Growth, Carbon Storage, and Optimal Rotation in Poplar Plantations: A Case Study on Clone and Planting Spacing Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Experimental Design
2.2. Tree Destructive Sampling and Biomass Investigation
2.3. Stem Analysis
2.4. Modeling of Tree Growth Dynamics
| NO. | Function Name | K * | Function * | Reference |
|---|---|---|---|---|
| 1 | Mitscherlich | 2 | Li et al. [29] | |
| 2 | Logistic | 3 | Yoshimoto [30] | |
| 3 | Gompertz | 3 | Yoshimoto [30] | |
| 4 | Johnson Schumacher | 3 | Fang et al. [31] | |
| 5 | Korf | 3 | Li et al. [29] | |
| 6 | Chapman-Richards | 3 | Liu and Li [32] | |
| 7 | Weibull | 4 | Deng et al. [33] |
2.5. Carbon Storage
2.6. Optimal Rotation Age
2.7. Statistical Analysis
3. Results
3.1. Tree Growth and Stand Volume
3.2. Biomass Production and Partitioning
3.3. Carbon Concentration and Storage
3.4. Quantitative Maturity Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jürgensen, C.; Kollert, W.; Lebedys, A. Assessment of Industrial Roundwood Production from Planted Forests; Planted Forests and Trees Working Papers (FAO) eng no. FP/48/E; FAO: Rome, Italy, 2014. [Google Scholar]
- Brown, S. Measuring carbon in forests: Current status and future challenges. Environ. Pollut. 2002, 116, 363–372. [Google Scholar] [CrossRef]
- Forest Resources Management Division. National Forest Resources Statistics-Main Results of the Eighth National Forest Resources Inventory in China; State Forestry Bureau: Beijing, China, 2015. [Google Scholar]
- Forest Resources Management Division. National Forestry and Grassland Development Statistical Bullet in 2018; State Forestry Bureau: Beijing, China, 2019. [Google Scholar]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO global forest resources assessment 2015. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Malhi, Y.; Meir, P.; Brown, S. Forests, carbon and global climate. Philos. Trans. A Math. Phys. Eng. Sci. 2002, 360, 1567–1591. [Google Scholar] [CrossRef]
- Nassi, O.D.N.; Guidi, W.; Ragaglini, G.; Tozzini, C.; Bonari, E. Biomass production and energy balance of a 12-year-old short-rotation coppice poplar stand under different cutting cycles. GCB Bioenergy 2010, 2, 89–97. [Google Scholar] [CrossRef]
- Hopmans, P.; Stewart, H.T.L.; Flinn, D.W. Impacts of harvesting on nutrients in a eucalypt ecosystem in southeastern Australia. Ecol. Manag. 1993, 59, 29–51. [Google Scholar] [CrossRef]
- Swamy, S.L.; Mishra, A.; Puri, S. Comparison of growth, biomass and nutrient distribution in five promising clones of Populus deltoides under an agrisilviculture system. Bioresour. Technol. 2006, 97, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycle 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhang, X.S. Performance of 14 hybrid poplar clones grown in Beijing, China. Biomass Bioenergy 2010, 34, 906–911. [Google Scholar] [CrossRef]
- Fortier, J.; Gagnon, D.; Truax, B.; Lambert, F. Biomass and volume yield after 6 years in multiclonal hybrid poplar riparian buffer strips. Biomass Bioenergy 2010, 34, 1028–1040. [Google Scholar] [CrossRef]
- Benomar, L.; DesRochers, A.; Larocque, G.R. The effects of spacing on growth, morphology and biomass production and allocation in two hybrid poplar clones growing in the boreal region of Canada. Trees 2012, 26, 939–949. [Google Scholar] [CrossRef]
- Khan, G.S.; Chaudhry, A.K. Effect of spacing and plant density on the growth of poplar (Populus deltoides) trees under agro-forestry system. Pak. J. Agric. Sci. 2007, 44, 321–327. [Google Scholar]
- Nelson, N.D.; Berguson, W.E.; McMahon, B.G.; Cai, M.; Buchman, D.J. Growth performance and stability of hybrid poplar clones in simultaneous tests on six sites. Biomass Bioenergy 2018, 118, 115–125. [Google Scholar] [CrossRef]
- Bogeat-Triboulot, M.; Brosché, M.; Renaut, J.; Jouve, L.; Le Thiec, D.; Fayyaz, P.; Vinocur, B.; Witters, E.; Laukens, K.; Teichmann, T. Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol. 2007, 143, 876–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinno, B.D.; Thomas, B.R.; Bélanger, N. Predicting the productivity of a young hybrid poplar clone under intensive plantation management in northern Alberta, Canada using soil and site characteristics. New For. 2010, 39, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Li, C.; Li, M.Y.; Liu, Z.Z. Influence of variable selection and forest type on forest aboveground biomass estimation using machine learning algorithms. Forests 2019, 10, 1073. [Google Scholar] [CrossRef] [Green Version]
- Ceulemans, R.; Deraedt, W. Production physiology and growth potential of poplars under short-rotation forestry culture. Ecol. Manag. 1999, 121, 9–23. [Google Scholar] [CrossRef]
- Czapowskyj, M.M.; Safford, L.O. Site preparation, fertilization, and 10-year yields of hybrid poplar on a clearcut forest site in eastern Maine, USA. New For. 1993, 7, 331–344. [Google Scholar]
- Dickmann, D.I.; Isebrands, J.G.; Eckenwalder, J.E.; Richardson, J. Poplar Culture in North America, 1st ed.; NRC Research Press: Ottawa, ON, Canada, 2002; ISBN 0660189887. [Google Scholar]
- Tun, T.N.; Guo, J.; Fang, S.Z.; Tian, Y. Planting spacing affects canopy structure, biomass production and stem roundness in poplar plantations. Scand. J. Res. 2018, 33, 464–474. [Google Scholar] [CrossRef]
- Fang, S.Z.; Xu, X.Z.; Lu, S.X.; Tang, L.Z. Growth dynamics and biomass production in short-rotation poplar plantations: 6-year results for three clones at four spacings. Biomass Bioenergy 1999, 17, 415–425. [Google Scholar] [CrossRef]
- Yan, Y.F.; Fang, S.Z.; Tian, Y.; Deng, S.P.; Tang, L.Z.; Chuong, D.N. Influence of tree spacing on soil nitrogen mineralization and availability in hybrid poplar plantations. Forests 2015, 6, 636–649. [Google Scholar] [CrossRef] [Green Version]
- Menezes, M.; Berger, U.; Worbes, M. Annual growth rings and long-term growth patterns of mangrove trees from the Bragança peninsula, North Brazil. Wetl. Ecol. Manag. 2003, 11, 233–242. [Google Scholar] [CrossRef]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A.; Vanclay, J.K. Forest Growth and Yield Modeling, 1st ed.; John Wiley and Sons: Manhattan, NY, USA, 2011; ISBN 978-0-470-66500-8. [Google Scholar]
- Phan, S.M.; Nguyen, H.T.T.; Nguyen, T.K.; Lovelock, C. Modelling above ground biomass accumulation of mangrove plantations in Vietnam. Ecol. Manag. 2019, 432, 376–386. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Method Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Li, F.R.; Zhao, B.D.; Su, G.L. A derivation of the generalized Korf growth equation and its application. J. For. Res. 2000, 11, 81–88. [Google Scholar]
- Yoshimoto, A. Application of the logistic, gompertz, and richards growth functions to gentan probability analysis. J. For. Res. 2001, 6, 265–272. [Google Scholar] [CrossRef]
- Fang, L.; Liu, S.Q.; Huang, Z.Y. Uncertain Johnson–Schumacher growth model with imprecise observations and k-fold cross-validation test. Soft Comput. 2020, 24, 2715–2720. [Google Scholar] [CrossRef]
- Liu, Z.G.; Li, F.R. The generalized Chapman-Richards function and applications to tree and stand growth. J. For. Res. 2003, 14, 19–26. [Google Scholar]
- Deng, C.; LV, Y.; Lei, Y.C.; Zhang, J. Study on individual tree diameter growth models with the relative diameter as competition indicator. For. Resour. Manag. 2011, 40–43. [Google Scholar] [CrossRef]
- Schwerz, F.; Eloy, E.; Elli, E.F.; Caron, B.O. Reduced planting spacing increase radiation use efficiency and biomass for energy in black wattle plantations: Towards sustainable production systems. Biomass Bioenergy 2019, 120, 229–239. [Google Scholar] [CrossRef]
- Balatinecz, J.J.; Kretschmann, D.E.; Leclercq, A. Achievements in the utilization of poplar wood–guideposts for the future. For. Chron. 2001, 77, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Ghezehei, S.B.; Nichols, E.G.; Hazel, D.W. Early clonal survival and growth of poplars grown on North Carolina Piedmont and Mountain Marginal Lands. Bioenergy Res. 2016, 9, 548–558. [Google Scholar] [CrossRef]
- Pliura, A.; Zhang, S.Y.; Mackay, J.; Bousquet, J. Genotypic variation in wood density and growth traits of poplar hybrids at four clonal trials. Ecol. Manag. 2007, 238, 92–106. [Google Scholar] [CrossRef]
- Huang, Q.F.; Sun, Q.X.; Wu, Z.M.; Xiang, Y. A study on principal felling age of poplar plantation on Changjiang River beach. Sci. Silvae Sin. 2002, 39, 154–158. [Google Scholar]
- Fang, S.; Yang, W.Z. Interclonal and within-tree variation in wood properties of poplar clones. J. Res. 2003, 14, 263–268. [Google Scholar]
- Fang, S.Z.; Yang, W.Z.; Fu, X.X. Variation of microfibril angle and its correlation to wood properties in poplars. J. Res. 2004, 15, 261–267. [Google Scholar]
- Debell, D.S.; Singleton, R.; Harrington, C.A.; Gartner, B.L. Wood density and fiber length in young populus stems: Relation to clone, age, growth rate, and pruning. Wood Fiber Sci. 2002, 34, 529–539. [Google Scholar]
- De Boever, L.; Vansteenkiste, D.; Van Acker, J.; Stevens, M. End-use related physical and mechanical properties of selected fast-growing poplar hybrids (Populus trichocarpa × P. deltoides). Ann. Sci. 2007, 64, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, I.; Denaud, L.; Marchal, R.; Darmawan, W. Ten new poplar cultivars provide laminated veneer lumber for structural application. Ann. Sci. 2015, 72, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Nazerian, M.; Ghalehno, M.D.; Kashkooli, A.B. Effect of wood species, amount of juvenile wood and heat treatment on mechanical and physical properties of laminated veneer lumber. J. Appl. Sci. 2011, 11, 980–987. [Google Scholar] [CrossRef]
- Haouzali, H.E.; Marchal, R.; Bléron, L.; Kifani-Sahban, F.; Butaud, J. Mechanical properties of laminated veneer lumber produced from ten cultivars of poplar. Eur. J. Wood Prod. 2020. [Google Scholar] [CrossRef]
- Jiang, B.; Yuan, W.G.; Qi, L.Z.; Zhu, J.R. Studies on key techniques for culturing large timber of poplar in flood land. Sci. Silvae Sin. 2001, 38, 68–75. [Google Scholar]
- Tang, W.P. Density Control Techniques and Successive Planting Effect of Poplar Plantation in Jianghan Plain. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2009. [Google Scholar]
- Babst, F.; Bouriaud, O.; Papale, D.; Gielen, B.; Janssens, I.A.; Nikinmaa, E.; Ibrom, A.; Wu, J.; Bernhofer, C.; Kostner, B. Above-ground woody carbon sequestration measured from tree rings is coherent with net ecosystem productivity at five eddy-covariance sites. New Phytol. 2014, 201, 1289–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Chen, A.P.; Peng, C.H.; Zhao, S.Q.; Ci, L.J. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Z.; Xue, J.N.; Tang, L. Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J. Environ. Manag. 2007, 85, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.S.; Aref, I.M.; Al-Mefarrej, H.; El-Juhany, L.I. Effect of spacing on the biomass production and allocation in Conocarpus erectus L. trees grown in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2008, 15, 315–322. [Google Scholar]
- Fang, S.Z.; Li, H.L.; Sun, Q.X.; Chen, L.B. Biomass production and carbon stocks in poplar-crop intercropping systems: A case study in northwestern Jiangsu, China. Agrofor. Syst. 2010, 79, 213–222. [Google Scholar] [CrossRef]
- Zheng, W.J.; Bao, W.K.; Gu, B.; He, X.; LI, L. Carbon concentration and its characteristics in terrestrial higher plants. Chin. J. Ecol. 2007, 26, 307–313. [Google Scholar]
- Demirbas, A. Relationships between lignin contents and fixed carbon contents of biomass samples. Energy Convers. Manag. 2003, 44, 1481–1486. [Google Scholar] [CrossRef]
- Benomar, L.; Desrochers, A.; Larocque, G.R. Changes in specific leaf area and photosynthetic nitrogen-use efficiency associated with physiological acclimation of two hybrid poplar clones to intraclonal competition. Can. J. Res. 2011, 41, 1465–1476. [Google Scholar] [CrossRef]
- Mitchell, C.P. Ecophysiology of short rotation forest crops. Biomass Bioenergy 1992, 2, 25–37. [Google Scholar] [CrossRef]
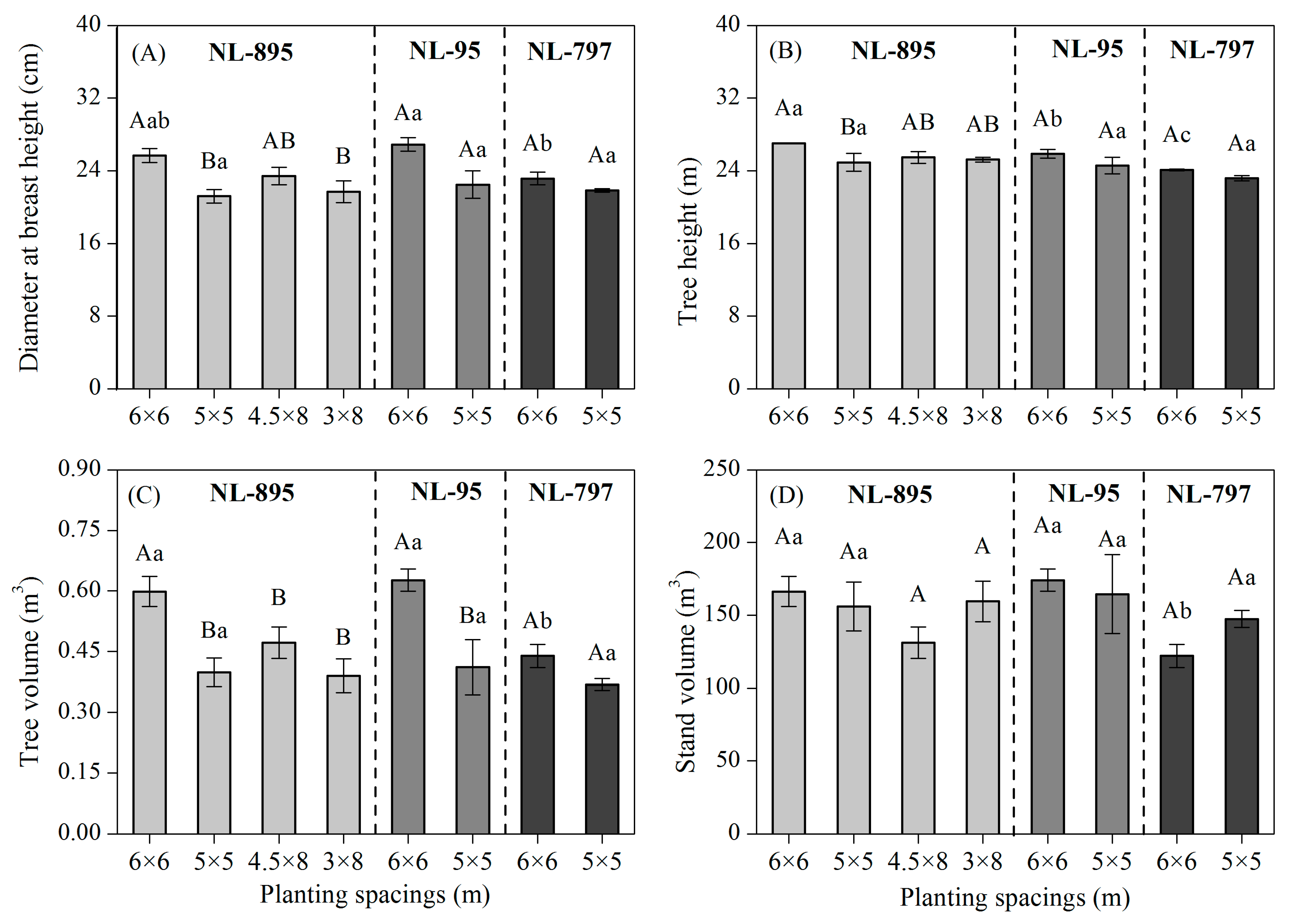
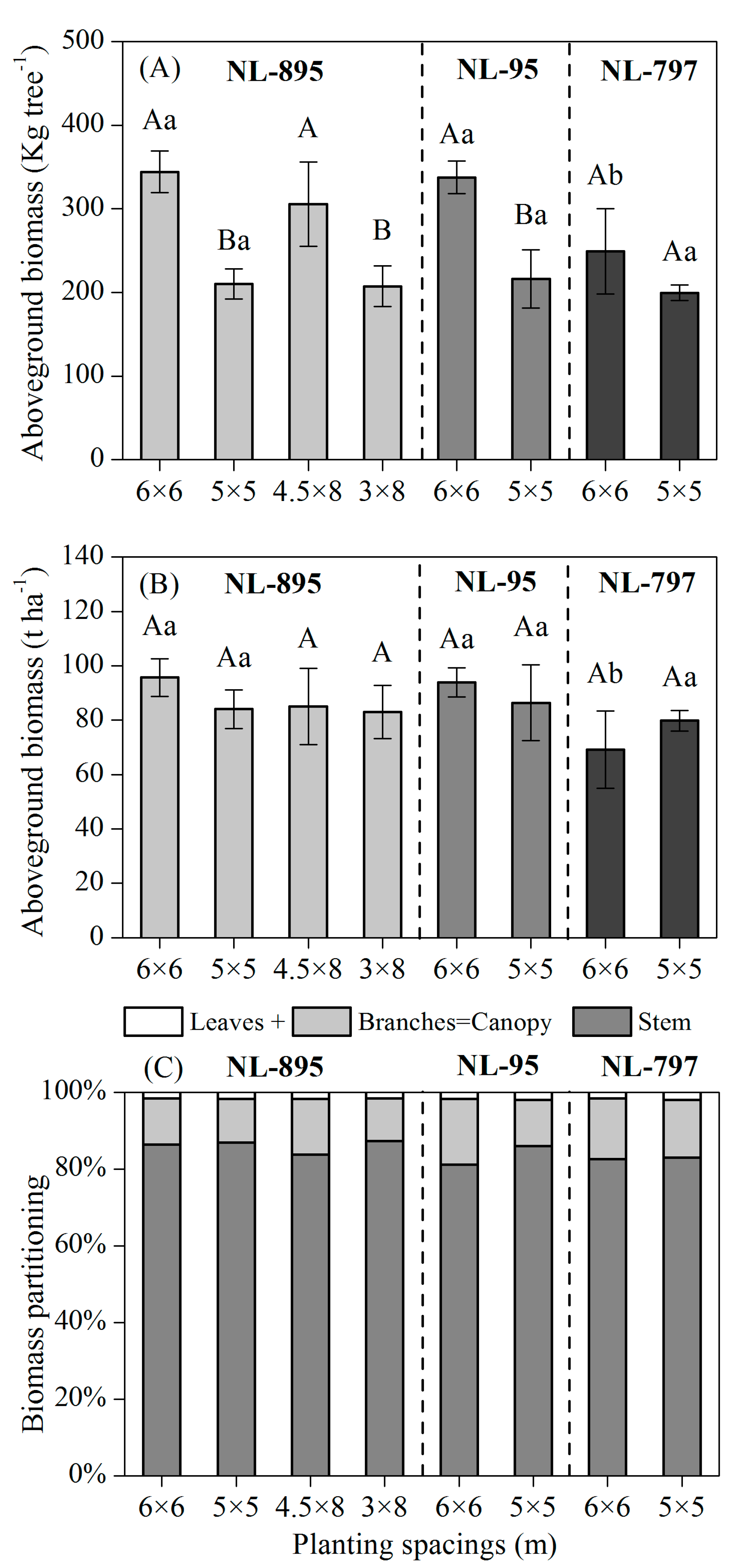
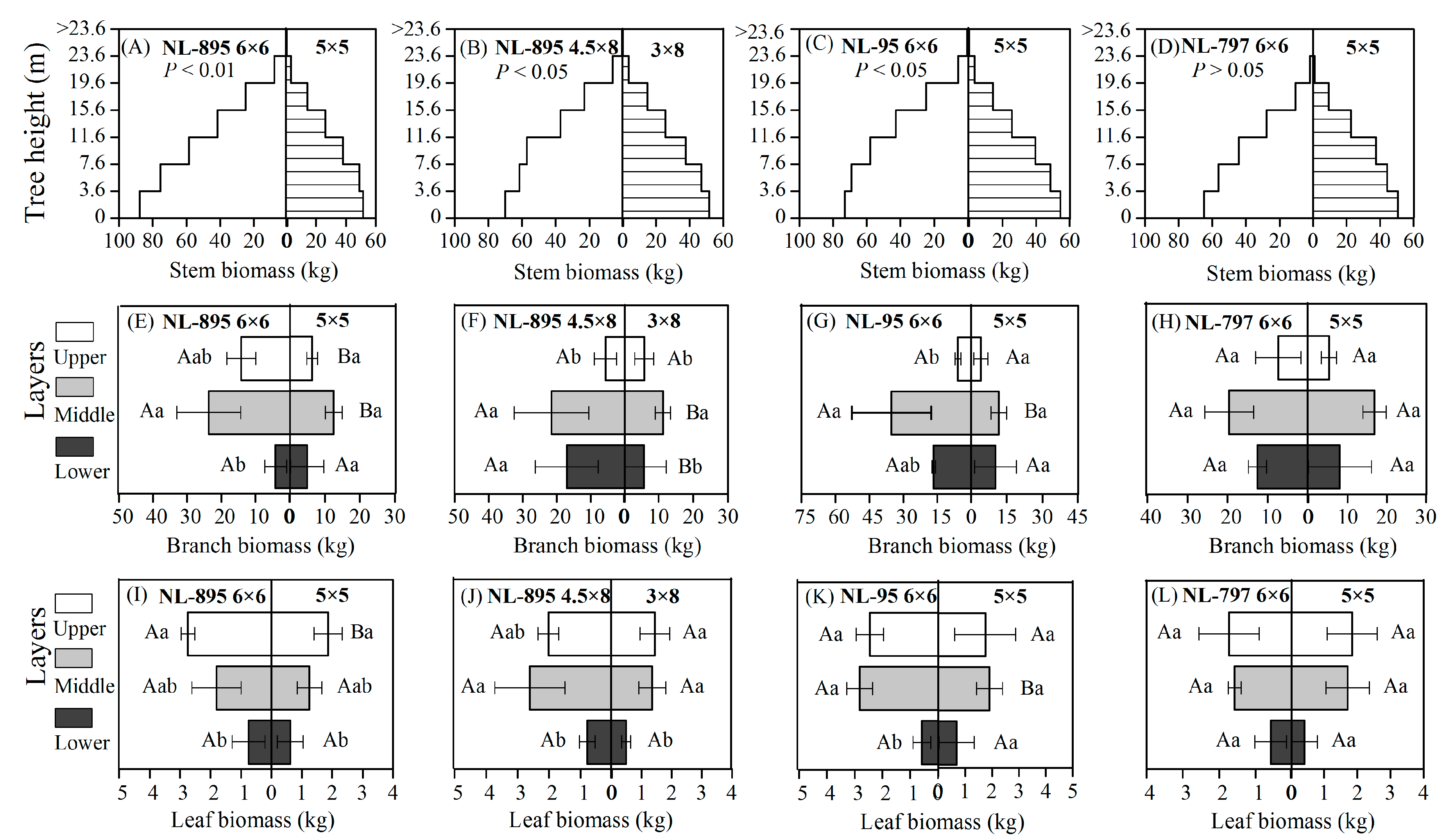
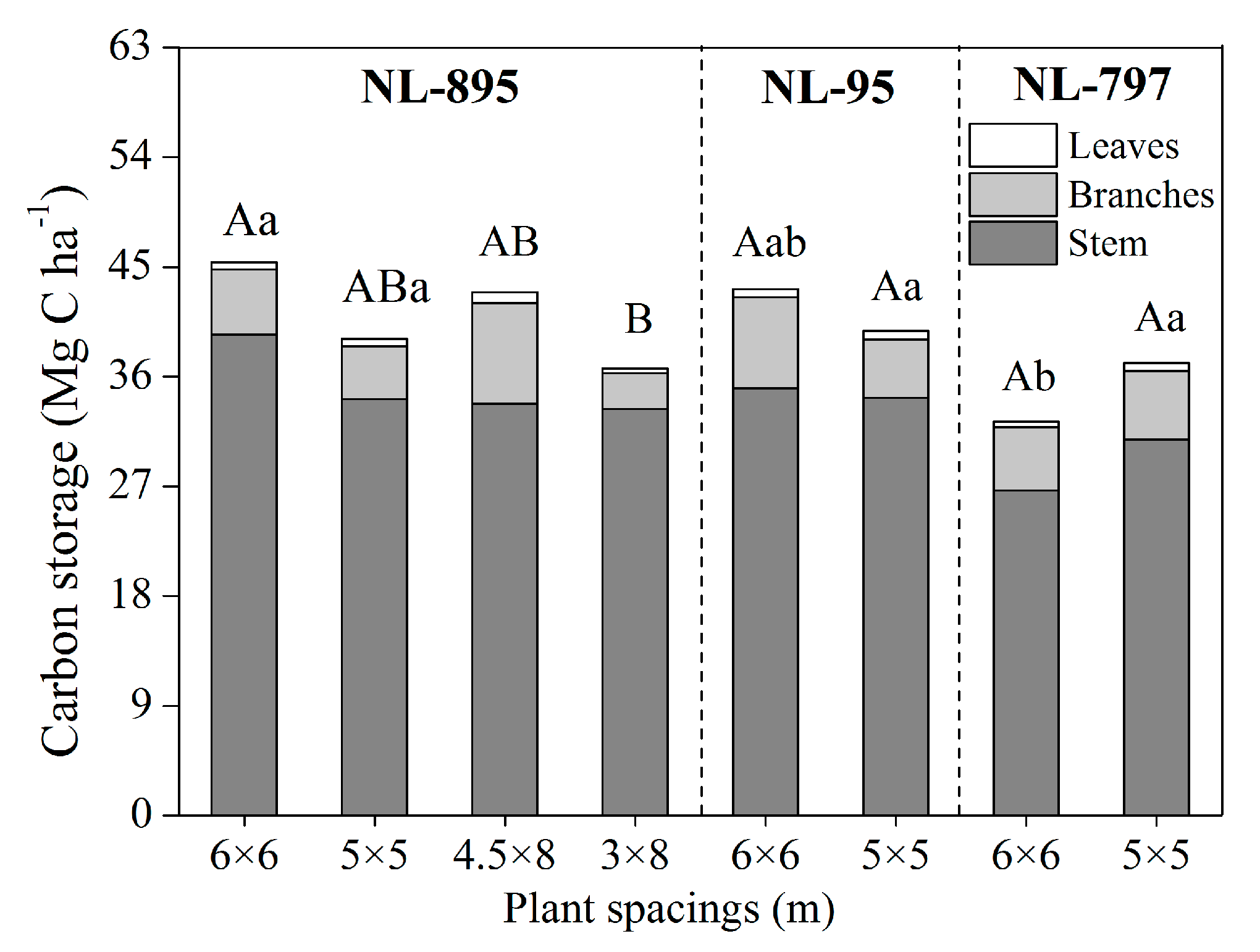
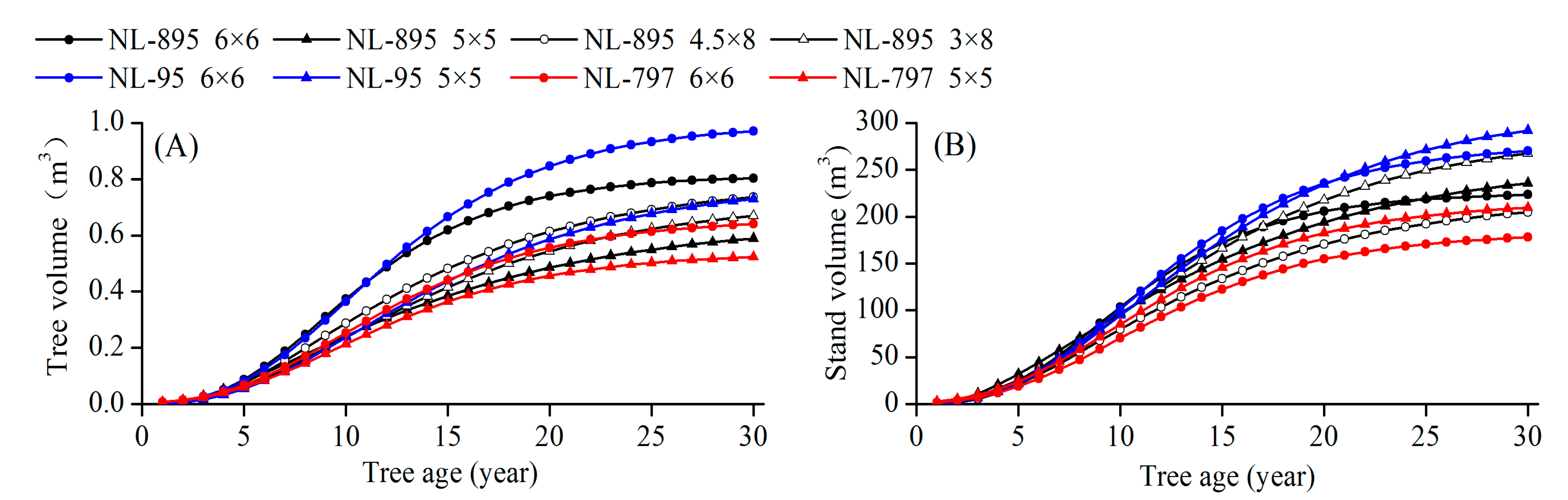
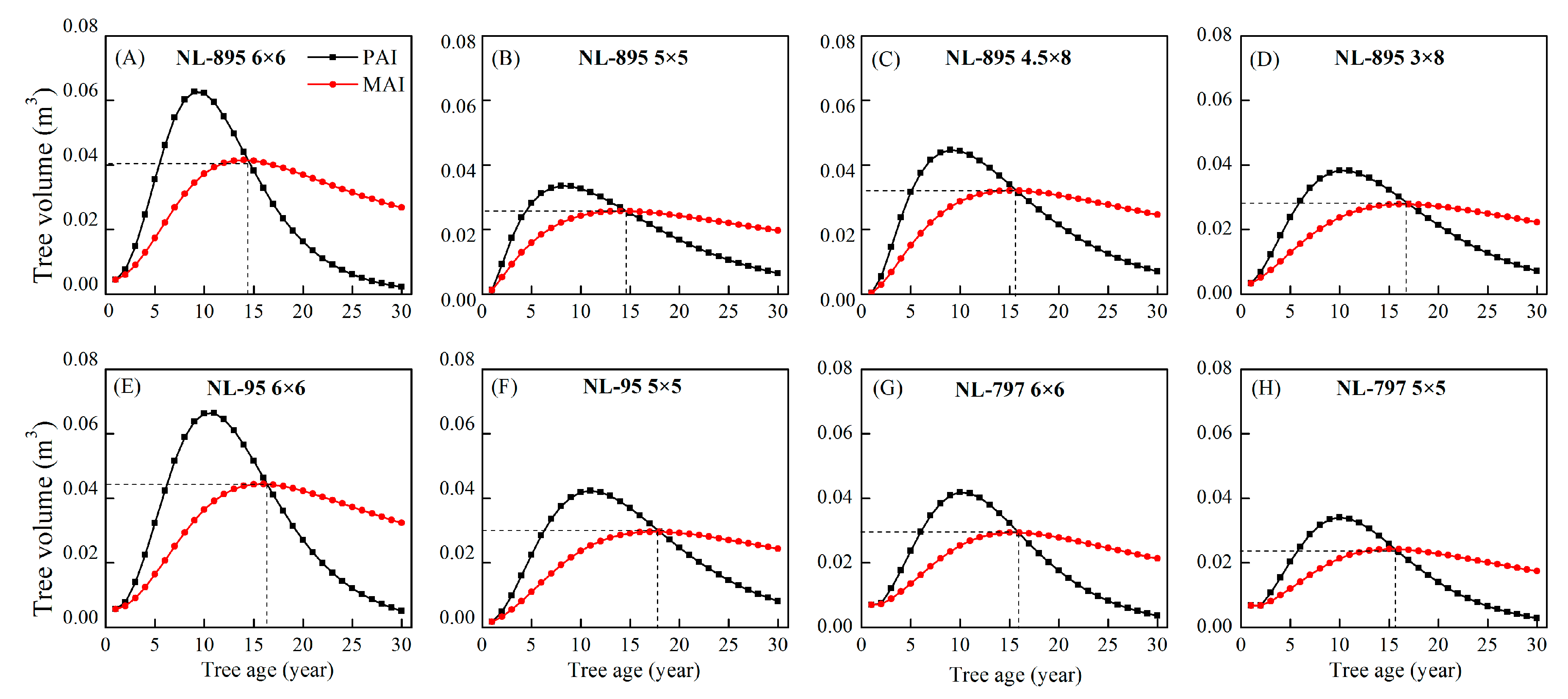
| Index | Treatment | df | Stand Age (years) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |||
| Diameter | Clone (C) | 2 | 0.35 | 0.11 | 0.34 | 0.58 | 0.72 | 1.37 | 2.40 | 2.72 | 3.25 | 3.55 | 3.95 * | 4.12 * | 3.79 * |
| At breast height | Spacing (S) | 3 | 0.86 | 0.76 | 0.74 | 0.54 | 1.25 | 2.75 | 3.82 * | 4.73 ** | 5.89 ** | 7.15 ** | 8.65 ** | 9.17 ** | 8.23 ** |
| C × S | 2 | 0.76 | 1.32 | 0.56 | 0.19 | 0.79 | 1.09 | 1.01 | 1.28 | 1.34 | 1.79 | 2.30 | 2.54 | 2.56 | |
| Tree height | C | 2 | 0.27 | 0.82 | 0.79 | 0.48 | 1.27 | 0.79 | 0.70 | 1.32 | 1.60 | 5.85 * | 13.41 * | 9.93 * | 8.89 * |
| S | 3 | 0.57 | 0.15 | 0.41 | 0.64 | 1.75 | 3.14 | 1.33 | 0.64 | 0.74 | 3.18 | 3.16 | 3.09 | 3.66 * | |
| C × S | 2 | 0.27 | 0.85 | 0.42 | 1.30 | 0.84 | 0.97 | 0.77 | 0.67 | 0.21 | 0.67 | 2.10 | 0.96 | 0.55 | |
| Tree volume | C | 2 | 2.04 | 0.09 | 0.26 | 0.49 | 1.19 | 2.36 | 4.08 * | 4.92 * | 5.34 * | 6.26 * | 7.21 * | 7.10 * | 6.33 * |
| S | 3 | 2.77 | 0.51 | 0.44 | 0.47 | 1.28 | 3.25 * | 4.87 * | 6.81 * | 8.74 * | 10.72 * | 12.83 * | 12.77 * | 11.229 * | |
| C × S | 2 | 2.05 | 1.18 | 0.90 | 0.46 | 0.55 | 0.82 | 0.92 | 1.30 | 1.49 | 2.04 | 2.68 | 2.80 | 2.73 | |
| Poplar Clone | Planting Spacing (m) | Biomass Components | ||
|---|---|---|---|---|
| Leaf | Branch | Stem | ||
| NL-895 | 6 × 6 | 39.79 ± 0.14 Cb | 46.11 ± 0.28 Ba | 47.76 ± 0.25 Aa |
| 5 × 5 | 39.85 ± 0.23 Bb | 46.08 ± 0.29 Aa | 46.69 ± 0.26 Aa | |
| 4.5 × 8 | 40.11 ± 0.41 B | 46.01 ± 0.25 A | 46.88 ± 0.09 A | |
| 3 × 8 | 40.92 ± 0.58 B | 46.41 ± 0.39 A | 46.66 ± 0.27 A | |
| NL-95 | 6 × 6 | 41.23 ± 0.91 Bab | 46.15 ± 0.4 Aa | 46.01 ± 0.21 Ab |
| 5 × 5 | 41.11 ± 0.64 Bab | 46.4 ± 0.19 Aa | 46.09 ± 0.08 Aa | |
| NL-797 | 6 × 6 | 42.35 ± 0.4 Ba | 46.88 ± 0.18 Aa | 46.66 ± 0.09 Ab |
| 5 × 5 | 42.29 ± 0.31 Ba | 46.67 ± 0.25 Aa | 46.56 ± 0.21 Aa | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tian, Y.; Ding, S.; Lv, Y.; Samjhana, W.; Fang, S. Growth, Carbon Storage, and Optimal Rotation in Poplar Plantations: A Case Study on Clone and Planting Spacing Effects. Forests 2020, 11, 842. https://doi.org/10.3390/f11080842
Zhang Y, Tian Y, Ding S, Lv Y, Samjhana W, Fang S. Growth, Carbon Storage, and Optimal Rotation in Poplar Plantations: A Case Study on Clone and Planting Spacing Effects. Forests. 2020; 11(8):842. https://doi.org/10.3390/f11080842
Chicago/Turabian StyleZhang, Yanhua, Ye Tian, Sihui Ding, Yi Lv, Wagle Samjhana, and Shengzuo Fang. 2020. "Growth, Carbon Storage, and Optimal Rotation in Poplar Plantations: A Case Study on Clone and Planting Spacing Effects" Forests 11, no. 8: 842. https://doi.org/10.3390/f11080842





