Effects of Ageratina adenophora Invasion on the Understory Community and Soil Phosphorus Characteristics of Different Forest Types in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Community Investigation and Soil Sampling
2.3. Analysis of Soil P Indicators
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Understory Community
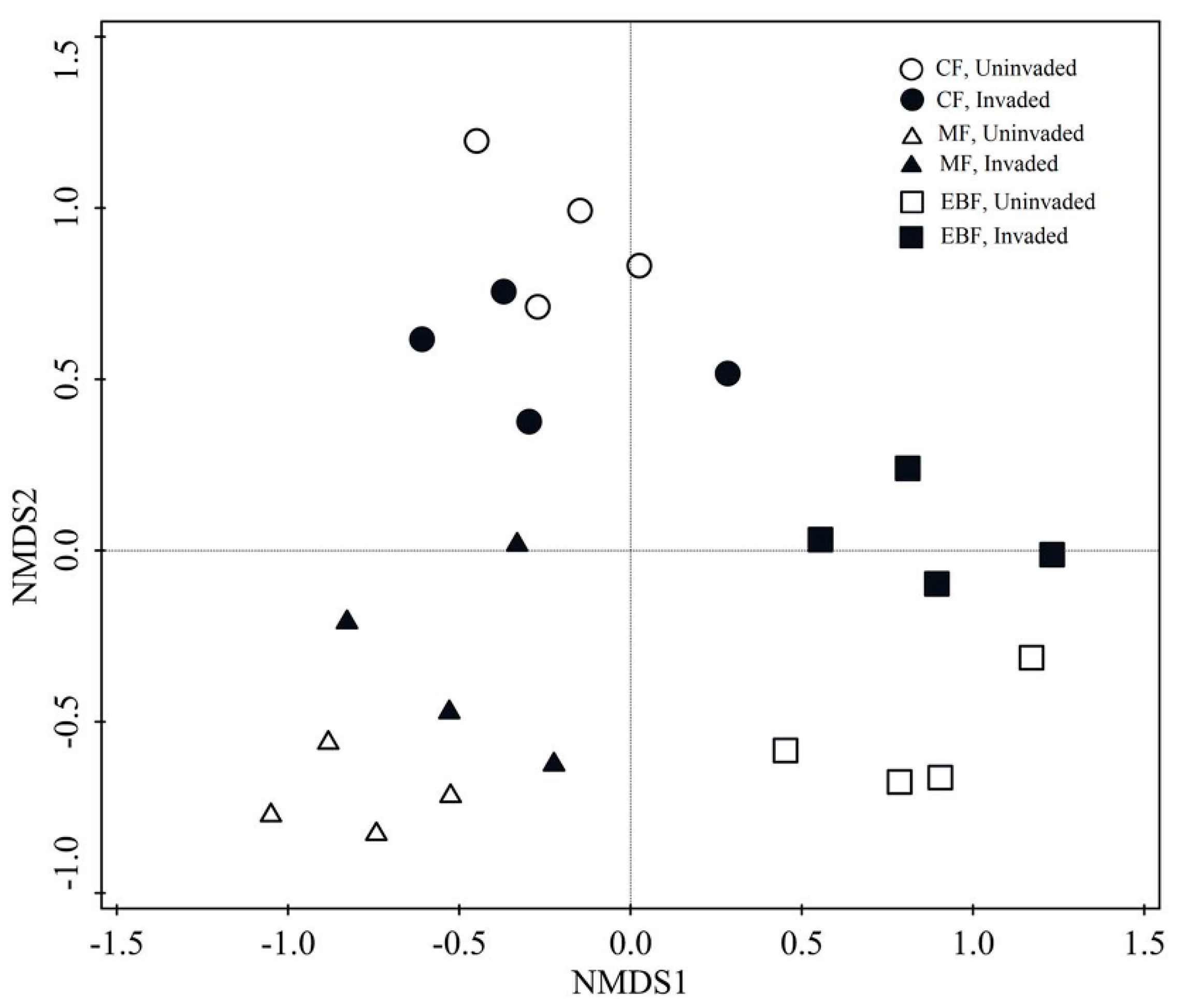
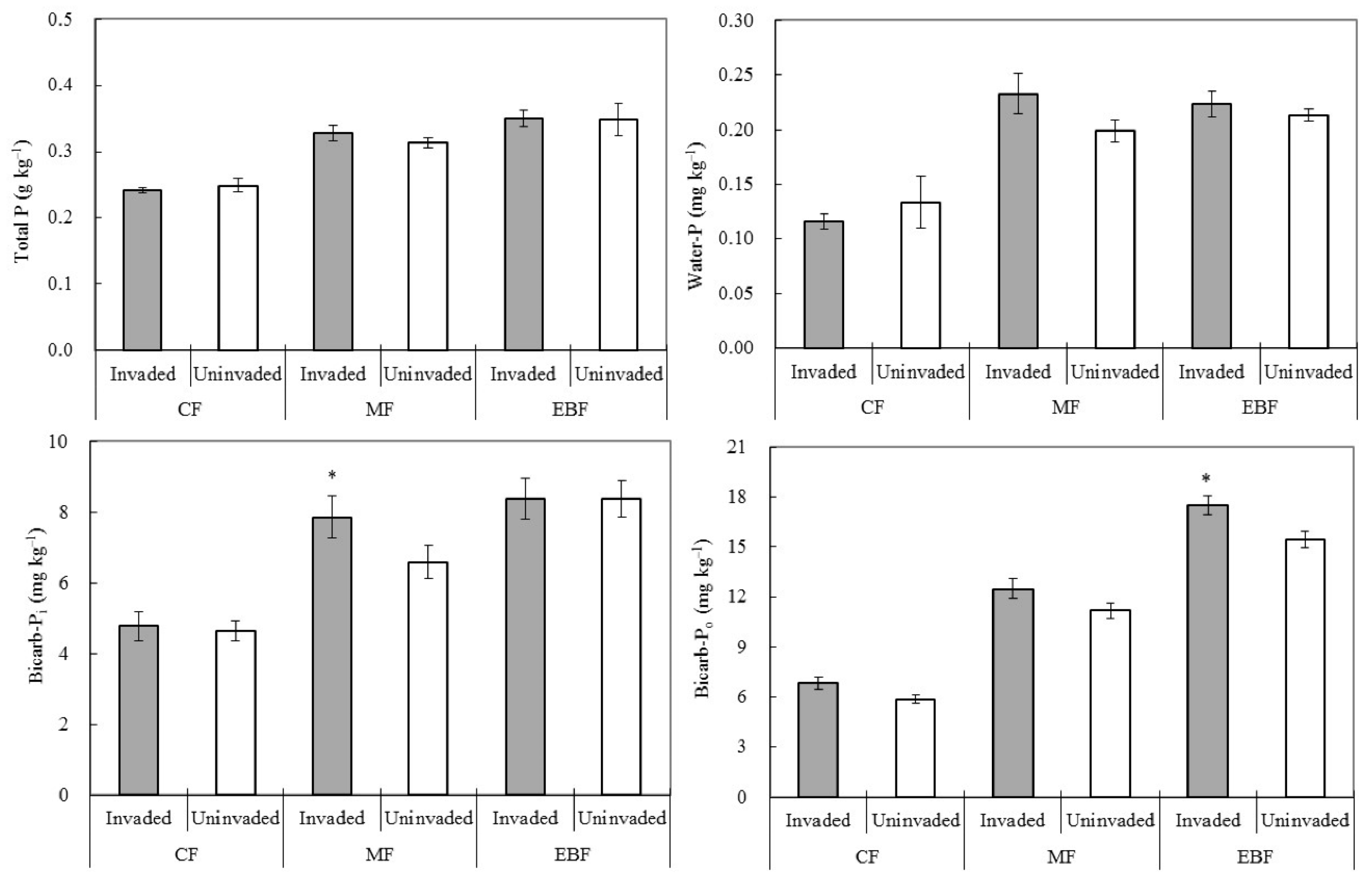
3.2. Response of Soil Phosphorus Status to Invasion
3.3. Relationship between Understory Community Properties and Soil P Status
4. Discussion
4.1. Effects of Invasion on the Understory Community under Different Forest Types
4.2. Effect of Invasion on Soil P Status in Different Forest Types
4.3. Relationships between Understory Vegetation and Soil P Status
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Little, C.J.; Altermatt, F. Species turnover and invasion of dominant freshwater invertebrates alter biodiversity–ecosystem-function relationship. Ecol. Monogr. 2018, 88, 461–480. [Google Scholar] [CrossRef] [Green Version]
- Courchamp, F.; Fournier, A.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Jeschke, J.M.; Russell, J.C. Invasion Biology: Specific Problems and Possible Solutions. Trends Ecol. Evol. 2017, 32, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGeoch, M.A.; Butchart, S.H.M.; Spear, D.; Marais, E.; Kleynhans, E.J.; Symes, A.; Chanson, J.; Hoffmann, M. Global indicators of biological invasion: Species numbers, biodiversity impact and policy responses. Divers. Distrib. 2010, 16, 95–108. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and Exotic Plant Invasion: From Molecules and Genes to Species Interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef]
- Landesman, W.J.; Nelson, D.M.; Fitzpatrick, M.C. Soil properties and tree species drive ß-diversity of soil bacterial communities. Soil Biol. Biochem. 2014, 76, 201–209. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Franzese, J.; Raffaele, E.; Blackhall, M.; Rodriguez, J.; Soto, A.Y. Changes in land cover resulting from the introduction of non-native pine modifies litter traits of temperate forests in Patagonia. J. Veg. Sci. 2020, 31, 223–233. [Google Scholar] [CrossRef]
- Bardgett, R.D. Plant trait-based approaches for interrogating belowground function. Biol. Environ. 2017, 117, 1–13. [Google Scholar]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant. Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Fried, G.; Carboni, M.; Mahaut, L.; Violle, C. Functional traits modulate plant community responses to alien plant invasion. Perspect. Plant. Ecol. Evol. Syst. 2019, 37, 53–63. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Loinaz, G.; Onaindia, M.; Amezaga, I.; Mijangos, I.; Garbisu, C. Relationship between vegetation diversity and soil functional diversity in native mixed-oak forests. Soil Biol. Biochem. 2008, 40, 49–60. [Google Scholar] [CrossRef]
- Šnajdr, J.; Dobiášová, P.; Urbanová, M.; Petránková, M.; Cajthaml, T.; Frouz, J.; Baldrian, P. Dominant trees affect microbial community composition and activity in post-mining afforested soils. Soil Biol. Biochem. 2013, 56, 105–115. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Perrett, C. Lantana camara L. (Verbenaceae) invasion effects on soil physicochemical properties. Biol. Fertil. Soils 2011, 47, 349–355. [Google Scholar] [CrossRef]
- Yu, F.-K.; Huang, X.-H.; Duan, C.-Q.; He, S.-Z.; Zhang, G.-S.; Liu, C.-E.; Fu, D.-G.; Shao, H.-B. Impacts of Ageratina adenophora invasion on soil physical-chemical properties of Eucalyptus plantation and implications for constructing agro-forest ecosystem. Ecol. Eng. 2014, 64, 130–135. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Sardans, J.; Zeng, C.-S.; Tong, C.; Wang, C.; Peñuelas, J. Impact of Plant Invasion and Increasing Floods on Total Soil Phosphorus and its Fractions in the Minjiang River Estuarine Wetlands, China. Wetlands 2016, 36, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhou, J.; Wang, L.; Cui, X.; Ning, C.; Wu, H.; Zhu, X.; Lin, G. Effects of short-term invasion of Spartina alterniflora and the subsequent restoration of native mangroves on the soil organic carbon, nitrogen and phosphorus stock. Chemosphere 2017, 184, 774–783. [Google Scholar] [CrossRef]
- Wu, C.; Mo, Q.; Wang, H.; Zhang, Z.; Huang, G.; Ye, Q.; Zou, Q.; Kong, F.; Liu, Y.; Geoff Wang, G. Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China. Ann. For. Sci. 2018, 75, 24. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Wu, X.; Huang, N.; Duan, C. Effects of the invasive herb Ageratina adenophora on understory plant communities and tree seedling growth in Pinus yunnanensis forests in Yunnan, China. J. For. Res. 2018, 23, 112–119. [Google Scholar] [CrossRef]
- Herr, C.; Chapuis-Lardy, L.; Dassonville, N.; Vanderhoeven, S.; Meerts, P. Seasonal effect of the exotic invasive plant Solidago gigantea on soil pH and P fractions. J. Plant. Nutr. Soil Sci. 2007, 170, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Chapuis-Lardy, L.; Vanderhoeven, S.; Dassonville, N.; Koutika, L.S.; Meerts, P. Effect of the exotic invasive plant Solidago gigantea on soil phosphorus status. Biol. Fertil. Soils 2006, 42, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Liu, W.; Wan, F.; Liu, B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant. Soil 2007, 294, 73–85. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Casanoves, F.; Pla, L.; Di Rienzo, J.A.; Díaz, S. FDiversity: A software package for the integrated analysis of functional diversity. Methods Ecol. Evol. 2011, 2, 233–237. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Lu, R.K. Analysis Method on Soil Agro-Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations1. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Canadian Society of Soil Science: Charlottetown, PEI, Canada, 1993; pp. 75–86. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. Biological and geochemical controls on phosphorus fractions in semiarid soils. Biogeochemistry 2001, 52, 155–172. [Google Scholar] [CrossRef]
- Pei, Z.; Eichenberg, D.; Bruelheide, H.; Kröber, W.; Kühn, P.; Li, Y.; von Oheimb, G.; Purschke, O.; Scholten, T.; Buscot, F.; et al. Soil and tree species traits both shape soil microbial communities during early growth of Chinese subtropical forests. Soil Biol. Biochem. 2016, 96, 180–190. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Ulrich, W. A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Model. Softw. 2011, 26, 173–178. [Google Scholar] [CrossRef]
- Chabrerie, O.; Loinard, J.; Perrin, S.; Saguez, R.; Decocq, G. Impact of Prunus serotina invasion on understory functional diversity in a European temperate forest. Biol. Invasions 2010, 12, 1891–1907. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Verheyen, K.; Vanhellemont, M.; Stock, T.; Hermy, M. Predicting patterns of invasion by black cherry (Prunus serotina Ehrh.) in Flanders (Belgium) and its impact on the forest understorey community. Divers. Distrib. 2007, 13, 487–497. [Google Scholar] [CrossRef]
- Tang, C.Q.; Zhao, M.-H.; Li, X.-S.; Ohsawa, M.; Ou, X.-K. Secondary succession of plant communities in a subtropical mountainous region of SW China. Ecol. Res. 2010, 25, 149–161. [Google Scholar] [CrossRef]
- Levine, J.M.; Vilà, M.; Antonio, C.M.D.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, W.; Almeida-Neto, M.; Gotelli, N.J. A consumer’s guide to nestedness analysis. Oikos 2009, 118, 3–17. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian Forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tecco, P.A.; Díaz, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Fargione, J.; Brown, C.S.; Tilman, D. Community assembly and invasion: An experimental test of neutral versus niche processes. Proc. Natl. Acad. Sci. USA 2003, 100, 8916–8920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- HuiNa, L.; WanXue, L.; Lian, D.; FangHao, W.; YuanYin, C. Invasive impacts of Ageratina adenophora (Asteraceae) on the changes of microbial community structure, enzyme activity and fertility in soil ecosystem. Sci. Agric. Sin. 2009, 42, 3964–3971. [Google Scholar]
- Fu, D.; Wu, X.; Duan, C.; Chadwick, D.R.; Jones, D.L. Response of soil phosphorus fractions and fl uxes to different vegetation restoration types in a subtropical mountain ecosystem. Catena 2020, 193, 104663. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- Daly, K.; Styles, D.; Lalor, S.; Wall, D.P. Phosphorus sorption, supply potential and availability in soils with contrasting parent material and soil chemical properties. Eur. J. Soil Sci. 2015, 66, 792–801. [Google Scholar] [CrossRef]
- Maguire, R.O.; Foy, R.H.; Bailey, J.S.; Sims, J.T. Estimation of the phosphorus sorption capacity of acidic soils in Ireland. Eur. J. Soil Sci. 2001, 52, 479–487. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Ai, R.; Jin, L. Impacts of native and exoticvegetation types on a cambisols properties in the mid-Yunnan Plateau. Chin. J. Soil Sci. 2011, 42, 852–858. [Google Scholar]
- Zhao, Q.; Zeng, D.-H.; Fan, Z.-P.; Yu, Z.-Y.; Hu, Y.-L.; Zhang, J. Seasonal variations in phosphorus fractions in semiarid sandy soils under different vegetation types. For. Ecol. Manag. 2009, 258, 1376–1382. [Google Scholar] [CrossRef]
- Turrión, M.-B.; López, O.; Lafuente, F.; Mulas, R.; Ruipérez, C.; Puyo, A. Soil phosphorus forms as quality indicators of soils under different vegetation covers. Sci. Total Environ. 2007, 378, 195–198. [Google Scholar] [CrossRef]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef] [PubMed]

| CF | MF | EBF | ||
|---|---|---|---|---|
| Tree layer | ||||
| Coverage | 0.66 ± 0.02b | 0.78 ± 0.04a | 0.82 ± 0.03a | |
| Height (m) | 9.75 ± 0.48b | 16.00 ± 0.41a | 15.00 ± 0.41a | |
| Basic soil properties | ||||
| pH | Invaded | 4.53 ± 0.03a | 4.37 ± 0.04b | 4.27 ± 0.06b |
| Uninvaded | 4.57 ± 0.03a | 4.39 ± 0.05b | 4.26 ± 0.05b | |
| SOC (g kg–1) | Invaded | 39.17 ± 1.49c | 45.25 ± 1.17 b | 49.93 ± 0.23a |
| Uninvaded | 37.82 ± 1.02c | 43.76 ± 0.82b | 47.77 ± 0.91a | |
| TN (g kg–1) | Invaded | 0.74 ± 0.04b | 1.19 ± 0.07a | 1.44 ± 0.12a |
| Uninvaded | 0.67 ± 0.07b | 1.08 ± 0.09a | 1.32 ± 0.09a | |
| S | H | E | FRic | FEve | FDiv | FDis | |
|---|---|---|---|---|---|---|---|
| Vegetation | 3.56 | 15.40 *** | 14.78 ** | 3.71 | 6.85 ** | 2.49 | 16.20 *** |
| Invasion | 7.10 * | 0.29 | 4.41 | 9.64 ** | 4.89 * | 1.36 | 9.57 ** |
| Vegetation × invasion | 1.92 | 3.76 | 0.19 | 1.76 | 0.29 | 0.77 | 17.94 *** |
| CF | MF | EBF | ||||
|---|---|---|---|---|---|---|
| Invaded | Uninvaded | Invaded | Uninvaded | Invaded | Uninvaded | |
| S | 9.00 ± 0.58 * | 14.67 ± 1.20 | 11.67 ± 0.67 | 13.00 ± 1.15 | 14.33 ± 2.33 | 15.67 ± 0.88 |
| H | 1.16 ± 0.03 * | 1.29 ± 0.02 | 1.31 ± 0.03 | 1.23 ± 0.03 | 1.07 ± 0.07 | 1.07 ± 0.02 |
| E | 0.53 ± 0.01 | 0.48 ± 0.02 | 0.54 ± 0.01 | 0.49 ± 0.02 | 0.41 ± 0.04 | 0.39 ± 0.02 |
| FRic | 11.33 ± 5.51 ** | 47.68 ± 1.32 | 15.78 ± 5.36 | 21.67 ± 3.35 | 31.05 ± 12.69 | 50.70 ± 12.82 |
| FEve | 0.56 ± 0.02 | 0.64 ± 0.05 | 0.46 ± 0.01 | 0.50 ± 0.04 | 0.45 ± 0.06 | 0.53 ± 0.02 |
| FDiv | 0.84 ± 0.08 | 0.93 ± 0.01 | 0.93 ± 0.01 | 0.94 ± 0.01 | 0.96 ± 0.02 | 0.97 ± 0.01 |
| FDis | 7.54 ± 0.44 * | 10.04 ± 0.91 | 8.96 ± 0.98 * | 5.65 ± 0.67 | 14.01 ± 0.66 * | 8.74 ± 1.00 |
| TP | Water-Pi | Bicarb-Pi | Bicarb-Po | Sm | MBC | |
|---|---|---|---|---|---|---|
| Vegetation | 38.59 *** | 27.80 *** | 245.85 *** | 259.31 *** | 6.65 ** | 14.14 ** |
| Invasion | 0.08 | 0.58 | 11.54 ** | 15.64 ** | 16.52 ** | 148.16 *** |
| Vegetation × invasion | 0.41 | 1.61 | 8.71 ** | 0.77 | 1.23 | 0.24 |
| k | Sm (mg kg−1) | MBC (mg kg−1) | ||
|---|---|---|---|---|
| CF | Invaded | 0.25 ± 0.01 | 1119 ± 74 * | 274 ± 22 ** |
| Uninvaded | 0.27 ± 0.01 | 1570 ± 87 | 416 ± 11 | |
| MF | Invaded | 0.21 ± 0.01 * | 1467 ± 11 | 313 ± 16 ** |
| Uninvaded | 0.29 ± 0.02 | 1632 ± 128 | 471 ± 10 | |
| EBF | Invaded | 0.27 ± 0.02 | 1665 ± 126 | 448 ± 19 |
| Uninvaded | 0.28 ± 0.01 | 1792 ± 74 | 494 ± 13 |
| Predictive Factors | TP | Water-Pi | Bicarb-Pi | Bicarb-Po | Sm | MBC |
|---|---|---|---|---|---|---|
| FRic | 4.90 * | 4.04 | 0.69 | 0.36 | 3.46 | 0.29 |
| Vegetation | 52.39 *** | 25.50 *** | 118.75 *** | 201.60 *** | 5.34 * | 15.56 ** |
| Invasion | 4.38 | 0.88 | 7.48 * | 1.71 | 1.40 | 26.76 *** |
| FRic × invasion | 6.11 * | |||||
| FEve | 1.10 | 0.01 | 0.12 | 0.34 | 0.13 | 0.06 |
| Vegetation | 27.56 *** | 11.22 ** | 62.62 *** | 151.81 *** | 4.99 * | 11.49 ** |
| Invasion | 0.65 | 0.31 | 4.45 | 9.14 ** | 5.41 * | 31.35 *** |
| FDiv | 0.29 | 0.01 | 0.01 | 0.98 | 0.17 | 0.16 |
| Vegetation | 27.18 *** | 18.27 *** | 79.95 *** | 187.41 *** | 4.56 * | 11.73 ** |
| Invasion | 0.19 | 0.41 | 4.53 | 17.07 ** | 7.32 * | 36.33 *** |
| FDis | 0.03 | 4.50 | 2.33 | 0.96 | 3.01 | 1.38 |
| Vegetation | 17.94 *** | 20.10 *** | 157.33 *** | 130.37 *** | 4.65 * | 5.92 * |
| Invasion | 0.29 | 0.16 | 15.72 ** | 0.28 | 0.22 | 14.52 ** |
| FDis × invasion | 12.20 ** | |||||
| S | 0.57 | 0.77 | 0.14 | 0.10 | 7.44 * | 1.32 |
| Vegetation | 30.14 *** | 20.25 *** | 84.15 *** | 189.40 *** | 3.87 * | 10.06 ** |
| Invasion | 0.44 | 1.19 | 4.31 | 9.35 ** | 2.42 | 24.56 *** |
| H | 0.00 | 0.11 | 6.96 * | 3.29 | 1.20 | 0.72 |
| Vegetation | 31.95 *** | 32.12 *** | 263.11 *** | 221.16 *** | 6.31 * | 17.56 *** |
| Invasion | 0.00 | 0.00 | 7.52 * | 1.99 | 0.94 | 6.16 * |
| H × invasion | 9.53 ** | 10.09 ** | ||||
| E | 0.50 | 0.81 | 0.33 | 2.14 | 0.98 | 0.07 |
| Vegetation | 23.97 *** | 18.87 *** | 101.24 *** | 158.59 *** | 2.92 | 8.94 ** |
| Invasion | 0.27 | 0.09 | 3.60 | 1.68 | 0.27 | 2.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Duan, C.; Fu, D.; Peng, P.; Zhao, L.; Jones, D.L. Effects of Ageratina adenophora Invasion on the Understory Community and Soil Phosphorus Characteristics of Different Forest Types in Southwest China. Forests 2020, 11, 806. https://doi.org/10.3390/f11080806
Wu X, Duan C, Fu D, Peng P, Zhao L, Jones DL. Effects of Ageratina adenophora Invasion on the Understory Community and Soil Phosphorus Characteristics of Different Forest Types in Southwest China. Forests. 2020; 11(8):806. https://doi.org/10.3390/f11080806
Chicago/Turabian StyleWu, Xiaoni, Changqun Duan, Denggao Fu, Peiyuan Peng, Luoqi Zhao, and Davey L. Jones. 2020. "Effects of Ageratina adenophora Invasion on the Understory Community and Soil Phosphorus Characteristics of Different Forest Types in Southwest China" Forests 11, no. 8: 806. https://doi.org/10.3390/f11080806
APA StyleWu, X., Duan, C., Fu, D., Peng, P., Zhao, L., & Jones, D. L. (2020). Effects of Ageratina adenophora Invasion on the Understory Community and Soil Phosphorus Characteristics of Different Forest Types in Southwest China. Forests, 11(8), 806. https://doi.org/10.3390/f11080806





