Slash Pile Burn Scar Restoration: Tradeoffs between Abundance of Non-Native and Native Species
Abstract
:1. Introduction
2. Materials and Methods
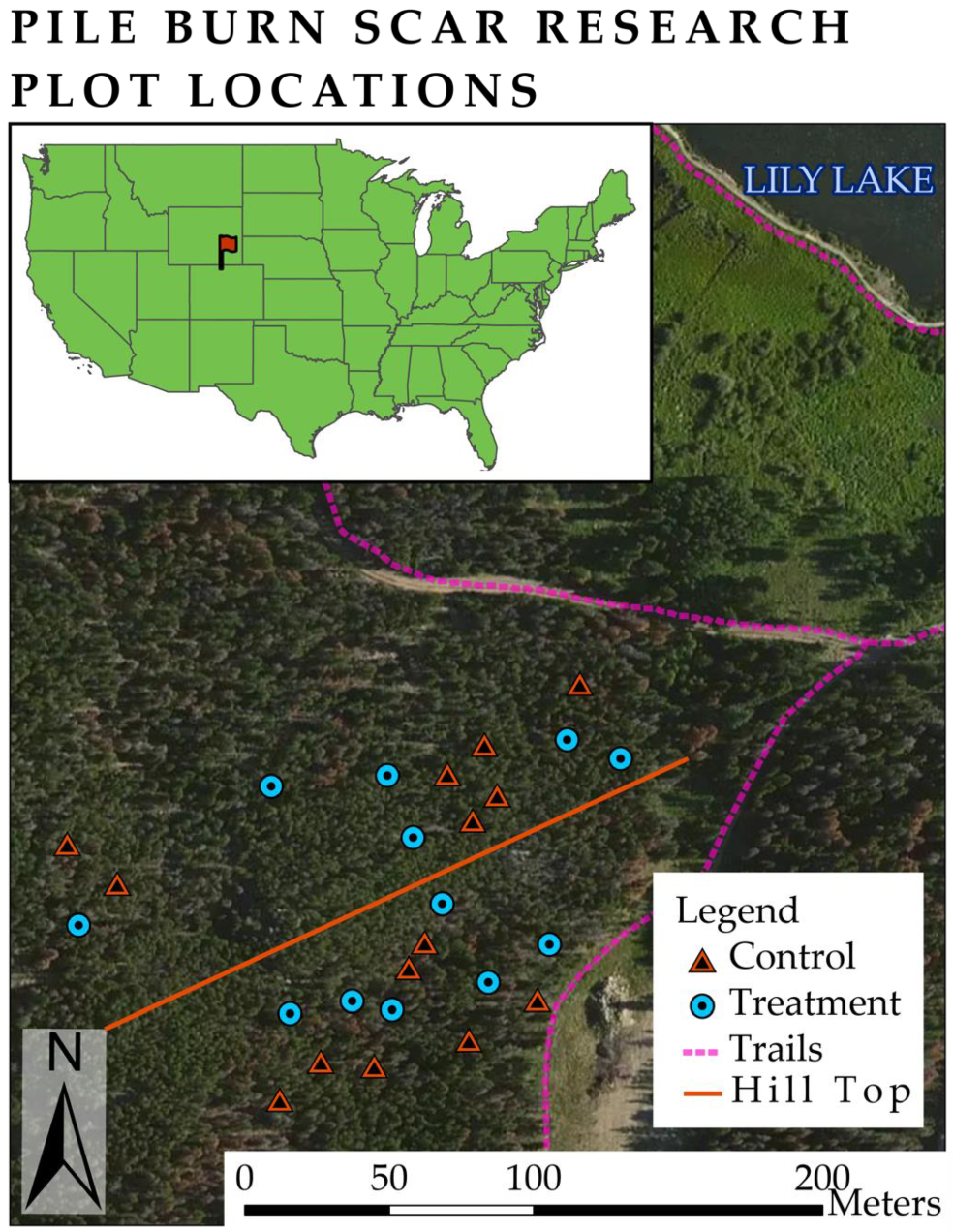
2.1. Site Selection and Plot Establishment
2.2. Restoration Methods
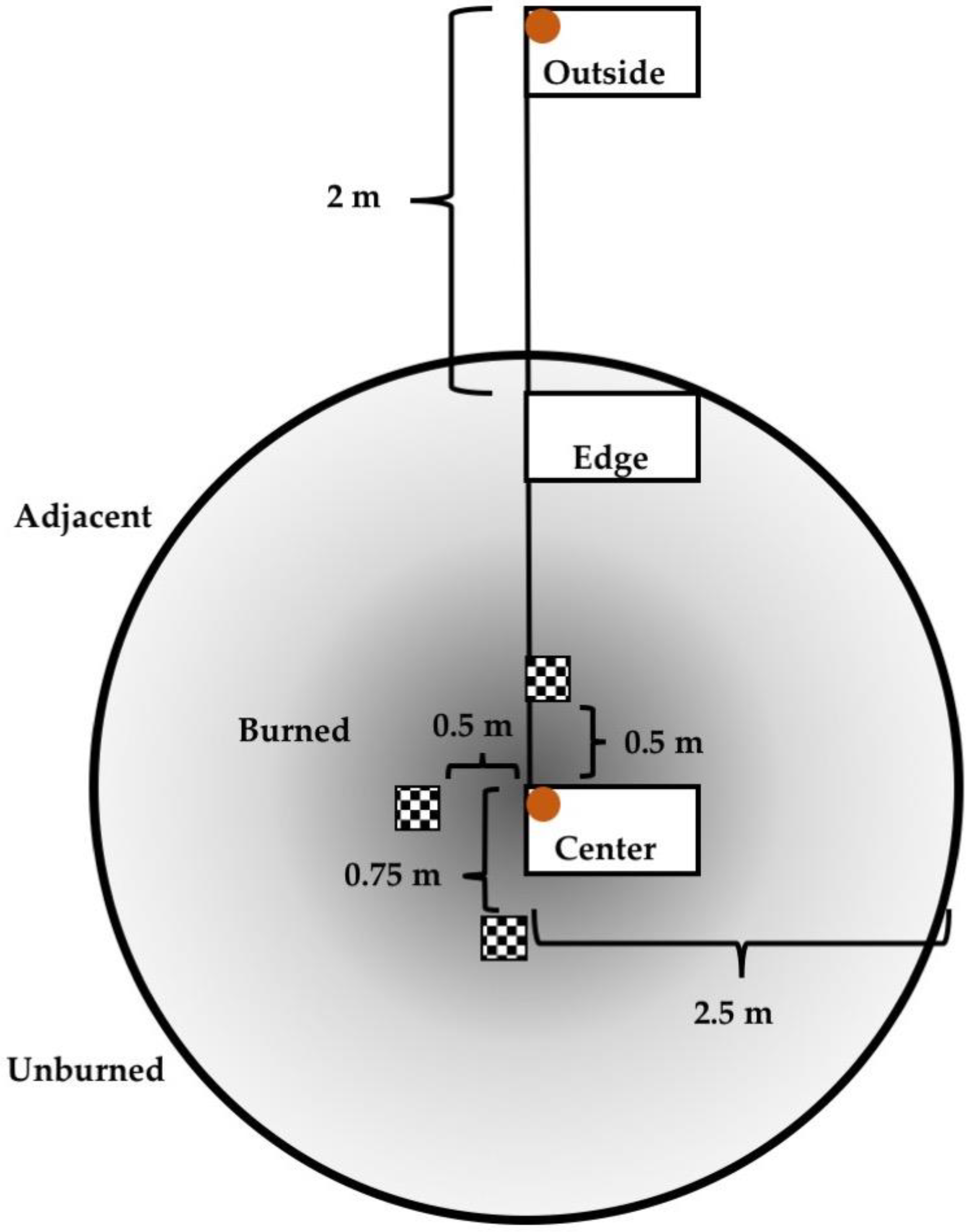
2.3. Vegetation Sampling
2.4. Seedling Establishment Study
2.5. Data Analysis
3. Results
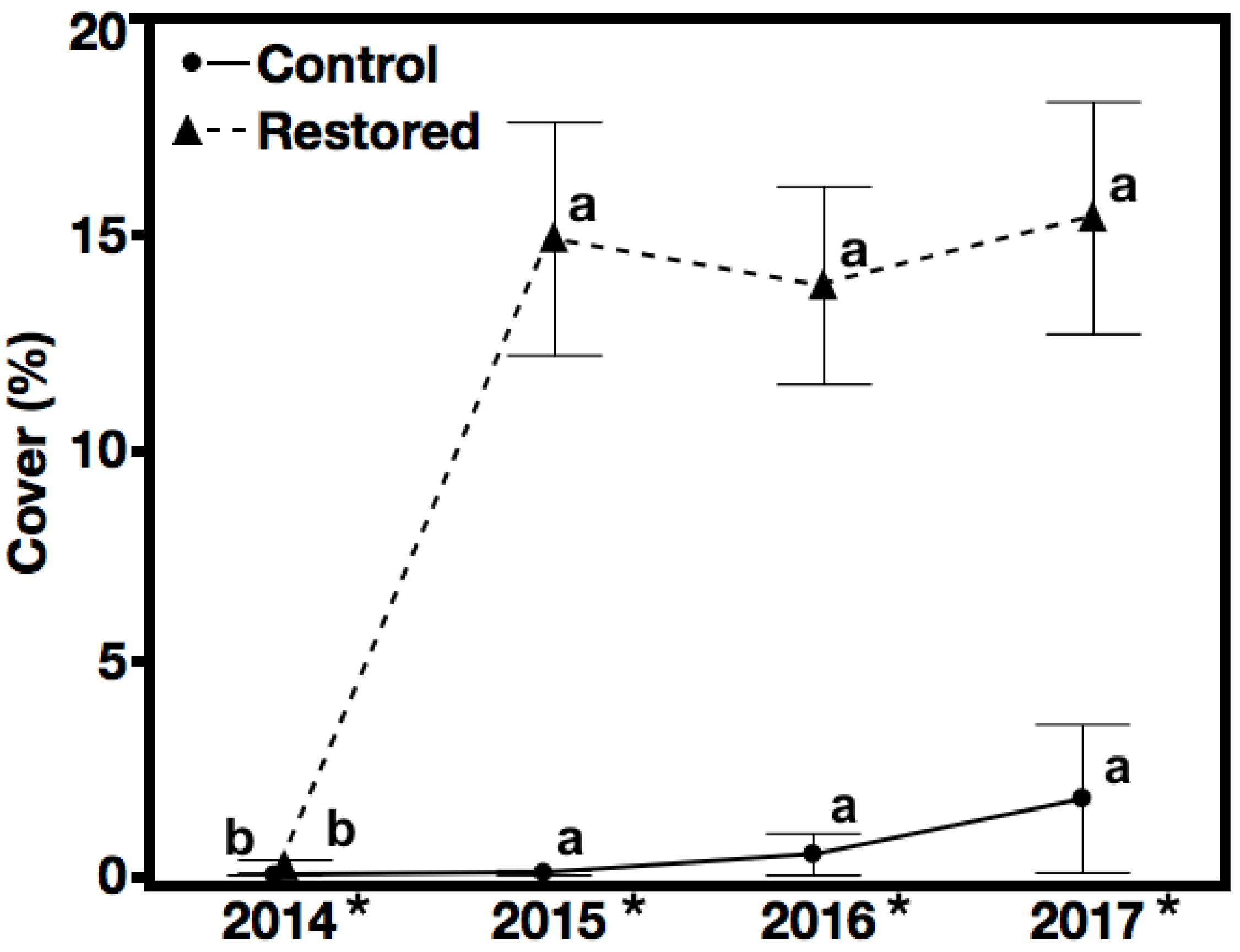
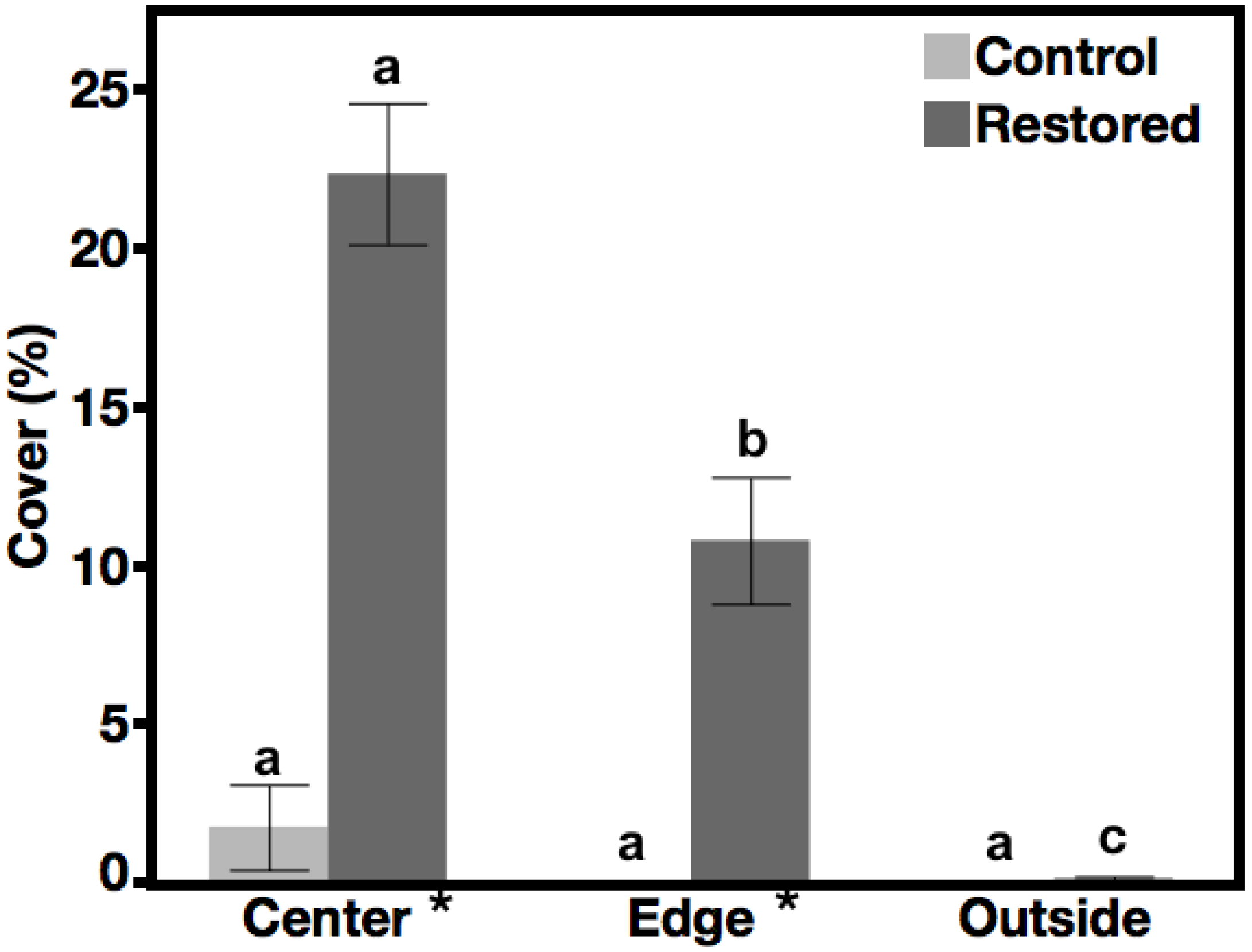
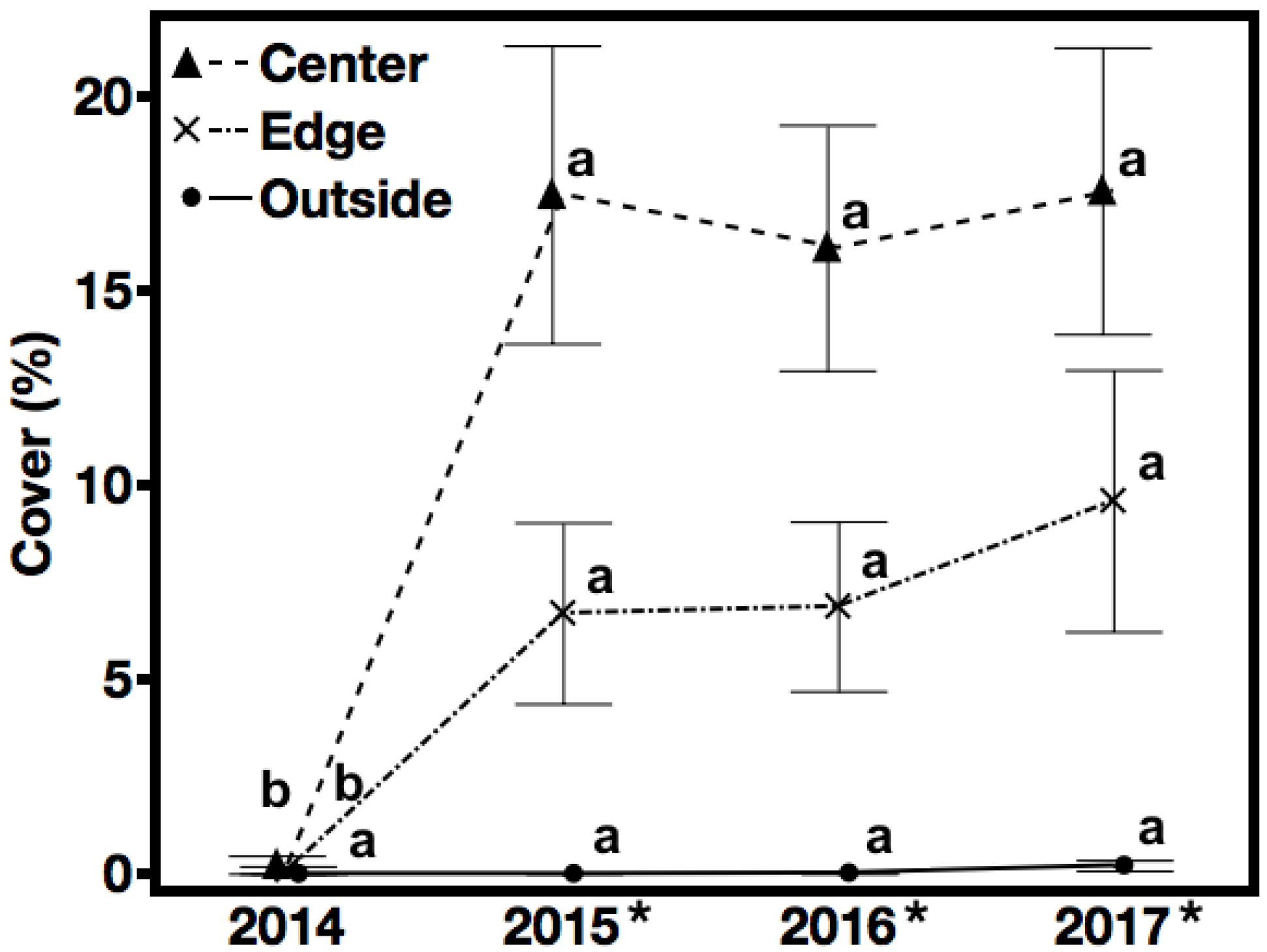
3.1. Seeded Species Cover—Hypothesis 1, Prediction 1
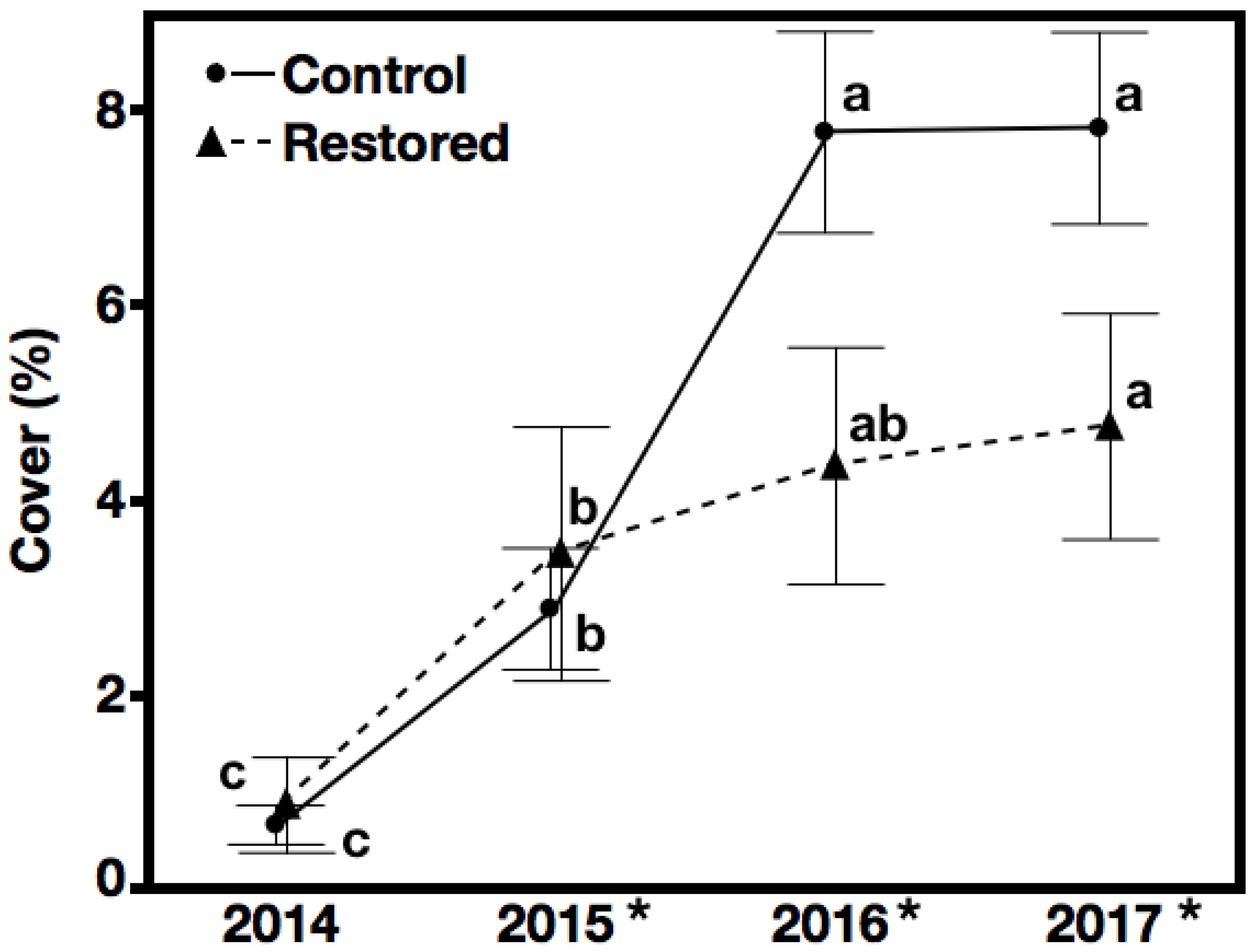
3.2. Non-Native Species Cover—Hypothesis 2, Prediction 2
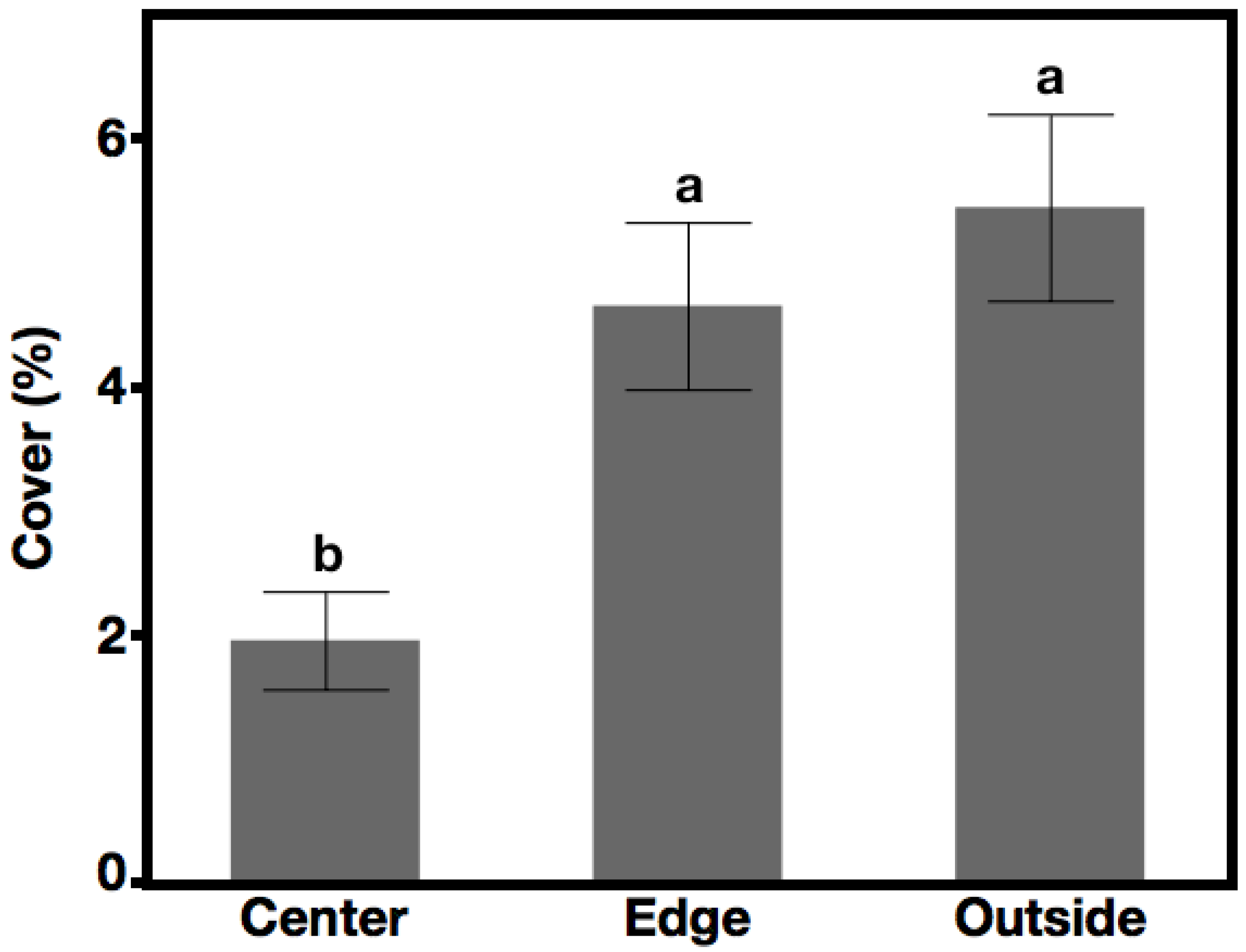
3.3. Native Species, Not Seeded Cover—Hypothesis 3, Prediction 3
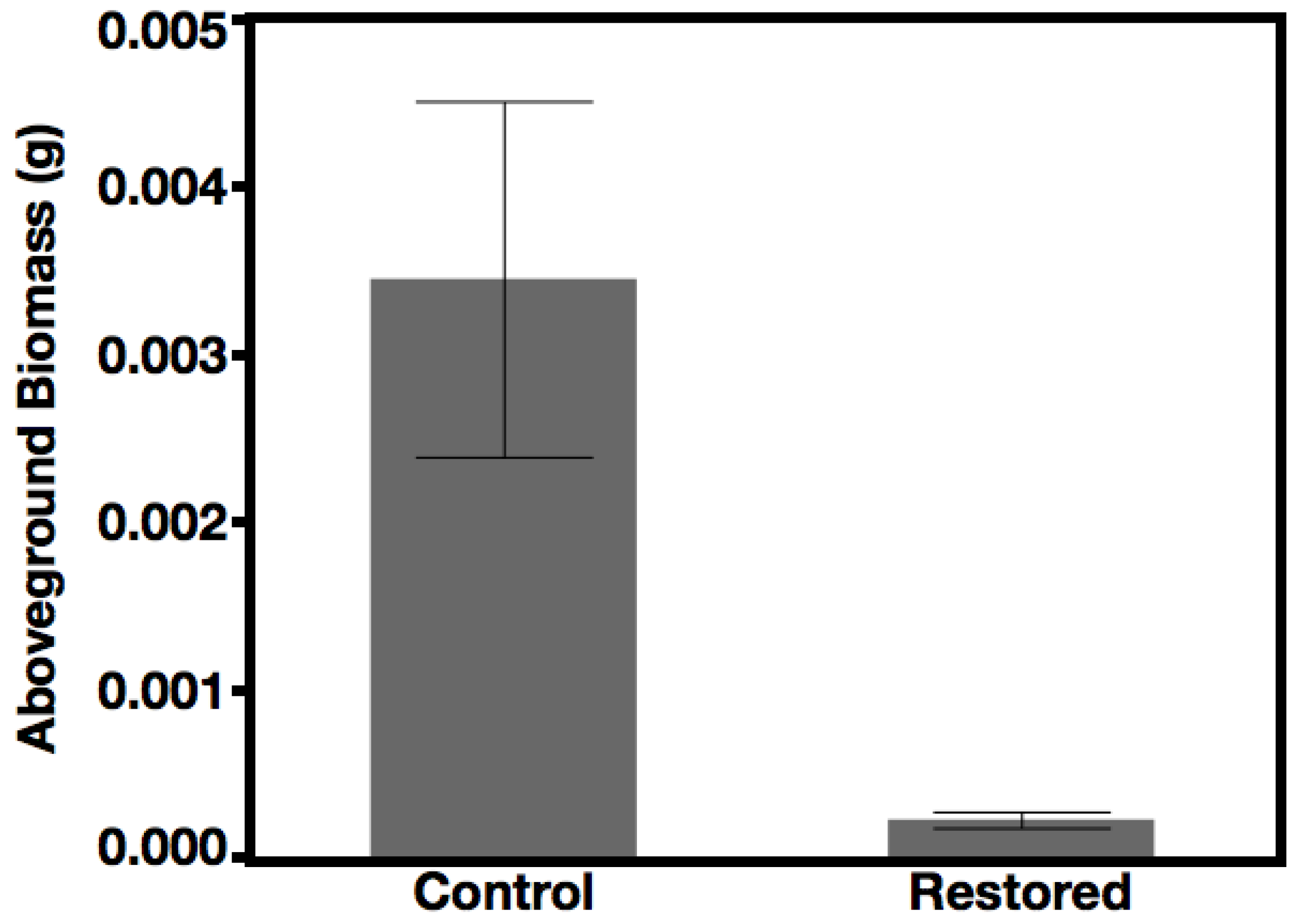
3.4. Recruitment and Productivity of Species Seeded Three Years After Restoration—Hypothesis 3, Prediction 4
4. Discussion
4.1. Hypothesis 1: Establishment of Seeded Species
4.2. Hypothesis 2: Suppression of Non-Native Species
4.3. Hypothesis 3: Suppression of Unseeded Native Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A




| Control | Restored | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Agrostis stolonifera L. | 0.1111 | 0.0499 | 0.0863 | 0.0426 |
| Bromus inermis Leyss. | 0.0486 | 0.0313 | 0 | 0 |
| Bromus tectorum L. | 0.0313 | 0.0198 | 0 | 0 |
| Cirsium arvense (L.) Scop. | 1.4861 | 0.4556 | 0.1310 | 0.0590 |
| Phleum pratense L. | 0.0799 | 0.0589 | 0.0744 | 0.0411 |
| Poa compressa L. | 0.5313 | 0.2156 | 0.4196 | 0.1706 |
| Rumex acetosella L. | 0.4306 | 0.2150 | 0 | 0 |
| Rumex crispus L. | 0 | 0 | 0.0685 | 0.0505 |
| Salsola collina Pall. | 0.0035 | 0.0035 | 0 | 0 |
| Taraxacum officinale F. H. Wigg. | 0.2118 | 0.0603 | 0.2440 | 0.1226 |
| Control | Restored | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Achillea millefolium L. | 0 | 0 | 0.0030 | 0.0030 |
| Agrostis scabra Willd. | 0.1840 | 0.0737 | 0.0149 | 0.0123 |
| Agrostis sp. | 0.2986 | 0.1071 | 0.0952 | 0.0531 |
| Androsace septentrionalis L. | 0.0139 | 0.0139 | 0.0506 | 0.0448 |
| Antennaria parvifolia Nutt. | 0.0035 | 0.0035 | 0 | 0 |
| Antennaria sp. | 0.0278 | 0.0278 | 0 | 0 |
| Arabis fendleri (S. Watson) Greene | 0 | 0 | 0.0030 | 0.0030 |
| Arctostaphylos uva-ursi (L.) Spreng | 0 | 0 | 0.0119 | 0.0119 |
| Arnica cordifolia Hook. | 0.0694 | 0.0539 | 0.0238 | 0.0238 |
| Arnica latifolia Bong. | 0 | 0 | 0.0149 | 0.0149 |
| Arnica sp. | 0.0660 | 0.0538 | 0 | 0 |
| Artemisia ludoviciana (Nutt). | 0.0278 | 0.0278 | 0.0119 | 0.0119 |
| Artemisia sp. | 0.0278 | 0.0278 | 0 | 0 |
| Astragalus sp. | 0.0035 | 0.0035 | 0 | 0 |
| Carex sp. | 1.0278 | 0.2183 | 0.8542 | 0.2923 |
| Chamerion angustifolium (L.) Holub | 0.0139 | 0.0139 | 0.0595 | 0.0313 |
| Chenopodium fremontii S. Watson | 0.1424 | 0.0758 | 0.0030 | 0.0030 |
| Chenopodium sp. | 0.0035 | 0.0035 | 0 | 0 |
| Corydalis aurea Willd. | 0.0035 | 0.0035 | 0.0506 | 0.0448 |
| Descurainia incana (Bernh. ex Fisch. & C.A. Mey.) Dorn | 0.0035 | 0.0035 | 0 | 0 |
| Potentilla fissa Nutt. | 0.0035 | 0.0035 | 0.0030 | 0.0030 |
| Epilobium ciliatum Raf. | 0.0243 | 0.0151 | 0 | 0 |
| Epilobium lactiflorum Hausskn. | 0.0174 | 0.0143 | 0.0030 | 0.0030 |
| Epilobium palustre L. | 0.0035 | 0.0035 | 0 | 0 |
| Epilobium sp. | 0.0243 | 0.0151 | 0.0060 | 0.0042 |
| Equisetum arvense L. | 0 | 0 | 0.0030 | 0.0030 |
| Erysimum capitatum (Douglas ex Hook.) Greene | 0.0486 | 0.0313 | 0 | 0 |
| Festuca saximontana Rydb. | 0.0139 | 0.0139 | 0.0476 | 0.0290 |
| Festuca sp. | 0 | 0 | 0.0030 | 0.0030 |
| Fragaria sp. | 0 | 0 | 0.0030 | 0.0030 |
| Fragaria virginiana Duchesne | 0 | 0 | 0.0060 | 0.0042 |
| Gayophytum diffusum Torr. & A. Gray | 0.0035 | 0.0035 | 0.0298 | 0.0138 |
| Grindelia subalpina Greene | 0.0035 | 0.0035 | 0.0030 | 0.0030 |
| Harbouria trachypleura (A. Gray) J.M. Coult. & Rose | 0 | 0 | 0.0298 | 0.0173 |
| Heterotheca villosa | 0.0278 | 0.0278 | 0 | 0 |
| Koeleria macrantha (Ledeb.) Schult. | 0.0278 | 0.0196 | 0 | 0 |
| Leymus ambiguus | 0.0174 | 0.0143 | 0 | 0 |
| Lupinus bakeri | 0 | 0 | 0.0030 | 0.0030 |
| Monolepis nuttalliana (Schult.) Greene | 0.0208 | 0.0147 | 0 | 0 |
| Oreochrysum parryi (A. Gray) Rydb. | 0.0278 | 0.0278 | 0.0238 | 0.0238 |
| Oxytropis sp. | 0.0660 | 0.0538 | 0.0030 | 0.0030 |
| Packera fendleri (A. Gray) W.A. Weber & Á. Löve | 0.0556 | 0.0391 | 0 | 0 |
| Penstemon sp. | 0.0035 | 0.0035 | 0 | 0 |
| Penstemon virens Pennell ex Rydb. | 0.1736 | 0.0660 | 0.0030 | 0.0030 |
| Phacelia alba Rydb. | 0.0035 | 0.0035 | 0.0685 | 0.0505 |
| Phacelia hastata Douglas ex Lehm. | 0 | 0 | 0.2649 | 0.1535 |
| Phacelia sericea (Graham) A. Gray | 0.1007 | 0.0605 | 0.0595 | 0.0462 |
| Phacelia sp. | 0 | 0 | 0.0030 | 0.0030 |
| Pinus contorta Douglas ex Loudon | 0.0486 | 0.0243 | 0.0446 | 0.0210 |
| Pinus sp. | 0.1111 | 0.0272 | 0.0655 | 0.0279 |
| Potentilla fissa Nutt. | 0.0208 | 0.0147 | 0.0238 | 0.0168 |
| Potentilla norvegica L. | 0 | 0 | 0.0238 | 0.0238 |
| Pseudognaphalium macounii (Greene) Kartesz | 0.0417 | 0.0310 | 0 | 0 |
| Pseudotsuga menziesii (Mirb.) Franco | 0.0035 | 0.0035 | 0.0060 | 0.0042 |
| Ribes sp. | 0.0035 | 0.0035 | 0.0238 | 0.0168 |
| Rosa woodsii Lindl. | 0.0451 | 0.0241 | 0 | 0 |
| Rubus idaeus L. | 0.4722 | 0.1943 | 0.1220 | 0.0651 |
| Sedum lanceolatum Torr. | 0.0521 | 0.0245 | 0.0863 | 0.0426 |
| Senecio eremophilus Richardson | 0.0035 | 0.0035 | 0.1280 | 0.1067 |
| Senecio sp. | 0.0035 | 0.0035 | 0 | 0 |
| Solidago simplex Kunth | 0.0660 | 0.0538 | 0 | 0 |
| Solidago sp. | 0 | 0 | 0.0714 | 0.0505 |
| Unk. Forb | 0.5208 | 0.1295 | 0.2619 | 0.1479 |
| Unk. Grass | 0.0625 | 0.0220 | 0.0476 | 0.0271 |
| Unk. Sedge | 0.7431 | 0.2190 | 0.6815 | 0.2218 |
References
- Adams, M.A. Mega-fires, tipping points and ecosystem services: Managing forests and woodlands in an uncertain future. For. Ecol. Manag. 2013, 294, 250–261. [Google Scholar] [CrossRef]
- Waltz, A.E.M.; Stoddard, M.T.; Kalies, E.L.; Springer, J.D.; Huffman, D.W.; Meador, A.S. Effectiveness of fuel reduction treatments: Assessing metrics of forest resiliency and wildfire severity after the Wallow Fire, AZ. For. Ecol. Manag. 2014, 334, 43–52. [Google Scholar] [CrossRef]
- Korb, J.E.; Johnson, N.C.; Covington, W.W. Slash pile burning effects on soil biotic and chemical properties and plant establishment: Recommendations for amelioration. Restor. Ecol. 2004, 12, 52–62. [Google Scholar] [CrossRef]
- Creech, M.N.; Kirkman, L.K.; Morris, L.A. Alteration and Recovery of Slash Pile Burn Sites in the Restoration of a Fire-Maintained Ecosystem. Restor. Ecol. 2012, 20, 505–516. [Google Scholar] [CrossRef]
- Fornwalt, P.J.; Rhoades, C.C. Rehabilitating Slash Pile Burn Scars in Upper Montane Forests of The Colorado Front Range. Nat. Areas J. 2011, 31, 177–182. [Google Scholar] [CrossRef]
- DeSandoli, L.; Turkington, R.; Fraser, L.H. Restoration of slash pile burn scars to prevent establishment and propagation of non-native plants. Can. J. For. Res. 2016, 46, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, C.C.; Fornwalt, P.J. Pile burning creates a fifty-year legacy of openings in regenerating lodgepole pine forests in Colorado. For. Ecol. Manag. 2015, 336, 203–209. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Fornwalt, P.J.; Paschke, M.W.; Shanklin, A.; Jonas, J.L. Recovery of small pile burn scars in conifer forests of the Colorado Front Range. For. Ecol. Manag. 2015, 347, 180–187. [Google Scholar] [CrossRef]
- Haskins, K.E.; Gehring, C.A. Long-term effects of burning slash on plant communities and arbuscular mycorrhizae in a semi-arid woodland. J. Appl. Ecol. 2004, 41, 379–388. [Google Scholar] [CrossRef]
- Hubbert, K.R.; Busse, M.; Overby, S.; Shestak, C.; Gerrard, R. Pile burning effects on soil water repellency, infiltration, and downslope water chemistry in the lake tahoe basin, USA. Fire Ecol. 2015, 11, 100–118. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Eschen, R.; Mortimer, S.R.; Lawson, C.S.; Edwards, A.R.; Brook, A.J.; Igual, J.M.; Hedlund, K.; Schaffner, U. Carbon addition alters vegetation composition on ex-arable fields. J. Appl. Ecol. 2007, 44, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Redmond, M.D.; Zelikova, T.J.; Barger, N.N. Limits to Understory Plant Restoration Following Fuel-Reduction Treatments in a Pion-Juniper Woodland. Environ. Manag. 2014, 54, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Havrilla, C.A.; Faist, A.M.; Barger, N.N. Understory Plant Community Responses to Fuel-Reduction Treatments and Seeding in an Upland Pinon-Juniper Woodland. Rangel. Ecol. Manag. 2017, 70, 609–620. [Google Scholar] [CrossRef]
- Kerns, B.K.; Day, M.A. Fuel Reduction, Seeding, and Vegetation in a Juniper Woodland. Rangel. Ecol. Manag. 2014, 67, 667–679. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.J.; Wragg, P.D.; Reich, P.B. Using revegetation to suppress invasive plants in grasslands and forests. J. Appl. Ecol. 2018, 55, 2362–2373. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Temperton, V.M.; Zirr, K. Order of Arrival and Availability of Safe Sites: An Example of Their Importance for Plant Community Assembly in Stressed Ecosystems. In Assembly Rules and Restoration Ecology: Bridging the Gap between Theory and Practice; Temperton, V.M., Hobbs, R.J., Nuttle, T., Halle, S., Eds.; Island Press: Washington, DC, USA, 2004; pp. 285–304. [Google Scholar]
- United States Department of Agriculture. Copeland Lake; SNOTEL Station 412; 1988–2017 Data. Available online: https://wcc.sc.egov.usda.gov/ (accessed on 9 November 2018).
- Daubenmire, R. A canopy-coverage method of vegetational analysis. Northwest Sci. 1959, 33, 43–64. [Google Scholar]
- United States Department of Agriculture-Natural Resources Conservation Service. The Plants Database. Available online: http://plants.usda.gov/ (accessed on 26 January 2016).
- Akaike, H. New look at statistical-model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Arredondo, J.T.; Jones, T.A.; Johnson, D.A. Seedling growth of Intermountain perennial and weedy annual grasses. J. Range Manag. 1998, 51, 584–589. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, E.; Perkins, L. Bromus inermis and Elymus canadensis but not Poa pratensis demonstrate strong competitive effects and all benefit from priority. Plant Ecol. 2014, 215, 1269–1275. [Google Scholar] [CrossRef]


| Seed Mix Composition | % Pure Live Seed | |
|---|---|---|
| Elymus elymoides (Raf.) Swezey | 24% | 80–90% |
| Elymus canadensis L. | 64% | 63–87% |
| Artemisia frigida Willd. | 12% | 85–90% |
| Seeded Species | Non-Native Species | Native Species Not Seeded | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Num df | Den df | F | p | Num df | Den df | F | p | Num df | Den df | F | p | |
| Treatment | 1 | 52.8 | 126.11 | <0.01 | 1 | 33.4 | 7.80 | 0.09 | 1 | 34.2 | 14.44 | 0.01 |
| Year | 3 | 118.0 | 45.65 | <0.01 | 3 | 204.0 | 13.59 | <0.01 | 3 | 190.0 | 54.05 | <0.01 |
| Position | 2 | 36.5 | 94.43 | <0.01 | 2 | 39.8 | 4.37 | 0.17 | 2 | 41.3 | 14.51 | <0.01 |
| Treatment * Year | 3 | 49.2 | 5.98 | 0.01 | 3 | 196.0 | 5.19 | 0.02 | 3 | 188.0 | 8.73 | <0.01 |
| Treatment * Position | 2 | 37.9 | 63.52 | <0.01 | 2 | 40.4 | 0.06 | 1.00 | 2 | 41.9 | 2.60 | 0.34 |
| Position * Year | 6 | 113.0 | 20.26 | <0.01 | 6 | 169.0 | 0.67 | 1.00 | 6 | 156.0 | 2.83 | 0.10 |
| Block | 1 | 39.7 | 3.01 | 0.54 | 1 | 20.7 | 0.27 | 1.00 | 1 | 22.7 | 5.69 | 0.15 |
| Block * Treatment | 1 | 15.5 | 0.51 | 1.00 | 1 | 18.6 | 0.22 | 1.00 | 1 | 19.4 | 0.80 | 0.76 |
| Block * Year | 3 | 49.2 | 0.97 | 1.00 | 3 | 196.0 | 1.65 | 1.00 | 3 | 188.0 | 0.50 | 0.76 |
| Block * Position | 2 | 37.9 | 1.96 | 0.77 | 2 | 40.4 | 0.39 | 1.00 | 2 | 41.9 | 4.19 | 0.15 |
| UTMX | 1 | 15.5 | 0.49 | 1.00 | 1 | 18.6 | 0.24 | 1.00 | 1 | 19.4 | 3.85 | 0.32 |
| UTMY | 1 | 15.5 | 1.25 | 1.00 | 1 | 18.6 | 0.43 | 1.00 | 1 | 19.4 | 2.06 | 0.50 |
| Survival | Productivity | |||||||
|---|---|---|---|---|---|---|---|---|
| Num df | Den df | F | p | Num df | Den df | F | p | |
| Treatment | 1 | 20 | 0.75 | 0.40 | 1 | 20 | 30.88 | <0.01 |
| Block | 1 | 20 | 5.74 | 0.13 | 1 | 20 | 0.91 | 0.14 |
| Treatment * Block | 1 | 20 | 1.80 | 0.39 | 1 | 20 | 1.97 | 0.47 |
| UTMX | 1 | 20 | 2.54 | 0.38 | 1 | 20 | 2.18 | 0.47 |
| UTMY | 1 | 20 | 5.00 | 0.15 | 1 | 20 | 2.18 | 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sexton, I.; Turk, P.; Ringer, L.; Brown, C.S. Slash Pile Burn Scar Restoration: Tradeoffs between Abundance of Non-Native and Native Species. Forests 2020, 11, 813. https://doi.org/10.3390/f11080813
Sexton I, Turk P, Ringer L, Brown CS. Slash Pile Burn Scar Restoration: Tradeoffs between Abundance of Non-Native and Native Species. Forests. 2020; 11(8):813. https://doi.org/10.3390/f11080813
Chicago/Turabian StyleSexton, Ian, Philip Turk, Lindsay Ringer, and Cynthia S. Brown. 2020. "Slash Pile Burn Scar Restoration: Tradeoffs between Abundance of Non-Native and Native Species" Forests 11, no. 8: 813. https://doi.org/10.3390/f11080813





