1719. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Original article: Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Zhu, G.P.; Fan, J.Y.; Wang, M.L.; Chen, M.; Qiao, H. The importance of the shape of receiver operating characteristic (ROC) curve in ecological niche model evaluation: Case study of Hlyphantria Cunea. J. Biosaf. 2017, 3, 184–190. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Krechemer, F.S. Potential global distribution of Diabrotica species and the risks for agricultural production. Pest Manag. Sci. 2018, 74, 2100–2109. [Google Scholar] [CrossRef]
- Hill, M.P.; Terblanche, J.S. Niche overlap of congeneric invaders supports a single-species hypothesis and provides insight into future invasion risk: Implications for global management of the Bactrocera dorsalis complex. PLoS ONE 2014, 9, e90121. [Google Scholar] [CrossRef] [Green Version]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef]
- Wei, J.; Li, X.; Lu, Y.; Zhao, L.; Zhang, H.; Zhao, Q. Modeling the potential global distribution of Phenacoccus madeirensis green under various climate change scenarios. Forests 2019, 10, 773. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.F.; Lu, H.J. Minimum viable population: A basic theory of conservation biology. Chin. J. Ecol. 1996, 15, 25–30. [Google Scholar]
- Williamson, M. Biological Invasions; Chapman & Hall: London, UK, 1996; pp. 8–10. [Google Scholar]
- Cadotte, M.W.; Mcmahon, S.M.; Fukami, T. Conceptual Ecology and Invasion Biology: Reciprocal Approaches to Nature; Springer: Dordrecht, The Netherlands, 2006; pp. 343–367. [Google Scholar]
- Webber, B.L.; Yates, C.J.; Le Maitre, D.C.; Scott, J.; Kriticos, D.J.; Ota, N.; McNeill, A.; Le Roux, J.J.; Midgley, G.F. Modelling horses for novel climate courses: Insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Divers. Distrib. 2011, 17, 978–1000. [Google Scholar] [CrossRef]
- Aragón, P.; Baselga, A.; Lobo, J.M. Global estimation of invasion risk zones for the western corn rootworm Diabrotica virgifera virgifera: Integrating distribution models and physiological thresholds to assess climatic favourability. J. Appl. Ecol. 2010, 47, 1026–1035. [Google Scholar] [CrossRef]
- An, X.-C.; Guo, Q.; Jiang, L.; Zhang, W.-P. The study of predation of Icerya aegyptiaca by Rodolia Pumila. J. Hunan Agric. Univ. 2011, 34, 407–410. [Google Scholar] [CrossRef]
- Caltagirone, L.E.; Doutt, R.L. The history of the vedalia beetle importation to California and its impact on the development of biological control. Annu. Rev. Entomol. 1989, 34, 1–16. [Google Scholar] [CrossRef]
- Hortal, J.; Roura-Pascual, N.; Sanders, N.J.; Rahbek, C. Understanding (insect) species distributions across spatial scales. Ecography 2010, 33, 51–53. [Google Scholar] [CrossRef]


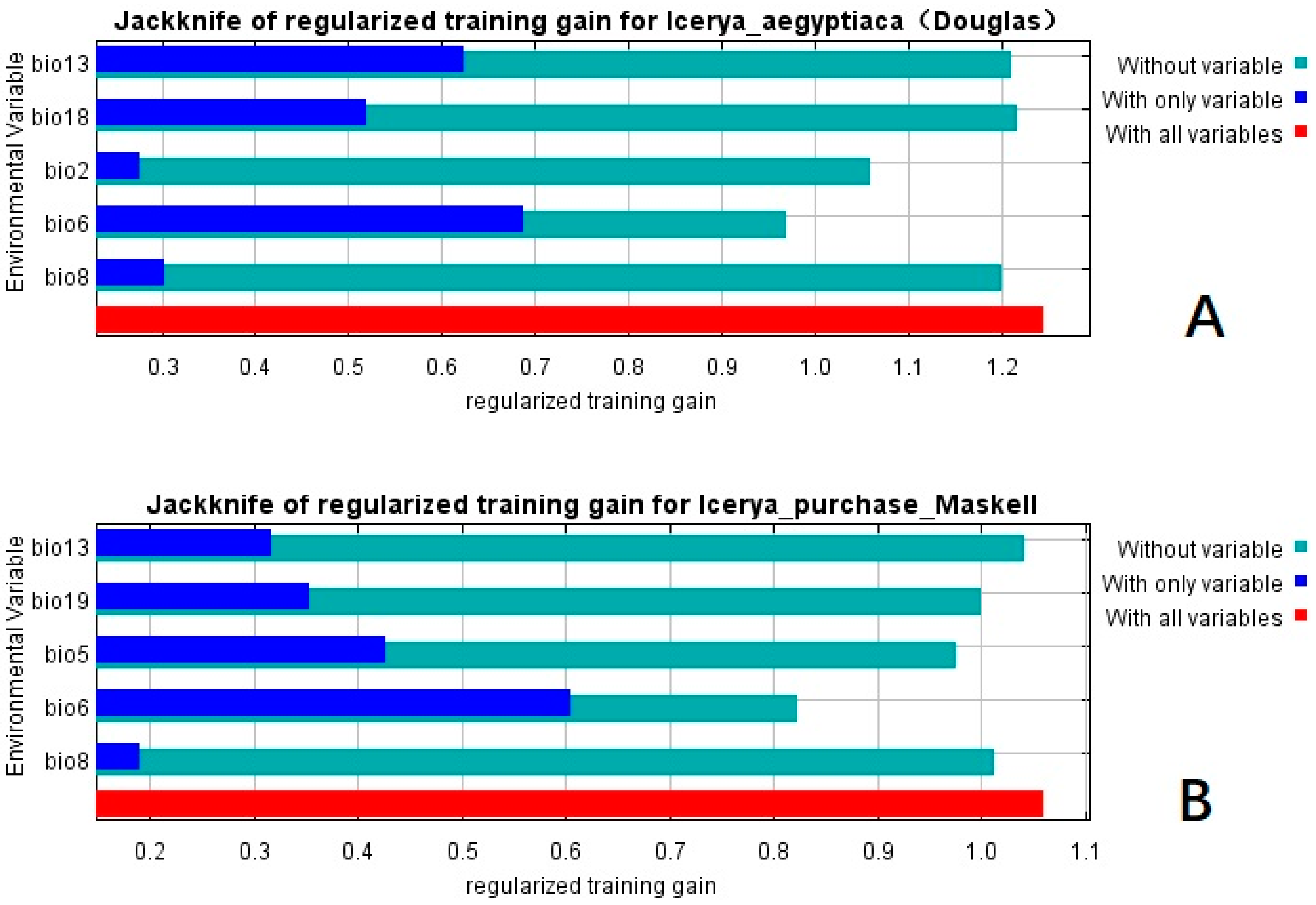





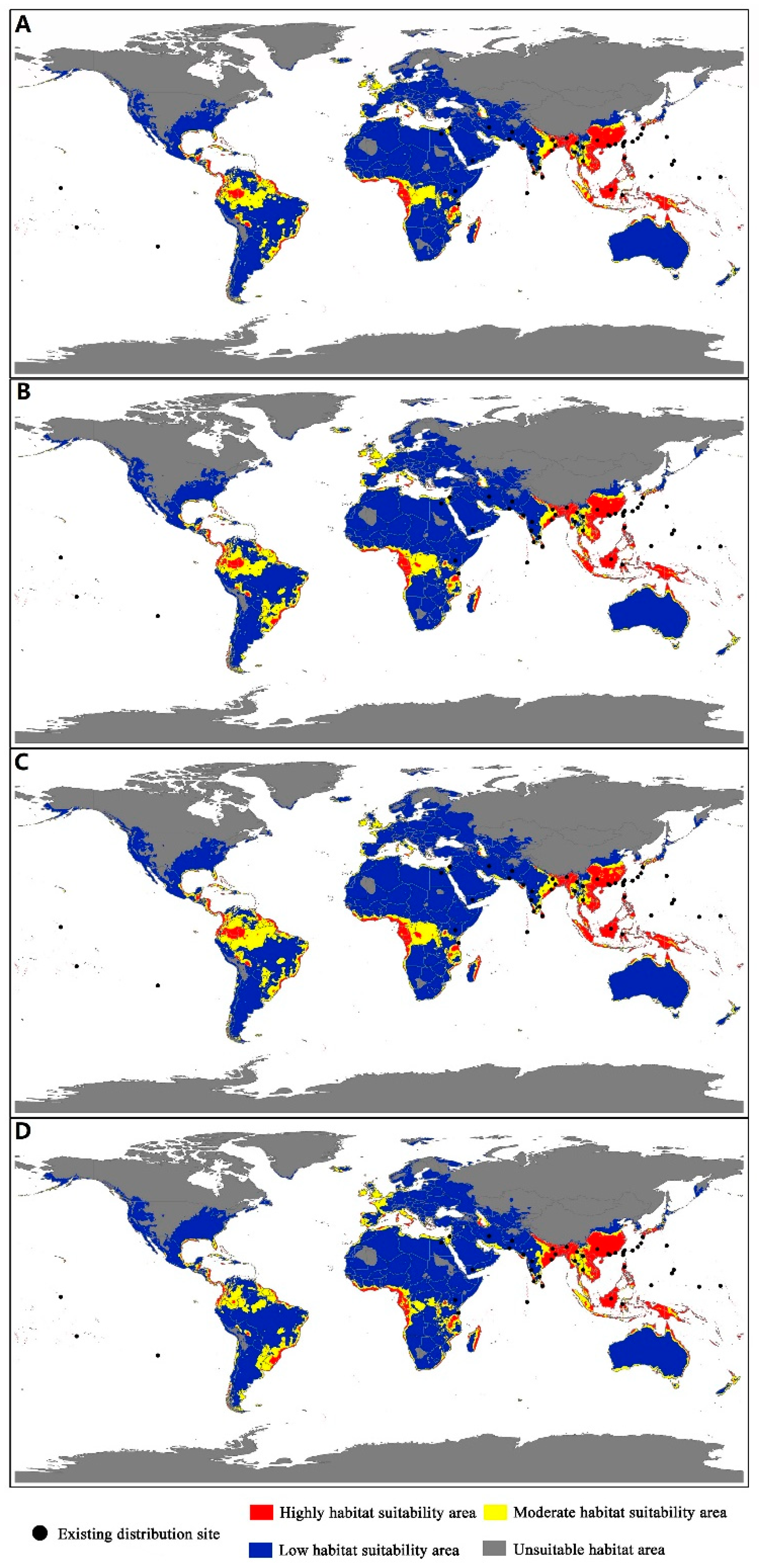

| Abbreviation | Bioclimate Variables | Abbreviation | Bioclimate Variables |
|---|---|---|---|
| bio01 | Annual mean temperature | bio11 | Mean temperature of coldest quarter |
| bio02 | Mean diurnal range | bio12 | Annual precipitation |
| bio03 | Isothermality | bio13 | Precipitation of wettest month |
| bio04 | Temperature seasonality | bio14 | Precipitation of driest month |
| bio05 | Max temperature of warmest month | bio15 | Precipitation seasonality |
| bio06 | Min temperature of coldest month | bio16 | Precipitation of wettest quarter |
| bio07 | Temperature annual range | bio17 | Precipitation of driest quarter |
| bio08 | Mean temperature of wettest quarter | bio18 | Precipitation of warmest quarter |
| bio09 | Mean temperature of driest quarter | bio19 | Precipitation of coldest quarter |
| bio10 | Mean temperature of warmest quarter |
| Species | Feature Classes | Regularization Multiplier | AUC | AUC Ratios (E = 0.05) | LPT |
|---|---|---|---|---|---|
| I. aegyptiaca | L, Q, P | 0.5 | 0.883 | 1.4828 | 0.0757 |
| I. purchasi | L, Q, H, P, T | 1.0 | 0.849 | 1.3813 | 0.0507 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shi, J. Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change. Forests 2020, 11, 684. https://doi.org/10.3390/f11060684
Liu Y, Shi J. Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change. Forests. 2020; 11(6):684. https://doi.org/10.3390/f11060684
Chicago/Turabian StyleLiu, Yang, and Juan Shi. 2020. "Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change" Forests 11, no. 6: 684. https://doi.org/10.3390/f11060684
APA StyleLiu, Y., & Shi, J. (2020). Predicting the Potential Global Geographical Distribution of Two Icerya Species under Climate Change. Forests, 11(6), 684. https://doi.org/10.3390/f11060684




