Use of Inoculator Bacteria to Promote Tuber melanosporum Root Colonization and Growth on Quercus faginea Saplings
Abstract
:1. Introduction
2. Materials and Methods
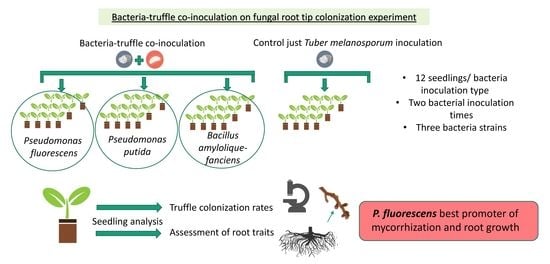
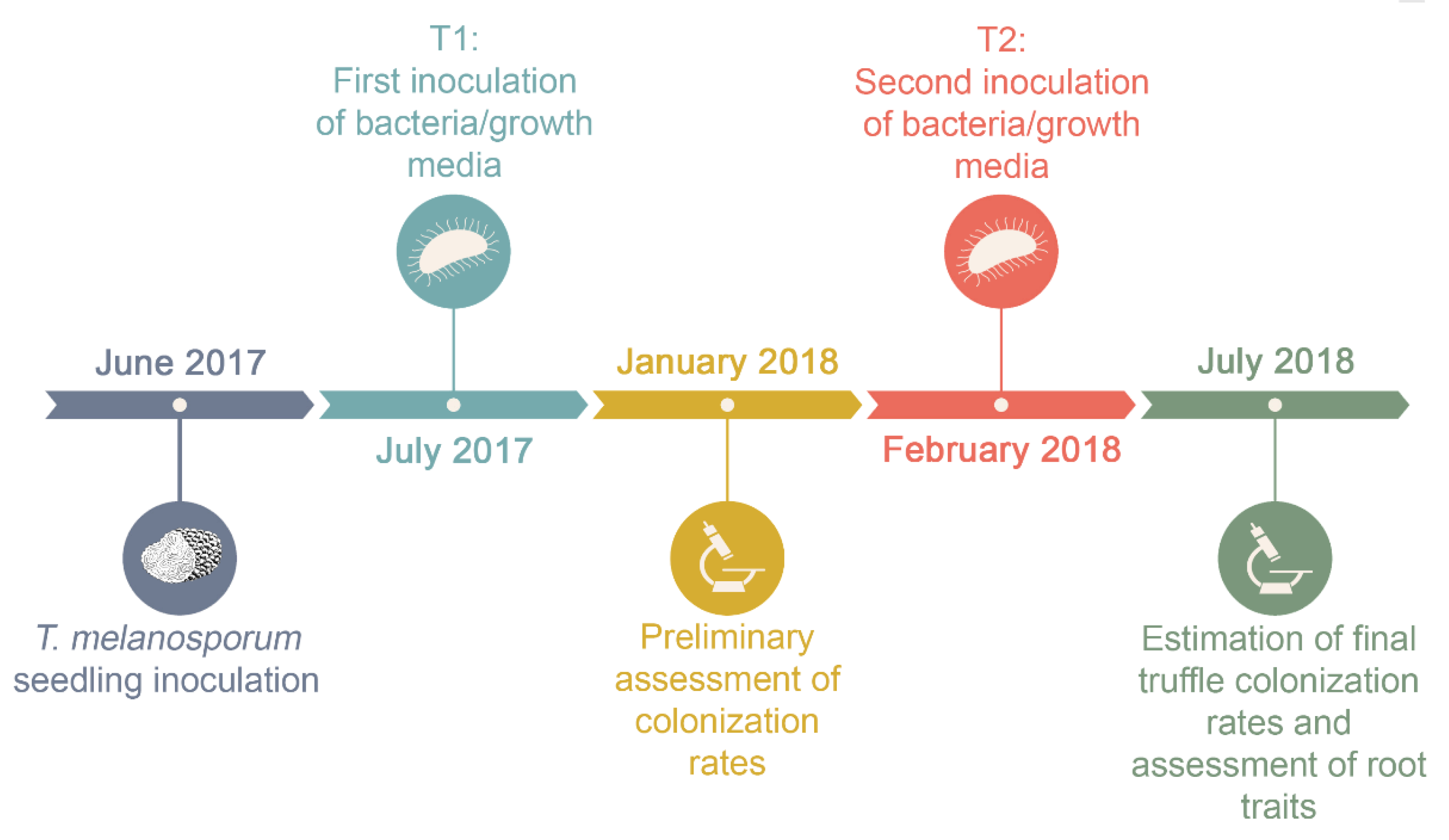
2.1. Experimental Design
2.2. Plant Material and Bacterial Inoculum
2.3. Seedling Root Trait Analyses
2.4. Estimation of T. melanosporum Colonization Rates
2.5. Statistical Analyses
3. Results
3.1. Effects of Bacteria and Inoculation Time on T. melanosporum Root Tip Colonization Rates
3.2. Bacteria and Inoculation Time Effects on Seedling Root Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonito, G.M.; Gryganskyi, A.P.; Trappe, J.M.; Vilgalys, R. A global meta-analysis of Tuber ITS rDNA sequences: Species diversity, host associations and long-distance dispersal. Mol. Ecol. 2010, 19, 4994–5008. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.; Murat, C.; Bonfante, P. Truffles: Much more than a prized and local fungal delicacy. FEMS Microbiol. Lett. 2006, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Büntgen, U.; Tegel, W.; Egli, S.; Stobbe, U.; Sproll, L.; Stenseth, N.C. Truffles and climate change. Front. Ecol. Environ. 2011, 9, 150–151. [Google Scholar] [CrossRef]
- Le Tacon, F.; Marcais, B.; Courvoisier, M.; Murat, C.; Montpied, P.; Becker, M. Climatic variations explain annual fluctuations in French Périgord black truffle wholesale markets but do not explain the decrease in black truffle production over the last 48 years. Mycorrhiza 2014, 24, 115–125. [Google Scholar] [CrossRef]
- Callot, G. La Truffe, la Terre, la Vie, INRA ed.; Quae: Versailles, France, 1999; 210p. [Google Scholar]
- Hall, I.R.; Zambonelli, A. The Cultivation of Mycorrhizal Mushrooms—Still the Next Frontier! In Mushroom Science XVIII; Zhang, J., Wang, H., Chen, M., Eds.; China Agricultural Press: Beijing, China, 2012; pp. 16–27. [Google Scholar]
- Hall, I.R.; Brown, G.T.; Zambonelli, A. Taming the Truffle; Timber Press: Portland, Oregon, 2007; 304p. [Google Scholar]
- Andrés-Alpuente, A.; Sánchez, S.; Martín, M.; Aguirre, A.J.; Barriuso, J.J. Comparative analysis of different methods for evaluating quality of Quercus ilex seedlings inoculated with Tuber melanosporum. Mycorrhiza 2014, 24, 29–37. [Google Scholar] [CrossRef]
- Donnini, D.; Benucci, G.M.N.; Bencivenga, M.; Baciarelli-Falini, L. Quality assessment of truffle-inoculated seedlings in Italy: Proposing revised parameters for certification. For. Syst. 2014, 23, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.R.; Colinas, C. Methodology for certification of Quercus ilex seedlings inoculated with Tuber melanosporum for commercial application. In Proceedings of the 1st International Conference in Mycorrhizae, Berkeley, CA, USA, 4–9 August 1996. [Google Scholar]
- Le Roux, C.; Tournier, E.; Lies, A.; Sanguin, H.; Chevalier, G.; Dupponois, R.; Mousain, D.; Prin, Y. Bacteria of the genus Rhodopseudomonas (Bradyrhizobiaceae): Obligate symbionts in mycelial cultures of the black truffles Tuber melanosporum and Tuber brumale. SpringerPlus 2016, 5, 1085. [Google Scholar] [CrossRef]
- Iotti, M.; Piattoni, F.; Zambonelli, A. Techniques for Host Plant Inoculation with Truffles and Other Edible Ectomycorrhizal Mushrooms. In Taming the Truffle; Timber Press: Portland, Oregon, 2007; pp. 145–161. [Google Scholar]
- Buzzini, P.; Gasparetti, C.; Turchetti, B.; Cramarossa, M.R.; Vaughan-Martini, A.; Martini, A.; Pagnoni, U.M.; Forti, L. Production of volatile organic compounds (VOCs) by yeasts isolated from the ascocarps of black (Tuber melanosporum Vitt.) and white (Tuber magnatum Pico) truffles. Arch. Microbiol. 2005, 184, 187–193. [Google Scholar] [CrossRef]
- Rivera, C.S.; Blanco, D.; Oria, R.; Venturini, M.E. Diversity of culturable microorganisms and occurrence of Listeria monocytogenes and Salmonella spp. in Tuber aestivum and Tuber melanosporum ascocarps. Food Microbiol. 2010, 27, 286–293. [Google Scholar] [CrossRef]
- Pacioni, G.; Leonardi, M.; Aimola, P.; Ragnelli, A.; Rubini, A.; Paolocci, F. Isolation and characterization of some mycelia inhabiting Tuber ascomata. Mycol. Res. 2007, 111, 1450–1460. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Gryndler, M.; Hršelová, H. Isolation of bacteria from ectomycorrhizae of Tuber aestivum Vittad. Acta Mycol. 2012, 47, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Sbrana, C.; Agnolucci, M.; Bedini, S.; Lepera, A.; Toffanin, A.; Giovannetti, M.; Nuti, M.P. Diversity of culturable bacterial populations associated to Tuber borchii ectomycorrhizas and their activity on T. borchii mycelial growth. FEMS Microbiol. Lett. 2002, 211, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, E.; Bertini, L.; Rossi, I.; Ceccaroli, P.; Saltarelli, R.; Guidi, C.; Zambonelli, A.; Stocchi, V. New evidence for bacterial diversity in the ascoma of the ectomycorrhizal fungus Tuber borchii Vittad. FEMS Microbiol. Lett. 2005, 247, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, E.; Guidi, C.; Bertaux, J.; Frey-Klett, P.; Garbaye, J.; Ceccaroli, P.; Saltarelli, R.; Zambonelli, A.; Stocchi, V. Occurrence and diversity of bacterial communities in Tuber magnatum during truffle maturation. Environ. Microbiol. 2007, 9, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Benucci, G.M.N.; Bonito, G.M. The truffle microbiome: Species and geography effects on bacteria associated with fruiting bodies of hypogeous Pezizales. Microb. Ecol. 2016, 72, 4–8. [Google Scholar] [CrossRef]
- Antony-Babu, S.; Deveau, A.; Van Nostrand, J.D.; Zhou, J.; Le Tacon, F.; Robin, C.; Frey-Klett, P.; Uroz, S. Black truffle-associated bacterial communities during the development and maturation of Tuber melanosporum ascocarps and putative functional roles. Environ. Microbiol. 2014, 16, 2831–2847. [Google Scholar] [CrossRef]
- Deveau, A.; Antony-Babu, S.; Le Tacon, F.; Robin, C.; Frey-Klett, P.; Uroz, S. Temporal changes of bacterial communities in the Tuber melanosporum ectomycorrhizosphere during ascocarp development. Mycorrhiza 2016, 26, 389–399. [Google Scholar] [CrossRef]
- Domínguez, J.A.; Martin, A.; Anriquez, A.; Albanesi, A. The combined effects of Pseudomonas fluorescens and Tuber melanosporum on the quality of Pinus halepensis seedlings. Mycorrhiza 2012, 22, 429–436. [Google Scholar] [CrossRef]
- Mamoun, M.; Olivier, J.M. Effect of soil Pseudomonas on colonization of hazel roots by the ecto-mycorrhizal species Tuber melanosporum and its competitors. Plant. Soil 1992, 139, 265–273. [Google Scholar] [CrossRef]
- Reyna, S. Truficultura Fundamentos y Técnicas; Mundi-Prensa: Madrid, Spain, 2012; 720p. [Google Scholar]
- Mello, A.; Ding, G.C.; Piceno, Y.M.; Napoli, C.; Tom, L.M.; De Santis, T.Z.; Andersen, G.L.; Smalla, K.; Bonfante, P. Truffle brûlés have an impact on the diversity of soil bacterial communities. PLoS ONE 2013, 8, e61945. [Google Scholar] [CrossRef] [PubMed]
- Duponnois, R.; Garbaye, J. Effect of dual inoculation of Douglas fir with the ectomycorrhizal fungus Laccaria laccata and mycorrhization helper bacteria (MHB) in two bare-root forest nurseries. Plant. Soil 1991, 138, 169–176. [Google Scholar] [CrossRef]
- Gamez, R.; Cardinale, M.; Montes, M.; Ramirez, S.; Schnell, S.; Rodriguez, F. Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006) on banana cv. Williams (Musa acuminata Colla). Microbiol Res. 2019, 220, 12–20. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Berná, L.M.; Lozano-Carrillo, C.; Andrino, A.; Morte, A. Beneficial native bacteria improve survival and mycorrhization of desert truffle mycorrhizal plants in nursery conditions. Mycorrhiza 2016, 26, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.A.; Medina, M.; Berrocal-Lobo, M.; Anriquez, A.; Albanesi, A. The combined effects of Pseudomonas fluorescens CECT 844 and the black truffle co-inoculation on Pinus nigra seedlings. IForest 2015, 8, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Mediavilla, O.; Olaizola, J.; Santos-Del-Blanco, L.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Mycorrhization between Cistus ladanifer L. and Boletus edulis Bull is enhanced by the mycorrhiza helper bacteria Pseudomonas fluorescens Migula. Mycorrhiza 2016, 26, 161–168. [Google Scholar] [CrossRef]
- De Vita, P.; Avio, L.; Sbrana, C.; Laidò, G.; Marone, D.; Mastrangelo, A.M.; Cattivelli, L.; Giovannetti, M. Genetic markers associated to arbuscular mycorrhizal colonization in durum wheat. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.B.; Zhang, Q. Issues in using the WinRHIZO system to determine physical characteristics of plant fine roots. Acta Ecol. Sin. 2009, 29, 36–38. [Google Scholar] [CrossRef]
- Agerer, R. (1987–2008) Colour Atlas of Ectomycorrhizae, 1st–13th ed.; Einhorn-Verlag: Munich, Germany, 2012. [Google Scholar]
- Oh, S.Y.; Lim, Y.W. Effect of fairy ring bacteria on the growth of Tricholoma matsutake in vitro culture. Mycorrhiza 2018, 28, 411–419. [Google Scholar] [CrossRef]
- Gryndler, M.; Beskid, O.; Hujslová, M.; Konvalinkovà, T.; Bukovskà, P.; Zemkovà, L.; Hršelová, H.; Jansa, J. Soil receptivity for ectomycorrhizal fungi: Tuber aestivum is specifically stimulated by calcium carbonate and certain organic compounds, but not mycorrhizospheric bacteria. Appl. Soil Ecol. 2017, 117–118, 38–45. [Google Scholar] [CrossRef]
- Tarkka, M.T.; Drigo, B.; Deveau, A. Mycorrhizal microbiomes. Mycorrhiza 2018, 28, 403–409. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Chavatte, M.; Clausse, M.L.; Courrier, S.; Le Roux, C.; Raaijmakers, J.; Martinotti, M.G.; Pierrat, J.C.; Garbaye, J. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 2005, 165, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Batista, B.D.; Lacava, P.T.; Ferrari, A.; Sousa Teixeira-Silva, N.; Bonatelli, M.L.; Tsui, S.; Mondin, M.; Watanabe Kitajima, E.; Odair Pereira, J.; Azevedo, J.L.; et al. Screening of tropically derived, multi-trait plant growth- promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol. Res. 2018, 206, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Labbé, J.L.; Weston, D.J.; Dunkirk, N.; Pelletier, D.A.; Tuskan, G.A. Newly identified helper bacteria stimulate ectomycorrhizal formation in Populus. Front. Plant. Sci. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Rincón, A.; Ruiz-Díez, B.; García-Fraile, S.; Lucas-García, J.A.; Fernández-Pascual, M.; Pueyo, J.J.; de Felipe, M.R. Colonisation of Pinus halepensis roots by Pseudomonas fluorescens and interaction with the ectomycorrhizal fungus Suillus granulatus. FEMS Microbiol. Ecol. 2005, 51, 303–311. [Google Scholar] [CrossRef] [Green Version]

| Bacterial Inoculation Treatment | Bacteria/Growth Media | T. melanosporum Mycorrhization Rates (%) | Average Mycorrhization Rates (%)/Inoculation Treatment Type |
|---|---|---|---|
| Bacteria in growth media | Pseudomonas fluorescens (F) | 35.1 ± 1.6 a | 33.4 ± 1.4 a |
| Pseudomonas putida (P) | 32.9 ± 2.5 ab | ||
| Bacillus amyloliquefaciens (B) | 32.2 ± 3.1 ab | ||
| Growth media without bacteria | P. fluorescens growth medium (CF) | 26.8 ± 1.8 b | 29.6 ± 1.2 a |
| P. putida growth medium (CP) | 33.0 ± 2.0 ab | ||
| B. amyloliquefaciens growth medium (CB) | 29.1 ± 2.5 ab | ||
| No inoculation | Control seedlings (CS) | 25.4 ± 2.7 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñuela, Y.; G. Alday, J.; Oliach, D.; Bolaño, F.; Colinas, C.; Bonet, J.A. Use of Inoculator Bacteria to Promote Tuber melanosporum Root Colonization and Growth on Quercus faginea Saplings. Forests 2020, 11, 792. https://doi.org/10.3390/f11080792
Piñuela Y, G. Alday J, Oliach D, Bolaño F, Colinas C, Bonet JA. Use of Inoculator Bacteria to Promote Tuber melanosporum Root Colonization and Growth on Quercus faginea Saplings. Forests. 2020; 11(8):792. https://doi.org/10.3390/f11080792
Chicago/Turabian StylePiñuela, Yasmine, Josu G. Alday, Daniel Oliach, Francesc Bolaño, Carlos Colinas, and José Antonio Bonet. 2020. "Use of Inoculator Bacteria to Promote Tuber melanosporum Root Colonization and Growth on Quercus faginea Saplings" Forests 11, no. 8: 792. https://doi.org/10.3390/f11080792