Effects of Changes in Biopolymer Composition on Moisture in Acetylated Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Acetylation
2.2.2. Low-Field Nuclear Magnetic Resonance (LFNMR) Characterization
2.2.3. Hydroxyl Accessibility
3. Results
3.1. General Mass Changes of Wood after Treatments
3.2. Effect of Hemicellulose Content Reduction on the Moisture in Acetylated Wood
3.3. Effect of Lignin Content Reduction on the Moisture in Acetylated Wood
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sathre, R.; Gustavsson, L. Using wood products to mitigate climate change: External costs and structural change. Appl. Energy 2009, 86, 251–257. [Google Scholar] [CrossRef]
- Wang, L.; Toppinen, A.; Juslin, H. Use of wood in green building: A study of expert perspectives from the UK. J. Clean. Prod. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- Milner, H.; Woodard, A. Sustainability of engineered wood products. In Sustainability of Construction Materials, 2nd ed.; Khatib, J.M., Ed.; Woodhead Publishing: Sawston Cambridge, UK, 2016; pp. 159–180. [Google Scholar]
- Skaar, C. Wood-Water Relations; Springer: Berlin, German, 1988. [Google Scholar]
- Kong, L.; Guan, H.; Wang, X. In Situ Polymerization of Furfuryl Alcohol with Ammonium Dihydrogen Phosphate in Poplar Wood for Improved Dimensional Stability and Flame Retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Wagner, L.; Bos, C.; Bader, T.K.; De Borst, K. Effect of Water on the Mechanical Properties of Wood Cell Walls—Results of a Nanoindentation Study. Bioresources 2015, 10, 4011–4025. [Google Scholar] [CrossRef] [Green Version]
- Thybring, E.E.; Kymäläinen, M.; Rautkari, L. Moisture in modified wood and its relevance for fungal decay. iForest Biogeosci. For. 2018, 11, 418–422. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood. In Wood Chemistry and Wood Composites; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Beck, G.; Thybring, E.E.; Thygesen, L.G.; Hill, C. Characterization of moisture in acetylated and propionylated radiata pine using low-field nuclearmagnetic resonance (LFNMR) relaxometry. Holzforschung 2018, 72, 225–233. [Google Scholar] [CrossRef]
- Hill, C.A.S. The reduction in the fibre saturation point of wood due to chemical modification using anhydride reagents: A reappraisal. Holzforschung 2008, 62, 423–428. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Hill, C.A.S.; Curling, S.; Ormondroyd, G.; Xie, Y. The water vapour sorption behaviour of acetylated birch wood: How acetylation affects the sorption isotherm and accessible hydroxyl content. J. Mater. Sci. 2013, 49, 2362–2371. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: West Sussex, UK, 2006. [Google Scholar]
- Moghaddam, M.S.; Wålinder, M.E.; Claesson, P.M.; Swerin, A. Wettability and swelling of acetylated and furfurylated wood analyzed by multicycle Wilhelmy plate method. Holzforschung 2016, 70, 69–77. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Hill, C.A.S. The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana. Holz Roh Werkst. 2002, 60, 329–332. [Google Scholar] [CrossRef]
- Beck, G.; Strohbusch, S.; Larnøy, E.; Militz, H.; Hill, C. Accessibility of hydroxyl groups in anhydride modified wood as measured by deuterium exchange and saponification. Holzforschung 2017, 72, 17–23. [Google Scholar] [CrossRef]
- Rowell, R.M.; Simonson, R.; Hess, S.; Plackett, D.V.; Cronshaw, D.; Dunningham, E. Acetyl distribution in acetylated whole wood and reactivity of isolated wood cell-wall components to acetic anhydride. Wood Fiber Sci. 1994, 26, 11–18. [Google Scholar]
- Ramsden, M.J.; Blake, F.S.R.; Fey, N.J. The effect of acetylation on the mechanical properties, hydrophobicity and dimensional stability of Pinus sylvestris. Wood Sci. Technol. 1997, 31, 97–104. [Google Scholar] [CrossRef]
- Chang, H.-T.; Chang, S.-T. Moisture excluding efficiency and dimensional stability of wood improved by acylation. Bioresour. Technol. 2002, 85, 201–204. [Google Scholar] [CrossRef]
- Rowell, R.M. Dimensional stability and fungal durability of acetylated wood. Komunikaty 2016, 59, 139–150. [Google Scholar]
- Stamm, A.J.; Baechler, R.H. Decay resistance and dimensional stability of five modified woods. For. Prod. J. 1960, 10, 22–26. [Google Scholar]
- Zelinka, S.L.; Kirker, G.T.; Bishell, A.B.; Glass, S.V. Effects of Wood Moisture Content and the Level of Acetylation on Brown Rot Decay. Forests 2020, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Mantanis, G. Chemical Modification of Wood by Acetylation or Furfurylation: A Review of the Present Scaled-up Technologies. Bioresources 2017, 12, 4478–4489. [Google Scholar] [CrossRef] [Green Version]
- Forest Products Laboratory. Wood Handbook-Wood as an Engineering Material; Forest Products Laboratory: Madison, WI, USA, 1999; p. 463. [Google Scholar]
- Hoadley, R.B. Understanding Wood: A Craftsman’s Guide to Wood Technology; The Taunton Press: Newtown, CT, USA, 2000. [Google Scholar]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in Iβ cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Hofstetter, K.; Hinterstoisser, B.; Salmén, L. Moisture uptake in native cellulose—The roles of different hydrogen bonds: A dynamic FT-IR study using Deuterium exchange. Cellulose 2006, 13, 131–145. [Google Scholar] [CrossRef]
- Thybring, E.E.; Thygesen, L.G.; Burgert, I. Hydroxyl accessibility in wood cell walls as affected by drying and re-wetting procedures. Cellulose 2017, 60, 665–2384. [Google Scholar] [CrossRef] [Green Version]
- Kulasinski, K.; Guyer, R.; Keten, S.; Derome, D.; Carmeliet, J. Impact of Moisture Adsorption on Structure and Physical Properties of Amorphous Biopolymers. Macromolecules 2015, 48, 2793–2800. [Google Scholar] [CrossRef]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Wood Chemistry and Wood Biotechnology; De Gruyter: Berlin, Germany, 2009. [Google Scholar]
- Asif, M. Sustainability of timber, wood and bamboo in construction. In Sustainability of Construction Materials; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Christensen, G.N.; Kelsey, K.E. The rate of sorption of water vapor by wood. Holz Roh Werkst 1959, 17, 178–188. [Google Scholar] [CrossRef]
- Engelund, E.T.; Thygesen, L.G.; Svensson, S.; Hill, C.A.S.; Thybring, E.E. A critical discussion of the physics of wood–water interactions. Wood Sci. Technol. 2012, 47, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Ringman, R.; Beck, G.; Pilgård, A. The Importance of Moisture for Brown Rot Degradation of Modified Wood: A Critical Discussion. Forests 2019, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Zhou, H.; Ma, E.; Wang, J. Effects of removal of different chemical components on moisture sorption property of Populus euramericana Cv. under dynamic hygrothermal conditions. Results Phys. 2018, 10, 61–68. [Google Scholar] [CrossRef]
- Fredriksson, M.; Thygesen, L.G. The states of water in Norway spruce (Picea abies (L.) Karst.) studied by low-field nuclear magnetic resonance (LFNMR) relaxometry: Assignment of free-water populations based on quantitative wood anatomy. Holzforschung 2017, 71, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688. [Google Scholar] [CrossRef] [Green Version]
- Terenzi, C.; Prakobna, K.; Berglund, L.A.; Furo, I. Nanostructural Effects on Polymer and Water Dynamics in Cellulose Biocomposites: 2H and 13C NMR Relaxometry. Biomacromolecules 2015, 16, 1506–1515. [Google Scholar] [CrossRef]
- Liu, H.E.; Liu, L.; Si, H.G.; Feng, H.; Han, Y.F. Chemical composition of wood of some Poplars. J. Zhejiang For. Coll. 1995, 12, 342–346. [Google Scholar]
- Elder, T.; Labbé, N.; Harper, D.P.; Rials, T. Time domain-nuclear magnetic resonance study of chars from southern hardwoods. Biomass Bioenergy 2006, 30, 855–862. [Google Scholar] [CrossRef]
- Yin, J.; Yuan, T.; Lu, Y.; Song, K.; Li, H.; Zhao, G.; Yin, Y. Effect of compression combined with steam treatment on the porosity, chemical compositon and cellulose crystalline structure of wood cell walls. Carbohydr. Polym. 2017, 155, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, J.; Xu, J.; Ma, E.; Cao, J. Hygroscopicity and dimensional stability of Populus euramericana Cv. modified by furfurylation combined with low hemicellulose pretreatment. J. Mater. Sci. 2019, 54, 13445–13456. [Google Scholar] [CrossRef]
- Elder, T.; Houtman, C. Time-domain NMR study of the drying of hemicellulose extracted aspen (Populus tremuloides Michx.). Holzforschung 2013, 67, 405–411. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Elder, T. Moisture in untreated, acetylated, and furfurylated Norway spruce studied during drying using time domain NMR. Wood Fiber Sci. 2013, 40, 309–320. [Google Scholar]
- Wålinder, M.; Omidvar, A.; Seltman, J.; Segerholm, K. Micromorphological studies of modified wood using a surface preparation technique based on ultraviolet laser ablation. Wood Mater. Sci. Eng. 2009, 4, 46–51. [Google Scholar] [CrossRef]
- Ou, R.; Xie, Y.; Wolcott, M.P.; Sui, S.; Wang, Q. Morphology, mechanical properties, and dimensional stability of wood particle/high density polyethylene composites: Effect of removal of wood cell wall composition. Mater. Des. 2014, 58, 339–345. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.; Ma, E. Static and dynamic sorption of lignin removed Populus euramericana. Tappi J. 2018, 17, 71–77. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Yu, S.; Yan, M.; Berglund, L. Optically Transparent Wood from a Nanoporous Cellulosic Template: Combining Functional and Structural Performance. Biomacromolecules 2016, 17, 1358–1364. [Google Scholar] [CrossRef]
- Zhu, M.; Song, J.; Li, T.; Gong, A.; Wang, Y.; Dai, J.; Yao, Y.; Luo, W.; Henderson, D.; Hu, L. Highly Anisotropic, Highly Transparent Wood Composites. Adv. Mater. 2016, 28, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Medina, L.; Li, Y.; Carosio, F.; Hajian, A.; Berglund, L.A. Nanostructured Wood Hybrids for Fire-Retardancy Prepared by Clay Impregnation into the Cell Wall. ACS Appl. Mater. Interfaces 2017, 9, 36154–36163. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Yang, F.; Tang, C.; Huang, Q.; Zhang, J. Impact of delignification on morphological, optical and mechanical properties of transparent wood. Compos. Part A Appl. Sci. Manuf. 2019, 117, 324–331. [Google Scholar] [CrossRef]
- Hoffmeyer, P.; Engelund, E.T.; Thygesen, L.G.; Thybring, E.E. Equilibrium moisture content (EMC) in Norway spruce during the first and second desorptions. Holzforschung 2011, 65, 875–882. [Google Scholar] [CrossRef]

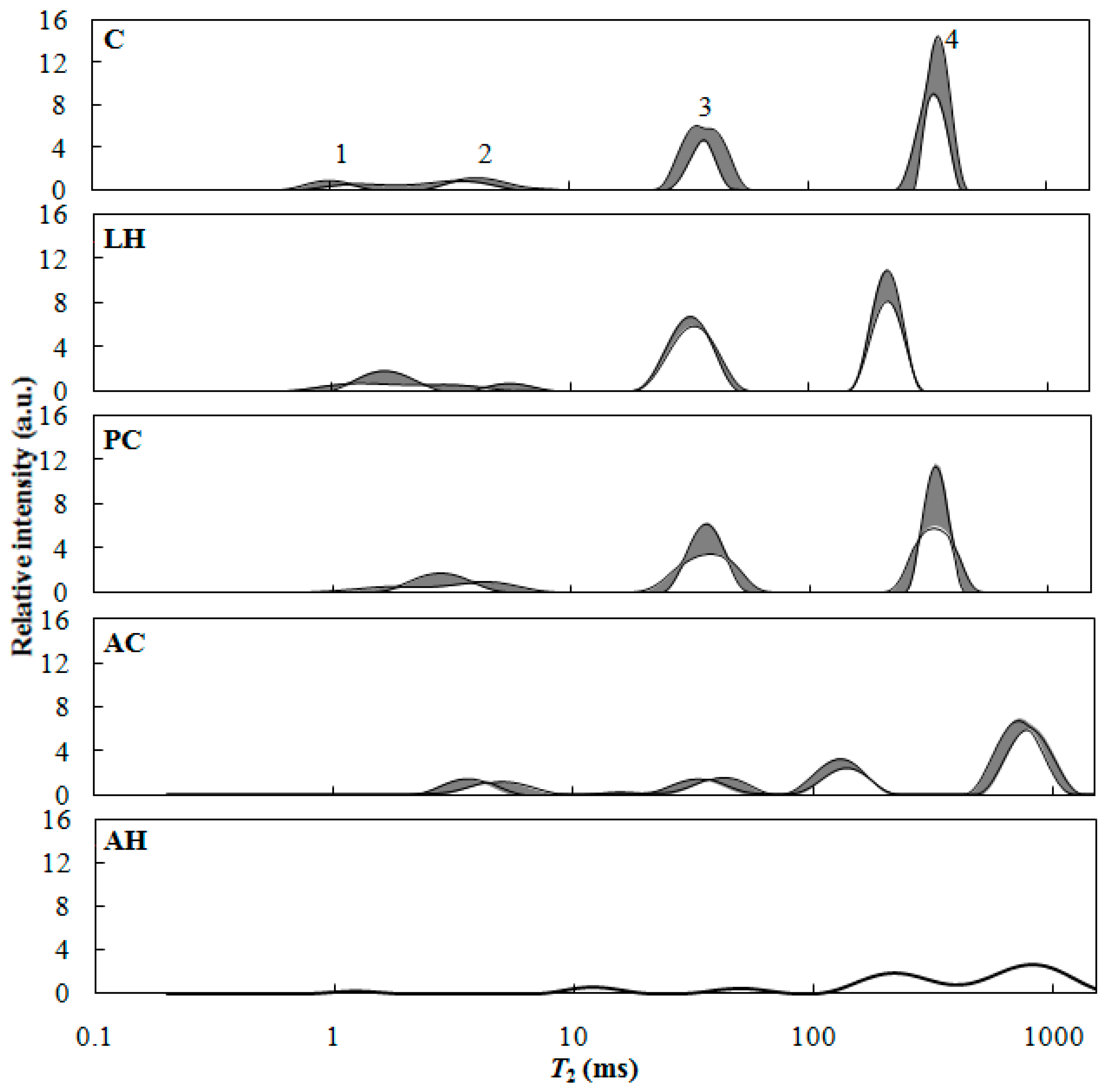
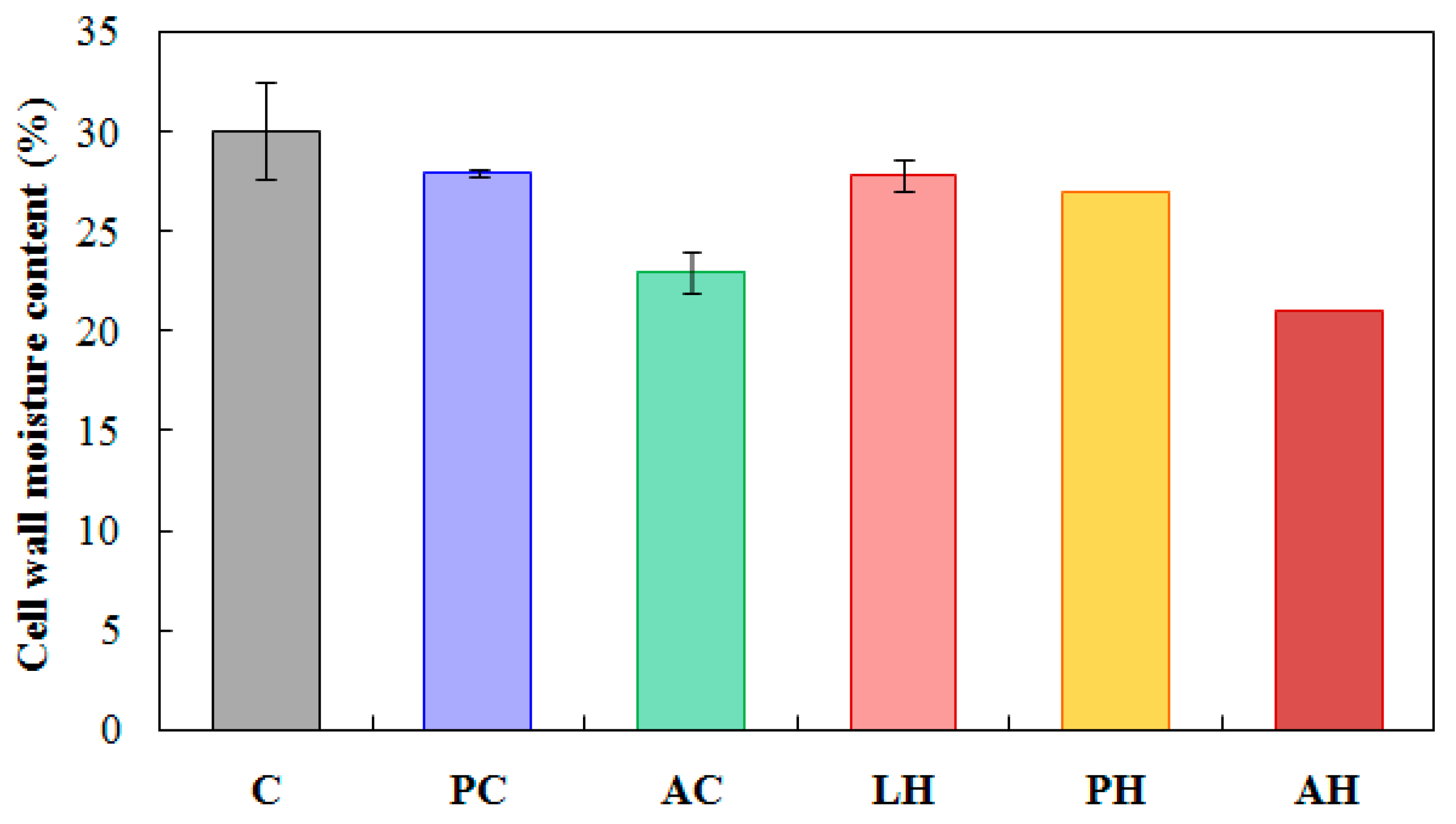


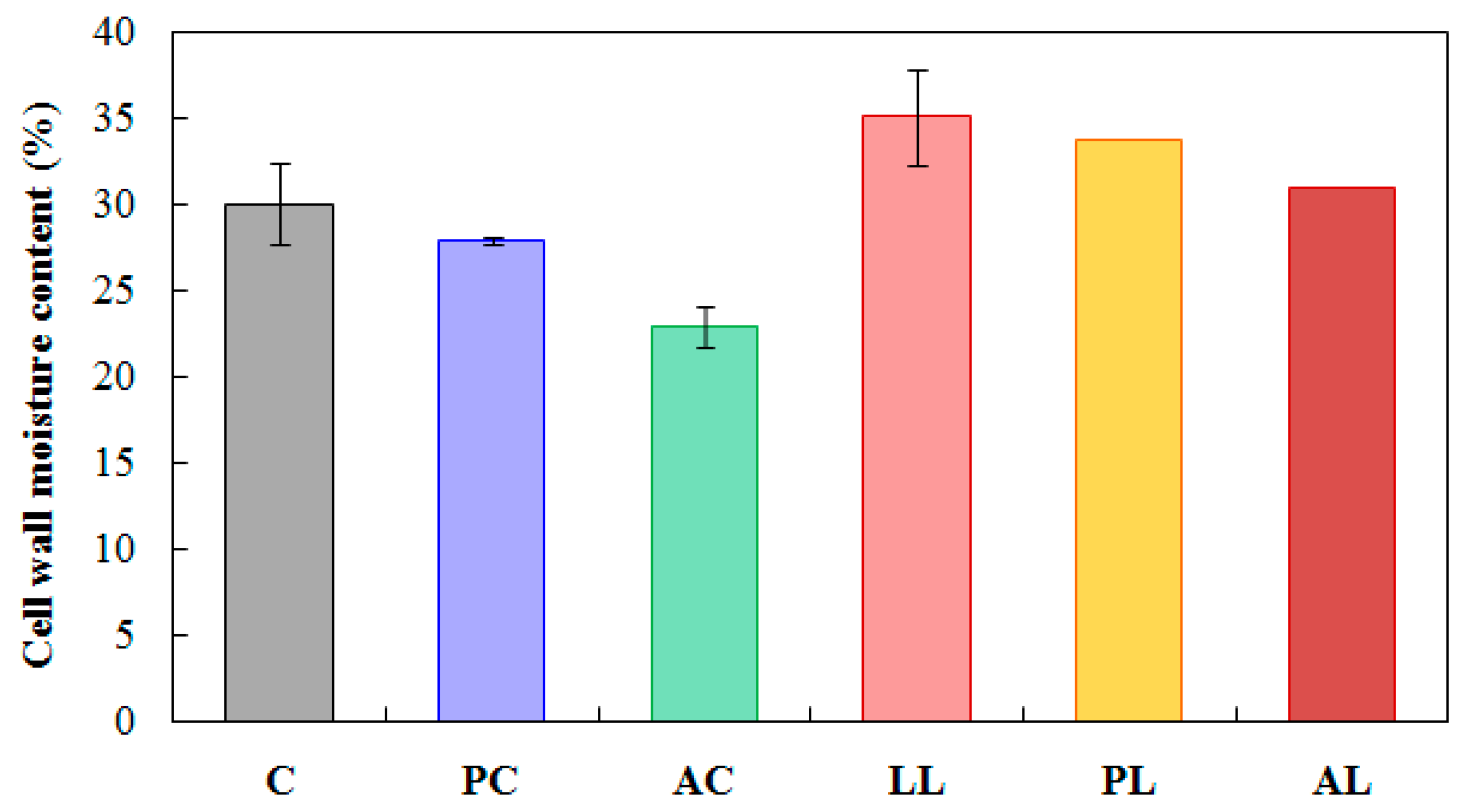
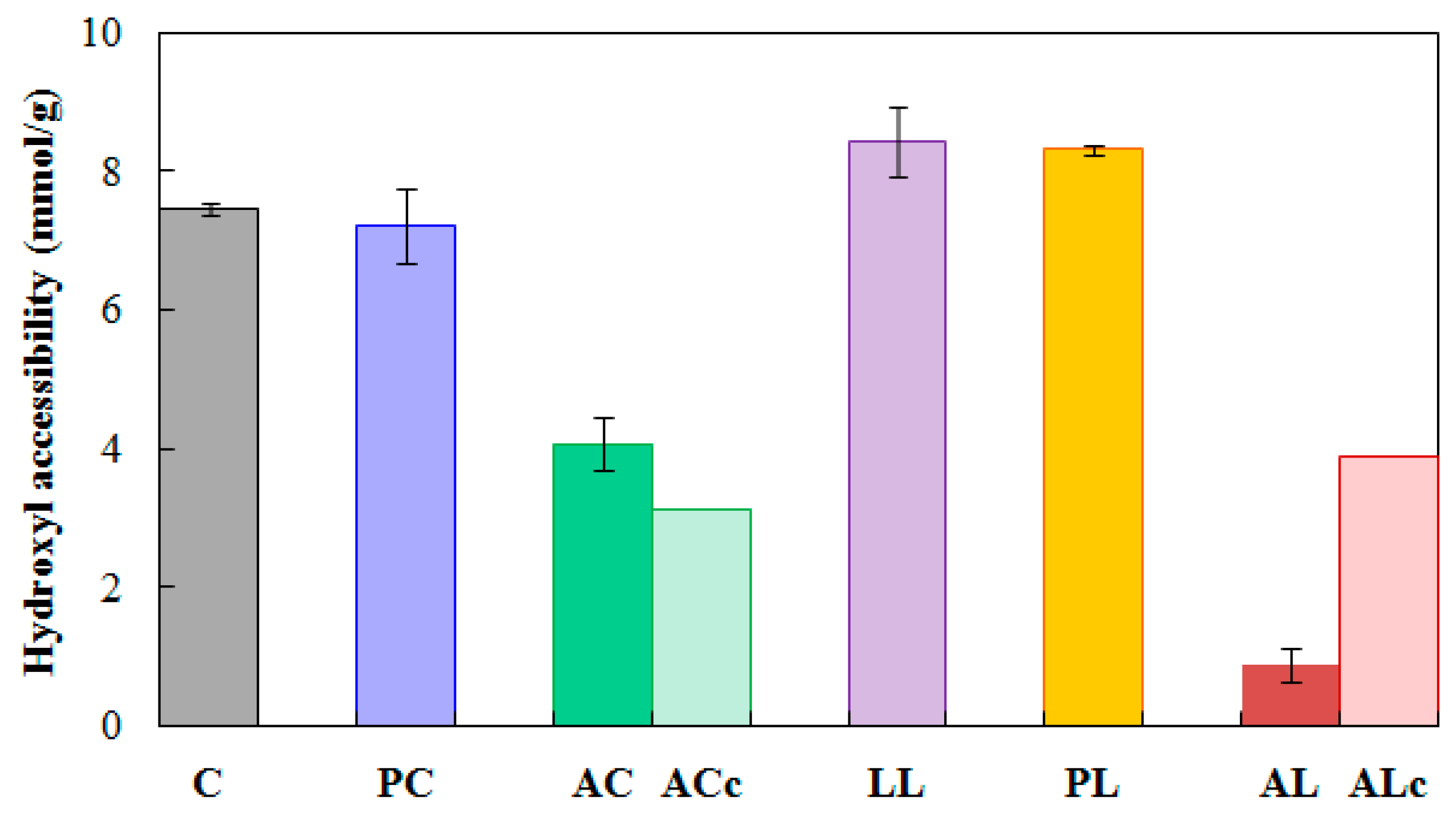
| Test | Replicate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | PC | AC | LH | PH | AH | LL | PL | AL | |
| LFNMR characterization | 3 | 3 | 3 | 3 | 1 | 1 | 3 | 1 | 1 |
| Hydroxyl accessibility | 3 | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 3 |
| Specimen | Control Wood | Partially Hemicellulose-Depleted | Partially Delignified |
|---|---|---|---|
| Mass change, pretreatment (% g g−1) a | 0 | −8.6 (+/−0.1) | −8.9 (+/−0.1) |
| Mass change, acetylation (% g g−1) a | 15.7 (+/−0.4) b | 15.3 (+/−0.7) c | 14.9 (+/−0.8) d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Thybring, E.E.; Fredriksson, M.; Ma, E.; Cao, J.; Digaitis, R.; Thygesen, L.G. Effects of Changes in Biopolymer Composition on Moisture in Acetylated Wood. Forests 2020, 11, 719. https://doi.org/10.3390/f11070719
Yang T, Thybring EE, Fredriksson M, Ma E, Cao J, Digaitis R, Thygesen LG. Effects of Changes in Biopolymer Composition on Moisture in Acetylated Wood. Forests. 2020; 11(7):719. https://doi.org/10.3390/f11070719
Chicago/Turabian StyleYang, Tiantian, Emil Engelund Thybring, Maria Fredriksson, Erni Ma, Jinzhen Cao, Ramūnas Digaitis, and Lisbeth Garbrecht Thygesen. 2020. "Effects of Changes in Biopolymer Composition on Moisture in Acetylated Wood" Forests 11, no. 7: 719. https://doi.org/10.3390/f11070719
APA StyleYang, T., Thybring, E. E., Fredriksson, M., Ma, E., Cao, J., Digaitis, R., & Thygesen, L. G. (2020). Effects of Changes in Biopolymer Composition on Moisture in Acetylated Wood. Forests, 11(7), 719. https://doi.org/10.3390/f11070719






