Community Structure and Functional Role of Limber Pine (Pinus flexilis) in Treeline Communities in Rocky Mountain National Park
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Selection
2.2. Tree Island Sampling
2.3. Solitary Tree Sampling
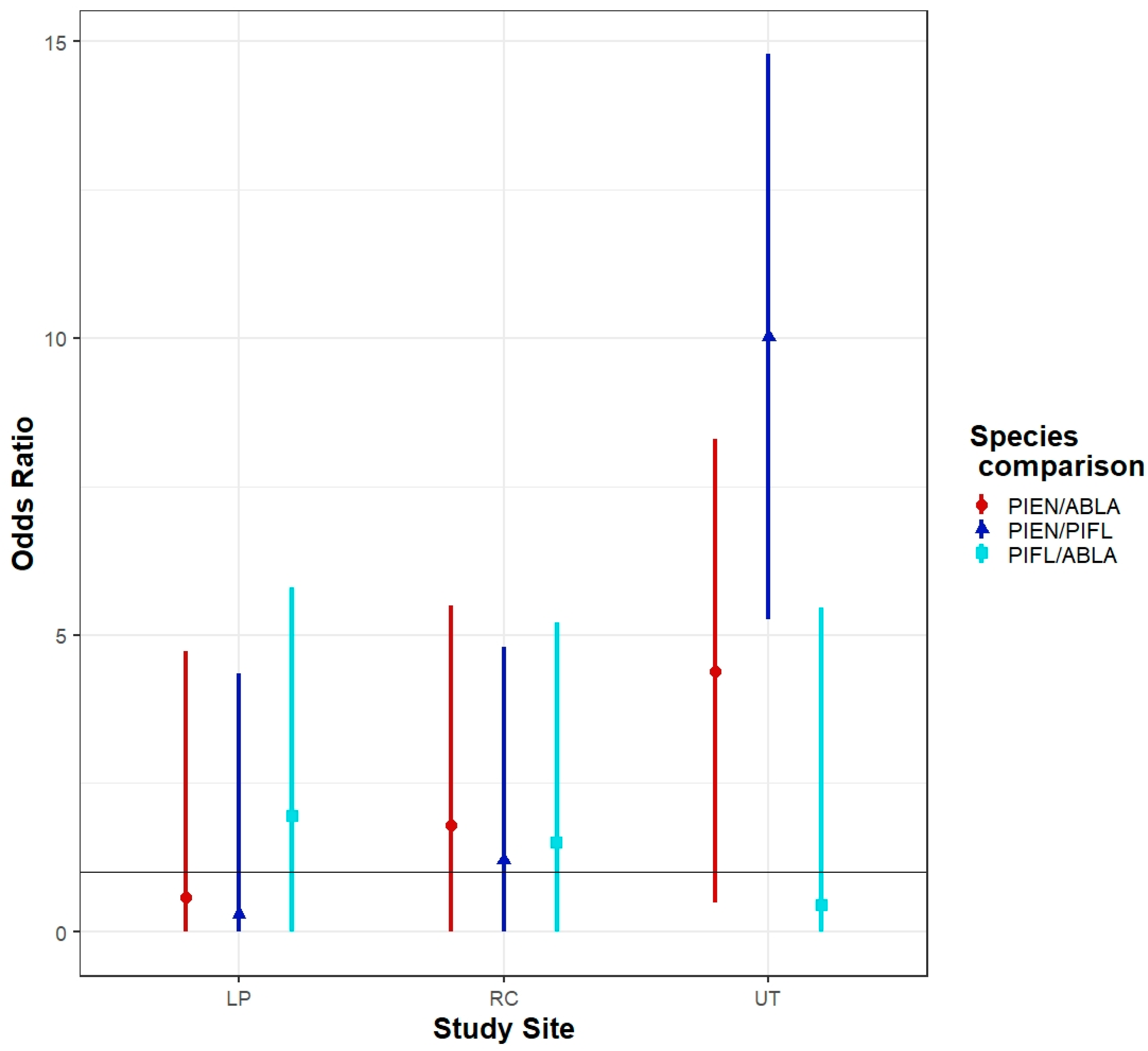
2.4. Statistical Analyses
3. Results
3.1. Community Structure
3.2. Species Composition of Tree Islands
3.3. Species Composition of Solitary Tree Plots
3.4. Windward Species Analyses
4. Discussion
4.1. Limber Pine as a Colonizer in the ATE
4.2. Role of Limber Pine in Tree Islands in the ATE
4.3. Differences among ATE Community Structure and Abiotic Characteristics
4.4. Considerations for the Conservation and Management of Limber Pine
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, W.S. Alpine vegetation in the vicinity of Long’s Peak. Bot. Gaz. 1908, 45, 319–337. [Google Scholar] [CrossRef] [Green Version]
- Rochefort, R.M.; Little, R.L.; Woodward, A.; Peterson, D.L. Changes in sub-alpine tree distribution in western North America: A review of climatic and other causal factors. Holocene 1994, 4, 89–100. [Google Scholar] [CrossRef]
- Baker, W.L.; Weisberg, P.J. Landscape analysis of the forest-tundra ecotone in Rocky Mountain National Park, Colorado. Prof. Geogr. 1995, 47, 361–374. [Google Scholar] [CrossRef]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resler, L.M. Geomorphic controls of spatial pattern and process at alpine treeline. Prof. Geogr. 2006, 58, 124–138. [Google Scholar] [CrossRef]
- Malanson, G.P.; Butler, D.R.; Fagre, D.B.; Walsh, S.J.; Tomback, D.F.; Daniels, L.D.; Resler, L.M.; Smith, W.K.; Weiss, D.J.; Peterson, D.L.; et al. Alpine treeline of western North America: Linking organism-to-landscape dynamics. Phys. Geogr. 2007, 28, 378–396. [Google Scholar] [CrossRef] [Green Version]
- Holtmeier, F.-K. What does the term “krummholz” really mean? Observations with special reference to the Alps and the Colorado Front Range. Mt. Res. Dev. 1981, 1, 253–260. [Google Scholar] [CrossRef]
- Ives, J.D.; Hansen-Bristow, K.J. Stability and instability of natural and modified upper timberline landscapes in the Colorado Rocky Mountains, USA. Mt. Res. Dev. 1983, 3, 149–155. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant. Life: Functional Plant. Ecology of High. Mountain Ecosystems, 2nd ed.; Springer: Berlin, Gemany, 2011; pp. 77–100. [Google Scholar]
- Elliott, G.P. Influences of 20th-century warming at the upper tree line contingent on local-scale interactions: Evidence from a latitudinal gradient in the Rocky Mountains, USA. Glob. Ecol. Biogeogr. 2011, 20, 46–57. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Sensitivity and response of Northern Hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Glob. Ecol. Biogeogr. 2005, 14, 395–410. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Piper, F.I.; Fajardo, A. Wind exposure and light exposure, more than elevation-related temperature, limit tree line seedling abundance on three continents. J. Ecol. 2016, 104, 1379–1390. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Treeline research—From the roots of the past to present time. A Review. Forests 2020, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef]
- Elliott, G.P.; Kipfmueller, K.F. Multi-scale influences of slope aspect and spatial pattern on ecotonal dynamics at upper treeline in the Southern Rocky Mountains, U.S.A. Arct. Antarct. Alp. Res. 2010, 42, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Hessl, A.E.; Baker, W.L. Spruce-fir growth form changes in the forest-tundra ecotone of Rocky Mountain National Park, Colorado, USA. Ecography 1997, 20, 356–367. [Google Scholar] [CrossRef]
- Resler, L.M.; Butler, D.R.; Malanson, G.P. Topographic shelter and conifer establishment and mortality in an alpine environment, Glacier National Park, Montana. Phys. Geogr. 2005, 26, 112–125. [Google Scholar] [CrossRef]
- Pyatt, J.C.; Tomback, D.F.; Blakeslee, S.C.; Wunder, M.B.; Resler, L.M.; Boggs, L.A.; Bevency, H.D. The importance of conifers for facilitation at treeline: Comparing biophysical characteristics of leeward microsites in whitebark pine communities. Arct. Antarct. Alp. Res. 2016, 48, 427–444. [Google Scholar] [CrossRef]
- Pansing, E.R.; Tomback, D.F.; Wunder, M.B.; French, J.P.; Wagner, A.C. Microsite and elevation zone effects on seed pilferage, germination, and seedling survival during early whitebark pine recruitment. Ecol. Evol. 2017, 7, 9027–9040. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Germino, M.J.; Johnson, D.M.; Reinhardt, K.; Smith, W.K.; Resler, L.M.; Bader, M.Y.; Sala, A.; Keuppers, L.M.; Broll, G.; et al. Seedling survival at timberline is critical to conifer mountain forest elevation and extent. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef]
- Germino, M.J.; Smith, W.K.; Resor, A.C. Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant. Ecol. 2002, 162, 157–168. [Google Scholar] [CrossRef]
- Smith, W.K.; Germino, M.J.; Hancock, T.E.; Johnson, D.M. Another perspective on altitudinal limits of alpine timberlines. Tree Phys. 2003, 23, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtmeier, F.K. Mountain Timberlines: Ecology, Patchiness, and Dynamics; Springer: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Holtmeier, F.-K.; Broll, G. The influence of tree islands and microtopography on pedoecological conditions in the forest-alpine tundra ecotone on Niwot Ridge, Colorado Front Range, U.S.A. Arct. Alp. Res. 1992, 24, 216–228. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lorti, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef]
- Batllori, E.; Camarero, J.J.; Ninot, J.M.; Gutiérrez, E. Seedling recruitment, survival and facilitation in alpine Pinus uncinata tree line ecotones. Implications and potential responses to climate warming. Glob. Ecol. Biogeogr. 2009, 18, 460–472. [Google Scholar] [CrossRef]
- Michelat, R.; Schöb, C.; Lortie, C.J.; Brooker, R.W.; Callaway, R.M. Partitioning net interactions among plants along altitudinal gradients to study community responses to climate change. Funct. Ecol. 2014, 28, 75–86. [Google Scholar] [CrossRef]
- Maher, E.L.; Germino, M.J.; Hasselquist, N.J. Interactive effects of tree and herb cover on survivorship, physiology, and microclimate of conifer seedlings at the alpine tree-line ecotone. Can. J. For. Res. 2005, 35, 567–574. [Google Scholar] [CrossRef]
- Barbeito, I.; Dawes, M.A.; Rixen, C.; Senn, J.; Bebi, P. Factors driving mortality and growth at treeline: A 30-year experiment of 92,000 conifers. Ecology 2012, 93, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Marr, J.W. The development and movement of tree islands near the upper limit of tree growth in the southern Rocky Mountains. Ecology 1977, 58, 1159–1164. [Google Scholar] [CrossRef]
- Peet, R.K. Forest vegetation of the Colorado Front Range. Vegetation 1981, 45, 3–75. [Google Scholar] [CrossRef]
- Benedict, J.B. Rates of tree-island migration, Colorado Rocky Mountains, USA. Ecology 1984, 65, 820–823. [Google Scholar] [CrossRef]
- Weisberg, P.J.; Baker, W.L. Spatial variation in tree regeneration in the forest-tundra ecotone, Rocky Mountain National Park, Colorado. Can. J. For. Res. 1995, 25, 1326–1339. [Google Scholar] [CrossRef]
- Humphries, H.C.; Bourgeron, P.S.; Mujica-Crapanzano, L.R. Tree spatial patterns and environmental relationships in the forest–alpine tundra ecotone at Niwot Ridge, Colorado, USA. Ecol. Res. 2008, 23, 589–605. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Layering in the Rocky Mountain treeline ecotone: Clonal conifer groups’ distribution, structure, and functional role. Trees 2017, 31, 953–965. [Google Scholar] [CrossRef]
- Sakulich, J. Reconstruction and spatial analysis of alpine treeline in the Elk Mountains, Colorado, USA. Phys. Geogr. 2015, 36, 471–488. [Google Scholar] [CrossRef]
- Knowles, P.; Grant, M.C. Age and size structure analyses of Engelmann spruce, ponderosa pine, lodgepole pine, and limber pine in Colorado. Ecology 1983, 64, 1–9. [Google Scholar] [CrossRef]
- Syring, J.; Farrell, K.; Businský, R.; Cronn, R.; Liston, A. Widespread genealogical nonmonophyly in species of Pinus subgenus Strobus. Syst. Biol. 2007, 56, 163–181. [Google Scholar] [CrossRef] [Green Version]
- Steele, R. Pinus flexilis James. In Silvics of North America; U.S. Department of Agriculture: Washington, DC, USA, 1990; Volume 1, pp. 348–354. [Google Scholar]
- Rebertus, A.J.; Burns, B.R.; Veblen, T.T. Stand dynamics of Pinus-flexilis-dominated sub-alpine forests in the Colorado Front Range. J. Veg. Sci. 1991, 2, 445–458. [Google Scholar] [CrossRef]
- Donnegan, J.A.; Rebertus, A.J. Rates and mechanisms of subalpine forest succession along an environmental gradient. Ecology 1999, 80, 1370. [Google Scholar] [CrossRef]
- Marr, J.W. Ecosystems of the east slope of the Front Range in Colorado. In University of Colorado Studies Series in Biology; Hulley, K.K., Ed.; University of Colorado Press: Boulder, CO, USA, 1961; Volume 8. [Google Scholar]
- Lepper, M.G. Carbon dioxide exchange in Pinus flexilis and P. strobiformis (Pinaceae). Madroño 1980, 27, 17–24. [Google Scholar]
- Schoettle, A.W.; Rochelle, S.G. Morphological variation of Pinus flexilis (Pinaceae), a bird-dispersed pine, across a range of elevations. Am. J. Bot. 2000, 87, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Schoettle, A.W. Ecological roles of five-needle pine in Colorado: Potential consequences of their loss. In Breeding and Genetic Resources of Five-Needle Pines: Growth, Adaptability and Pest Resistance; Proceedings RMRS-P32, IUFRO Working Party, Medford, Oregon, 2.02.15; Sniezko, R.A., Samman, S., Schlarbaum, S.E., Kriebel, H.B., Eds.; Rocky Mountain Research Station, Forest Service, U.S. Department of Agriculture: Medford, OR, USA, 2004; pp. 124–135. [Google Scholar]
- Tomback, D.F.; Kramer, K.A. Limber pine seed harvest by Clark’s nutcracker in the Sierra Nevada: Timing and foraging behavior. Condor 1980, 82, 467–468. [Google Scholar] [CrossRef]
- Lanner, R.M.; Vanderwall, S.B. Dispersal of limber pine seed by Clark’s nutcracker. J. For. 1980, 78, 637–639. [Google Scholar] [CrossRef]
- Tomback, D.F.; Schoettle, A.W.; Perez, M.J.; Grompone, K.M.; Mellmann-Brown, S. Regeneration and survival of whitebark pine after the 1988 Yellowstone Fires. In The Future of High-Elevation, Five-Needle White Pines in Western North America, Proceedings of the High Five Symposium, Missoula, MT, USA, 28–30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; Rocky Mountain Research Station, Forest Service, U.S. Department of Agriculture: Fort Collins, CO, USA, 2011; pp. 66–68. [Google Scholar]
- Williams, T.J.; Tomback, D.F.; Grevstad, N.; Broms, K. Temporal and energetic drivers of seed resource use by Clark’s nutcracker, keystone seed disperser of coniferous forests. Ecosphere 2020, 11, e03085. [Google Scholar] [CrossRef]
- Tomback, D.F. Foraging strategies of Clark’s nutcracker. Living Bird 1978, 16, 123–161. [Google Scholar]
- Tomback, D.F.; Taylor, C.L. Tourist impact on Clark’s Nutcracker foraging activities in Rocky Mountain National Park. In Toward the Year 2000; Proceedings of the Conference on Science in the National Parks, Fort Collins, Colorado; Singer, F.J., Ed.; George Wright Society, U.S. National Park Service: Hancock, MI, USA, 1987; pp. 158–172. [Google Scholar]
- Vander Wall, S.B. Foraging of Clark’s nutcrackers on rapidly changing pine seed resources. Condor 1988, 90, 621–631. [Google Scholar] [CrossRef]
- Tomback, D.F. Dispersal of whitebark pine seeds by Clark’s nutcracker: A mutualism hypothesis. J. Anim. Ecol. 1982, 51, 451–467. [Google Scholar] [CrossRef]
- Tomback, D.F. The impact of seed dispersal by Clark’s nutcracker on whitebark pine: Multi-scale perspective on a high mountain mutualism. In Mountain Ecosystems: Studies in Treeline Ecology; Broll, G., Keplin, B., Eds.; Springer Publishing: New York, NY, USA, 2005; pp. 181–201. [Google Scholar]
- Tomback, D.F. Seed dispersal by Corvids: Birds that build forests. In Why Birds Matter; Sekercioglu, C.H., Wenny, D.G., Whelan, C.J., Eds.; University of Chicago Press: Chicago, IL, USA, 2016; p. 368. [Google Scholar]
- Tomback, D.F. Post-fire regeneration of krummholz whitebark pine: A consequence of nutcracker seed caching. Madroño 1986, 33, 100–110. [Google Scholar]
- Tomback, D.F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellmann-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Tomback, D.F.; Linhart, Y.B. The evolution of bird-dispersed pines. Evol. Ecol. 1990, 4, 185–219. [Google Scholar] [CrossRef]
- Smithers, B.V.; North, M.P.; Millar, C.I.; Latimer, A.M. Leap frog in slow motion: Divergent responses of tree species and life stages to climatic warming in Great Basin subalpine forests. Glob. Chang. Biol. 2018, 24, E442–E457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, C.I.; Westfall, R.D.; Delany, D.L.; Flint, A.L.; Flint, L.E. Recruitment patterns and growth of high-elevation pines in response to climatic variability (1883–2013), in the western Great Basin, USA. Can. J. For. Res. 2015, 45, 1299–1312. [Google Scholar] [CrossRef]
- Smithers, B.V. Soil preferences in germination and survival of limber pine in the Great Basin White Mountains. Forests 2017, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Moyes, A.B.; Castanha, C.; Germino, M.J.; Kueppers, L.M. Warming and the dependence of limber pine (Pinus flexilis) establishment on summer soil moisture within and above its current elevation range. Oecologia 2013, 171, 271–282. [Google Scholar] [CrossRef]
- Moyes, A.B.; Germino, M.J.; Kueppers, L.M. Moisture rivals temperature in limiting photosynthesis by trees establishing beyond their cold-edge range limit under ambient and warmed conditions. New Phytol. 2015, 207, 1005–1014. [Google Scholar] [CrossRef]
- Kueppers, L.M.; Conlisk, E.; Castanha, C.; Moyes, A.B.; Germino, M.J.; de Valpine, P.; Torn, M.S.; Mitton, J.B. Warming and provenance limit tree recruitment across and beyond the elevation range of subalpine forest. Glob. Chang. Biol. 2017, 23, 2383–2395. [Google Scholar] [CrossRef] [Green Version]
- Monahan, W.B.; Cook, T.; Melton, F.; Connor, J.; Bobowski, B. Forecasting distributional responses of limber pine to climate change at management-relevant scales in Rocky Mountain National Park. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Baumeister, D.; Callaway, R.M. Facilitation by Pinus flexilis during succession: A hierarchy of mechanisms benefits other plant species. Ecology 2006, 87, 1816–1830. [Google Scholar] [CrossRef] [Green Version]
- Resler, L.M.; Tomback, D.F. Blister rust prevalence in krummholz whitebark pine: Implications for treeline dynamics, Northern Rocky Mountains, Montana, U.S.A. Arct. Antarct. Alp. Res. 2008, 40, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Tomback, D.F.; Chipman, K.G.; Resler, L.M.; Smith-McKenna, E.K.; Smith, C.M. Relative abundance and functional role of whitebark pine at treeline in the Northern Rocky Mountains. Arct. Antarct. Alp. Res. 2014, 46, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Tomback, D.F.; Resler, L.M.; Keane, R.; Pansing, E.R.; Andrade, A.; Wagner, A.C. Community structure, biodiversity, and ecosystem services in treeline whitebark pine communities: Potential impacts from a non-native pathogen. Forests 2016, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.C.; Tomback, D.F.; Resler, L.M.; Pansing, E.R. Whitebark pine prevalence and ecological function in the treeline communities of the Greater Yellowstone Ecosystem, USA: Potential disruption by white pine blister rust. Forests 2018, 9, 635. [Google Scholar] [CrossRef] [Green Version]
- Benkman, C.W. The impact of tree squirrels (Tamiasciurus) on limber pine seed dispersal adaptations. Evolution 1995, 49, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCutchen, H.E. Limber pine and bears. Great Basin Nat. 1996, 56, 90–92. [Google Scholar]
- Schoettle, A.W.; Burns, K.S.; Cleaver, C.M.; Connor, J.J. Proactive Limber Pine Conservation Strategy for the Greater Rocky Mountain National Park Area; Rocky Mountain Research Station, Forest Service, U.S. Department of Agriculture: Fort Collins, CO, USA, 2019. Available online: https://www.fs.usda.gov/treesearch/pubs/57621 (accessed on 2 May 2020).
- Connor, J.J.; Schoettle, A.W.; Burns, K.S.; Borgman, E. Limber pine conservation in Rocky Mountain National Park. Nutcracker Notes 2012–2013, 23, 13–15. [Google Scholar]
- Cleaver, C.M.; Jacobi, W.R.; Burns, K.S.; Means, R.E. Limber pine in the central and southern Rocky Mountains: Stand conditions and interactions with blister rust, mistletoe, and bark beetles. For. Ecol. Manag. 2015, 358, 139–153. [Google Scholar] [CrossRef]
- Tomback, D.F.; Achuff, P.; Schoettle, A.W.; Schwandt, J.W.; Mastrogiuseppe, R.J. The magnificent high-elevation five-needle white pines: Ecological roles and future outlook. In The Future of High-Elevation, Five-Needle White Pines in Western North America: Proceedings of the High Five Symposium, Proceedings of the RMRS-P-63, Missoula, MT, USA, 28–30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; Rocky Mountain Research Station, Forest Service, U.S. Department of Agriculture: Fort Collins, CO, USA, 2011; pp. 2–28. Available online: https://www.fs.usda.gov/treesearch/pubs/38188 (accessed on 2 May 2020).
- Klutsch, J.G.; Goodrich, B.A.; Schoettle, A.W. Limber pine forests on the leading edge of white pine blister rust distribution in Northern Colorado. In The Future of High-Elevation, Five-Needle White Pines in Western North America: Proceedings of the High Five Symposium, Proceedings of the RMRS-P-63, Missoula, MT, USA, 28–30 June 2010; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; Rocky Mountain Research Station, Forest Service, U.S. Department of Agriculture: Fort Collins, CO, USA, 2011. Available online: https://www.fs.usda.gov/treesearch/pubs/38227 (accessed on 8 June 2020).
- Smith, C.M.; Langor, D.W.; Myrholm, C.; Weber, J.; Gillies, C.; Stuart-Smith, J. Changes in white pine blister rust infection and mortality in limber pine over time. Can. J. For. Res. 2013, 43, 919–928. [Google Scholar] [CrossRef]
- Jones, B.; Gutsell, R.; Barnhardt, L.; Gould, J.; Smith, C.; Langor, D.; Ostermann, K. Alberta Limber Pine Recovery Plan 2014–2019; Alberta Species at Risk Recovery Plan No. 35; Alberta Environment and Sustainable Resource Development: Edmonton, AB, Canada, 2014; p. 61.
- Salas, D.; Stevens, J.; Schulz, K. Rocky Mountain National Park, Colorado 2001–2005 Vegetation Classification and Mapping Project Report; U.S. Bureau of Reclamation Technical Memorandum 8260-05-02; Remote Sensing and GIS Group Technical Service Center Bureau of Reclamation: Denver, CO, USA, 2005. [Google Scholar]
- Carsey, K.S.; Tomback, D.F. Growth form distribution and genetic-relationships in tree clusters of Pinus flexilis, a bird-dispersed pine. Oecologia 1994, 98, 402–411. [Google Scholar] [CrossRef]
- Signorell, A. DescTools: Tools for Descriptive Statistics. R Package Version 0.99.36. Available online: https://cran.r-project.org/package=DescTools (accessed on 1 July 2020).
- Hope, R.M. RMisc: Ryan Miscellaneous. R Package Version 1.5. Available online: https://cran.r-project.org/package=Rmisc (accessed on 1 July 2020).
- Whitlock, M.C.; Schluter, D. The Analysis of Biological Data, 2nd ed.; W.H. Freeman and Company: New York, NY, USA, 2015; pp. 179–256. [Google Scholar]
- Rita, H.; Komonen, A. Odds ratio: An ecologically sound tool to compare proportions. Annales Zoologici Fennici 2008, 45, 66–72. [Google Scholar] [CrossRef]
- Service, R.F. As the West Goes Dry. Science 2004, 303, 1124–1127. [Google Scholar] [CrossRef]
- Mote, P.W.; Hamlet, A.F.; Clark, M.P.; Lettenmaier, D.P. Declining mountain snowpack in western North America. Bull. Am. Meteor. 2005, 86, 39–50. [Google Scholar] [CrossRef]
- Mote, P.W. Climate-driven variability and trends in mountain snowpack in western North America. J. Clim. 2006, 19, 6209–6220. [Google Scholar] [CrossRef]
- Charles, L.H. Effects of Climate Change on Snowpack, Glaciers, and Water Resources in the Northern Rockies. In Climate Change and Rocky Mountain Ecosystems, 1st ed.; Halofsky, J.E., Peterson, D.L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 63, p. 263. [Google Scholar]
- Pansing, E.R.; Tomback, D.F. Survival of whitebark pine seedlings grown from direct seeding: Implications for regeneration and restoration under Climate Change. Forests 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Tranquillini, W. Physiological Ecology of the Alpine Timberline: Tree Existence at High. Altitudes with Special Reference to the European Alps; Springer: Berlin, Germany, 1979. [Google Scholar]
- Shemesh, H.; Boaz, B.E.; Millar, C.I.; Bruns, T.D. Symbiotic interactions above treeline of long-lived pines: Mycorrhizal advantage of limber pine (Pinus flexilis) over Great Basin bristlecone pine (Pinus longaeva) at the seedling stage. J. Ecol. 2020, 108, 908–916. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- McIntire, E.J.B.; Fajardo, A. Facilitation as a ubiquitous driver of biodiversity. New Phytol. 2014, 201, 403–416. [Google Scholar] [CrossRef]
- Tomback, D.F.; Blakeslee, S.C.; Wagner, A.C.; Wunder, M.B.; Resler, L.M.; Pyatt, J.C.; Diaz, S. Whitebark pine facilitation at treeline: Potential interactions for disruption by an invasive pathogen. Ecol. Evol. 2016, 6, 5144–5157. [Google Scholar] [CrossRef]
- Gill, R.A.; Campbell, C.S.; Karlinsey, S.M. Soil moisture controls Engelmann spruce (Picea engelmannii) seedling carbon balance and survivorship at timberline in Utah, USA. Can. J. For. Res. 2015, 45, 1845–1852. [Google Scholar] [CrossRef] [Green Version]
- Andrus, R.A.; Harvey, B.J.; Rodman, K.C.; Hart, S.J.; Veblen, T.T. Moisture availability limits subalpine tree establishment. Ecology 2018, 99, 567–575. [Google Scholar] [CrossRef]
- Kearns, H.S.J.; Jacobi, W.R. The distribution and incidence of white pine blister rust in central and southeastern Wyoming and northern Colorado. Can. J. For. Res. 2007, 37, 462–472. [Google Scholar] [CrossRef]
- Smith, E.K.; Resler, L.M.; Vance, E.A.; Carstensen, L.W.; Kolivras, K.N. Blister rust incidence in treeline whitebark pine, Glacier National Park, U.S.A.: Environmental and topographic influences. Arct. Antarct. Alp. Res. 2011, 43, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Cleaver, C.M.; Schoettle, A.W.; Burns, A.W.; Connor, J.J. Limber pine conservation strategy: Recommendations for Rocky Mountain National Park. In Proceedings of the 63rd Annual Western International Forest Disease Work Conference, Newport, OR, USA, 21–25 September 2015; Ramsey, A., Palacios, P., Eds.; Rocky Mountain Research Station, USDA Forest Service: Fort Collins, CO, USA, 2015; pp. 81–82. [Google Scholar]
- Tomback, D.F.; Resler, L.M. Invasive pathogens at treeline: Consequences for treeline dynamics. Phys. Geogr. 2007, 28, 397–418. [Google Scholar] [CrossRef]







| Study Site | Area (ha) | Mode Elevation (m) | Aspect (s) | Prevailing Wind Direction |
|---|---|---|---|---|
| Rainbow Curve | 2.74 | 3376 | NW | 283 (WNW) |
| Ute Trail | 6.62 | 3472 | SE, E | 282 (WNW) |
| Longs Peak | 8.54 | 3410 | N, NE, E | 268 (W) |
| Battle Mountain | 3.35 | 3456 | N, NW, W | 227 (SW) |
| Single-Tree Island Metrics | |||
| Study Site (No. Islands, %) | Median Island Length (cm) (95% CI) | Median Island Width (cm) (95% CI) | Median Island Height (cm) (95% CI) |
| Rainbow Curve (15, 38.5%) | 300 | 260 | 200 |
| (245, 400) | (180, 340) | (140, 245) | |
| Ute Trail (21, 53.8%) | 180 | 205 | 120 |
| (115, 340) | (130, 285) | (65, 145) | |
| Longs Peak (23, 54.8%) | 235 | 200 | 115 |
| (170, 305) | (150, 240) | (95, 155) | |
| Battle Mountain (27, 81.8%) | 170 | 160 | 110 |
| (130, 240) | (115, 255) | (60, 210) | |
| Multi-Tree Island Metrics | |||
| Rainbow Curve (24, 61.5%) | 1018 | 378 | 265 |
| (430, 1550) | (255, 510) | (180, 380) | |
| Ute Trail (18, 46.2%) | 1128 | 715 | 250 |
| (790, 2235) | (560, 1100) | (190, 300) | |
| Longs Peak (19, 45.2%) | 560 | 385 | 130 |
| (305, 745) | (200, 645) | (75, 160) | |
| Battle Mountain (6, 18.2%) | 275 | 248 | 135 |
| (95, 475) | (90, 425) | (45, 305) | |
| Study Site | No. Krummholz (%) | No. Krummholz-Upright (%) | No. Upright (%) |
|---|---|---|---|
| Rainbow Curve | 6 (15.4) | 31 (79.5) | 2 (5.1) |
| Ute Trail | 18 (46.2) | 15 (38.5) | 6 (15.4) |
| Longs Peak | 23 (54.8) | 19 (45.2) | 0 |
| Battle Mountain | 15 (45.5) | 13 (39.4) | 5 (15.2) |
| Study Site (Number of Solitary Tree Plots) | Limber Pine Solitary
—Engelmann Spruce Solitary
(95% CI) | Limber Pine Solitary
—Subalpine Fir Solitary
(95% CI) |
|---|---|---|
| Longs Peak (38) | 0.607 * | 0.683 * |
| (0.430, 0.784) | (0.523, 0.842) | |
| Rainbow Curve (39) | 0.588 * | 0.776 * |
| (0.409, 0.768) | (0.643, 0.909) | |
| Ute Trail (39) | 0.574 * | 0.537 * |
| (0.396, 0.752) | (0.352, 0.722) | |
| Battle Mountain (33) | 0.633 * | 0.749 * |
| (0.446, 0.819) | (0.593, 0.904) |
| Study Site | Limber Pine | Engelmann Spruce | Subalpine Fir |
|---|---|---|---|
| Longs Peak | −0.278 * | 0.066 | 0.247 * |
| (−0.482, −0.074) | (−0.101, 0.232) | (0.085, 0.409) | |
| Rainbow Curve | −0.440 * | −0.397 * | 0.249 * |
| (−0.637, −0.243) | (−0.595, −0.199) | (0.107, 0.391) | |
| Ute Trail | −0.579 * | 0.496 * | 0.083 |
| (−0.756, −0.402) | (0.310, 0.680) | (−0.096, 0.263) | |
| Battle Mountain | 0.206 * | −0.161 * | −0.045 |
| (0.068, 0.344) | (−0.287, −0.036) | (−0.116, 0.026) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindewald, L.A.; Tomback, D.F.; Neumeyer, E.R. Community Structure and Functional Role of Limber Pine (Pinus flexilis) in Treeline Communities in Rocky Mountain National Park. Forests 2020, 11, 838. https://doi.org/10.3390/f11080838
Sindewald LA, Tomback DF, Neumeyer ER. Community Structure and Functional Role of Limber Pine (Pinus flexilis) in Treeline Communities in Rocky Mountain National Park. Forests. 2020; 11(8):838. https://doi.org/10.3390/f11080838
Chicago/Turabian StyleSindewald, Laurel A., Diana F. Tomback, and Eric R. Neumeyer. 2020. "Community Structure and Functional Role of Limber Pine (Pinus flexilis) in Treeline Communities in Rocky Mountain National Park" Forests 11, no. 8: 838. https://doi.org/10.3390/f11080838





